Abstract
Due to their antimicrobial activity, parabens (i.e. alkyl esters of p-hydroxybenzoic acid) are widely used as preservatives in several industries (pharmaceutical, food, cosmetic). Although being extremely effective, their usage is hampered by their low aqueous solubility. Several formulation strategies can be applied to enhance their solubility, one of which is formation of water-soluble cyclodextrin (CD) complexes. Formation of inclusion complexes has been proved to be a good approach to increase solubility of lipophilic drugs and other active ingredients. Some research has been done in this field. However, a complete and comprehensive study on how the alkyl chain length of parabens influences the complex formation, aggregation and formation of insoluble complexes is still lacking. Phase-solubility studies showed that all the very water-soluble hydroxypropylated CDs form linear (AL) type phase-solubility profiles with all tested parabens. The poorly soluble βCD did also form AL-type profiles with methyl and ethyl paraben while the βCD complexes of propyl and butyl paraben have limited solubility in water and, thus displayed B-type profiles. The paraben complexes of αCD and γCD all had limited solubility in water and, thus, displayed B-type phase-solubility profiles. Fourier-transformed infrared spectroscopy, Differential scanning calorimetry and X-ray powder diffraction were applied to elucidate the nature of the solid phases from the phase-solubility studies. They consistently showed the presence of solid pure paraben over the CD concentration range studied when AL-type profiles were observed, and precipitation of poorly soluble paraben/CD complexes when B-type were observed (i.e. during and after the B-type plateau region). These studies demonstrate that the composition of solid phases is related to the type of phase-solubility profile. It was also shown that in aqueous CD solutions, paraben solubilization increase with increasing side chain length (i.e. methyl < ethyl < propyl < butyl), as well as, with increasing size of the CD cavity (i.e. αCD < βCD < γCD). This statement is valid for linear region of phase-solubility diagrams (i.e. A- and B-type).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkyl esters of p-hydroxybenzoic acid (so called parabens) are commonly used as preservatives in the cosmetic, food and pharmaceutical industry [1, 2]. However, their use is hampered by their very low aqueous solubility [3,4,5]. To overcome these limitations several approaches can be used, one of which is the formation of cyclodextrin (CD) complexes [6,7,8]. The ability of CDs to form water-soluble complexes and their very favorable toxicological profile has made them versatile and useful solubilizing excipients [5, 9, 10]. In aqueous solutions, CDs are able to solubilize many hydrophobic drugs through inclusion complex formation, by including a lipophilic moiety of the drug molecule into the central cavity of the CD or by formation of non-inclusion complexes [4, 6]. However, in some cases, interaction of guest molecules with CD can decrease their apparent aqueous solubility, leading to the precipitation of drug/CD complexes at certain drug/CD ratio and thus limiting their usage [11].
Interaction of parabens with native CDs [3, 5, 12,13,14,15,16] and their derivatives [4, 17,18,19,20,21,22] has been studied previously. However, a comprehensive and systematic understanding of CDs behavior regarding complex formation, aggregation and formation of insoluble complexes with parabens and other guest molecules in general is still missing. Here we present a systematic study of how the parent CDs and their hydroxypropylated derivatives solubilize various parabens (Fig. 1). We address how the diameter of the CD cavity affects the complexation, how the parabens affect CD solubility in aqueous media, and the composition of the solid phase formed. The solid phases from various phase-solubility studies were used to identify and characterize the different stages of the paraben/CD systems through analytical techniques that include fourier transformed infrared spectroscopy (FTIR), differential scanning calorimetry (DSC) and X-ray powder diffraction (XRPD). Characterization of the solid phase reveals its composition, solubility and crystalline structure.
Materials and methods
Materials
The unsubstituted native α-cyclodextrin (αCD), β-cyclodextrin (βCD) and γ-cyclodextrin (γCD), as well as, 2-hydroxypropyl-βCD (HPβCD) DS 4.2 (MW 1380), were kindly provided by Janssen Pharmaceutica (Beerse, Belgium). 2-Hydroxypropyl-αCD (HPαCD) with degree of substitution 3.6 (MW 1180) and 2-hydroxylpropyl-γCD (HPγCD) DS 4.2 (MW 1540) were purchased from Wacker Chemie (Burghausen, Germany). Methyl- (MP), ethyl- (EP), propyl- (PP), and butyl- (BP) paraben were kindly provided by Janssen Pharmaceutica (Beerse, Belgium). Milli-Q water (Millipore, Billerica, MA) was used to prepare CD solutions and mobile phases. The solvent used for analysis (acetonitrile) was of HPLC grade and obtained from Sigma-Aldrich (St. Louis, Missouri, USA).
Cyclodextrin solutions preparation
Aqueous stock solutions of CDs were prepared by sonication in a sonication bath at 60 °C for 60 min and then the solutions were allowed to cool to room temperature. The test solutions were prepared by dilution of these stock solutions. The CD concentration range depended on the intrinsic solubility of CD being tested. The parabens were chemically stable during the preparation of the test solutions and their storage.
Quantitative determination of CD/paraben samples
A reverse-phase ultra high-performance liquid chromatography system (UHPLC) from Dionex Softron GmbH (Germering, Germany) was used for quantification of CDs and parabens. The Ultimate 3000 series consisting of a LPG-3400SD pump with a built-in degasser, a WPS-3000 autosampler, a TCC-3100 column compartment, and a Corona® ultra RS detector. Phenomenex Kinetex C18 150 × 4.60 mm 5 µm column (stationary phase) with a matching HPLC Security Guard (Phenomenex, Cheshire, UK) were used. The mobile phase used consisted of acetonitrile and water (50:50). The flow rate was set to 1.0 mL/min and temperature of the column to 30 °C. The injection volume was 10 µL. Chromatogram were evaluated using Chromeleon version 7.2 SR4 (ThermoFisher Scientific, MA, USA).
We are able to quantify the actual concentration of CDs present in solution after each experiment with the help of a Corona detector as described in our previous publications [5, 23]. This constitutes a novelty to previous approach where theoretical CD concentrations were used to draw phase-solubility diagrams.
Phase-solubility experiments
An isothermal saturation method was used to get phase-solubility profiles for the paraben/CD systems. An excess amount of the solid paraben (i.e. amount of paraben needed to assure the presence of solid phase at the equilibrium stage) to be tested and 3 mL of an aqueous CD solution of given concentration were added to each vial. The procedure was identical for all 6 CDs tested and the 4 parabens and each study was performed in triplicate. The suspensions formed were kept at 25 °C under constant stirring for 48 h. After equilibrium was attained the mixtures were centrifuged (3000 rpm/10 min) using Rotina 35R (Hetich Zentrifugen, Germany). The supernatants were filtered through 0.45 µm Phenex-RC filters (Phenomenex, Cheshire, UK) and diluted with Milli-Q water before they were analyzed by UHPLC. Solid phases were collected and allowed to dry at 35 °C for two days and used for the solid-state analysis experiments (DSC, FTIR and XPRD).
Phase-solubility profiles were determined according to the method of Higuchi and Connors [24]. The apparent stability constant (K1:1, Eq. 1) and the complexation efficiency (CE, Eq. 2) were determined from the slope of the linear phase-solubility diagrams (i.e. plots of the total drug solubility ([D]t) versus concentration of CD in liquid phase ([CD]liq) in moles per liter) [25]:
where S0 is the intrinsic solubility of the respective paraben.
The maximum increase (in percentage) in apparent solubility of paraben per mM of CD (increase in solubility, IS) for each paraben/CD system was calculated using Eq. 3:
where Parabenmax corresponds to highest solubility of complex and CDmax the actual concentration of CD (measured in the liquid phase at equilibrium) needed to achieve it. These values were calculated and are valid for linear parts of the phase-solubility diagrams.
Fourier transformed infrared (FTIR) spectroscopy
FT-IR spectra of the raw materials (i.e. CDs and parabens) as well as solid phases from phase-solubility studies were recorded at room temperature on a Thermo Nicolet iZ10 spectrometer (Thermo Scientific, Madison, USA) over the range of 600–4000 cm−1 with the resolution of 4 cm−1.
Differential scanning calorimetry (DSC)
The DSC thermograms were recorded on Netzsch DSC 214 polyma (Netzsch GmbH, Germany). Samples of parabens/CDs complex, pure native CDs and their hydroxypropylated derivatives, as well as the dried solid phases from the phase solubility studies, were placed in closed pierced aluminum crucibles and an identical empty one used as reference. The samples were analyzed in the temperature range 25–200 °C at a rate of 10°/min under nitrogen atmosphere.
X-ray powder diffraction (XRPD) studies
X-ray powder diffractometry (Empyrean, PANalytical) was used to identify the crystal structures present in the solid phases. Wide angle XRPD using CuKα radiation with the voltage and working current of 45 kV and 40 mA, respectively, was employed. The scan speed, 2θ scan range and step size was set at 0.0691°/s, 4–55° and 0.013°, respectively. Native CDs, pure parabens (as received), solid phase of parabens submitted to solubility experiment (SE) and solid phases from phase-solubility experiments containing native CDs and parabens were tested.
Results and discussion
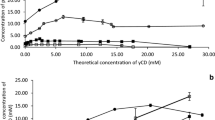
Phase-solubility studies of parabens with a wide range of cyclodextrins [4, 5, 12, 18, 20,21,22] reveal that we can expect two types of phase-solubility diagrams (i.e. A-type and B-type in Fig. 2) giving two phases (i.e. a liquid and a solid) after centrifugation.
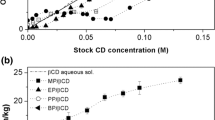
Traditionally only the liquid phase is characterized and only with respect to the amount of guest (e.g., paraben) brought into solution. Thus, for the large majority of the available phase-solubility studies of guest molecule/CD interactions characterization of the solid phase and the amount of CD in solution has been omitted. Here we wish to correlate the composition of both the liquid and solid phase with the type of phase-solubility diagram obtained. For that purpose, we have utilized a UHPLC system equipped with a Corona detector allowing simultaneous quantification of both CD and parabens in one analysis for full characterization of the composition of the liquid phase. This constituted one of the novelties of this work as contrarily to most publications in the field (which are based on theoretical CD concentration), our graphs and calculated parameters are based on liquid phase CD concentrations at equilibrium. In Fig. 3 we can observe how different phase-solubility diagrams when they are represented by actual or theoretical CD concentrations. It is actually just valid for B-types as for A-types the theoretical and actual CD concentrations remain practically the same throughout the phase-solubility experiments.
The solid phases where characterized using a range of solid state characterization techniques (i.e. FTIR, DSC and XPRD). In general, the solid phases analyzed where selected at the highest CD concentration for the A-type profiles, while at least two CD concentrations were tested for the B-type profiles (i.e. before and after the plateau region).
Phase-solubility studies
Formation of a paraben/CD complex normally enhanced the paraben solubility in aqueous media. To evaluate the paraben/CD interaction phase-solubility diagrams were constructed by plotting the concentration of CD in liquid phase (actual CD concentration) versus concentration of the dissolved paraben as determined by UHPLC (Figs. 4, 5). From these diagrams the slopes were determined, using all the CD concentration range (for A-type diagrams) or just in the initial linear portion where actual and theoretical CD concentrations are coincident (for B-type diagrams). Then apparent stability constants (K1:1) and the complexation efficiencies (CE) were determined from the slopes (Table 1). Previous studies showed that native CDs have low aqueous solubility in comparison to their hydroxypropylated derivatives and that the native CDs can self-assemble and form aggregates. This behavior will thus to some extent be an integral part of every phase-solubility profile and should be taken into consideration during their interpretation [26, 27].
Native cyclodextrins
Table 1 depicts that generally for aqueous native CD solutions, all parabens displayed BS-type phase-solubility profiles. This means that the paraben/CD complexes have limited solubility in the aqueous complexation media. In fact, this is a common and already expected behavior of the unsubstituted CDs, due to relatively strong binding of the CD molecules in the crystal state and, consequently, their rather limited aqueous solubility.
Three characteristic regions in the BS-type profiles can be identified (Fig. 2). In the initial linear region, the increasing CD concentration promotes formation of water-soluble paraben/CD complexes that increase the total solubility of the parabens. The second region is where the maximum paraben solubility (the sum of free paraben and paraben in complex) is achieved. Finally, a third region is observed where all the solid paraben has been consumed and the increase of CD concentration results in formation of insoluble paraben/CD complexes. The only exceptions from this trend were observed for the MP/βCD and EP/βCD systems, where AL-type phase-solubility profiles were observed (probably due to the low solubility of βCD and, consequently, low βCD concentrations used in the experiments) and the PP/γCD and BP/γCD systems that were of Bi-type phase-solubility profiles were observed (Fig. 4). The Bi-type profile is similar to the BS-type except that the formed complexes are so insoluble that they do not provide any increase in the paraben solubility [6].
The AL phase-solubility profile provides a linear increase of solubility with increasing CD concentration meaning that throughout all the concentration range, soluble complexes are formed.
In the case of MP and the native CDs, a slope greater than unity was registered. This may indicate that more than one MP molecule (i.e. paraben2/CD, paraben3/CD, etc.) is involved in the complex formation, aggregates have been formed or, in other words, that higher order complexes with respect to the paraben are being formed [6]. For the same reason, we observe in Table 1 that higher order complexes were formed for the EP/γCD system. All remaining systems studied (native paraben/CD complexes) could be described using model assuming a simple 1:1 stoichiometry with slopes of less than unity. This is an indication that the complex formation follows a 1:1 stoichiometry, but not a definite proof. These observations correlates with previous findings [5].
The analysis of IS and slope values suggest that overall γCD was the best native CD to be used as solubilizer of parabens (overall both IS and slope values increased with increasing CD cavity size). Exception was registered for BP where βCD was the best option. Table 1 also shows that generally IS increased with increasing alkyl chain meaning that CDs are better solubilizing agents the larger the side chain of parabens is (MP < EP < PP < BP).
Hydroxypropylated cyclodextrins
As expected all the hydroxypropylated CDs (HP-CDs) tested were able to solubilize the parabens and the apparent solubility of MP, EP, PP and BP increases linearly with increasing CD concentrations regardless of CD cavity size (Table 1). All phase-solubility diagrams for the HP-CDs have straight lines exemplifying AL-type profiles (Fig. 5). Several authors have reported similar profile type results for HPβCD solubilization of parabens [4, 20, 28] .
The phase-solubility profiles of all four parabens and HPαCD can be interpreted using a model assuming a simple 1:1 stoichiometry. This also applied to the phase-solubility diagrams obtained with PP and BP and HPβCD.
However, both MP and EP gave phase-solubility profiles with slopes greater that unity with HPβCD. Furthermore, all four parabens with HPγCD gave phase-solubility profiles with slopes greater that unity. This also indicates that higher order complexes are being formed. As in the case of the native CDs, the maximum increase of apparent parabens solubility per mM of HP-CDs increased with increasing paraben lipophilicity (i.e. increasing side chain length) as well as with increasing CD diameter of central cavity (i.e. αCD < βCD < γCD).
Apart from the differences in their profiles, mainly BS-type for native CDs and AL-type for their HP derivatives, latter ones showed slope and IS values quite similar to the native CDs showing that they were not so much better solubilizing agents for all the parabens tested (except the case of BP/γCD and BP/HPγCD were difference is considerable). In fact, αCD provided greater IS values than HPαCD. In spite of this it should be emphasized that HP-CDs provide a greater concentration range and allow for higher concentrations of dissolved parabens.
Fourier transform infrared spectroscopy (FTIR)
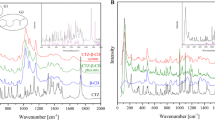
FTIR was used to elucidate the interaction between parabens and CDs, as shifts of the vibrational wavelengths of the components due to presence of inclusion complexes could be expected [29]. The IR data from solid phases from the phase-solubility studies are summarized in Table 2 and some examples are shown in Figs. 6 and 7.
The spectrum of each paraben (solid phase collected from the solubility experiments: SE) was determined and characteristic peaks selected: around 3300/3200 cm−1 (O–H); ≈ 3000 cm−1 (phenyl group); ≈ 1680 cm− 1 (C=O) and finally ≈ 770 cm−1 (C–H) [30]. These were used as reference peaks and compared against spectra obtained for all tested systems. Changes in these characteristic bands, either by disappearing/shifting or reducing their intensity, can reveal an interaction between CD and paraben with formation of a new compound (e.g., inclusion complexes).
Due to the extensive number of samples, one representative CD concentration point was analyzed in the case of A-type systems (highest CD concentration point) while for the B-type system two CD concentrations points were used (before and after plateau region).
Native cyclodextrins
Figure 6 shows representative FTIR spectra of the evaluated systems but it confers the trend of all studied samples (Table 2). At the top are spectra for a BS-type system (MP/αCD 1%) and for an AL-type (MP/βCD 1.5%). Comparing them with reference materials (MPSE, αCD and βCD) it is observed that both solid phases are dominated by MP (i.e. no detectable MP/βCD complexes are present), as their spectra are identical to the one of MP and no characteristic bands from CDs are visible. However, the MP/αCD system is different. As shown in Table 2, the second studied point for this system (MP/αCD at 10% αCD, immediately after the plateau region) showed slight shifts of characteristic paraben peaks, most likely due to the presence of αCD or insoluble MP/αCD complex.
Lower section of Fig. 6 shows FTIR spectrum of a Bi-type system (PP/γCD at 10% γCD, after plateau) and some differences of the characteristic PP peaks are noticed when compared to the reference (PPSE). At this stage the PP/CD ratio is extremely low so it might occur that in this spectrum the barely observed PP peaks are overlapped by the CD peaks.
Some similarities to the γCD spectra were found (especially in the fingerprint region) but also small differences (especially for the carbonyl group). This data suggests that the solid phase from PP/γCD at 10% γCD is probably composed by a new entity (most likely PP/γCD inclusion complex) or a mixture of PP/γCD inclusion complex and pure PP.
Hydroxypropylated cyclodextrins
In general, the observations made for the αCD and βCD systems (i.e. MP/αCD at 1% αCD and MP/βCD at 1.5% βCD) are also valid for the HP-CD systems shown in Fig. 7. As the phase-solubility studies show, all HP-CDs displayed AL-type diagrams for all studied parabens. For that reason, FTIR results (Fig. 7) shown that the spectrum of each solid phase from the paraben/HP-CD phase-solubility system is almost identical to the one of corresponding paraben with no significant shifts of the most characteristic peaks. This is typical and characteristic of all the paraben/HP-CD systems studied and suggests that solid phases consist of pure paraben. The non-existence of any HP-CD peaks in the IR spectra of the solid phases, further underlines that no solid paraben/HP-CD complexes were formed.
Differential scanning calorimetry (DSC) studies
DSC is one of the basic techniques used to study solid CD complexes, as interactions between drug/CD in the solid phase can be detected by shifting/disappearing of melting points of the individual components. The obtained phase-solubility data suggested formation of very water-soluble paraben/CD complexes in the case of HP-CDs but formation of insoluble paraben/CD complexes in case of the unsubstituted (i.e. native) CDs. DSC experiments of the obtained solid phases were carried out. To trace their state, solid samples representing all experimental studied systems were tested.
Native cyclodextrins
DSC results (Fig. 8) show typical thermal curves from crystalline anhydrous substances for parabens, with a well-defined sharp endothermic peak corresponding to the paraben melting point. As it is already described in the literature [1, 13] that melting points of parabens decrease with increasing alkyl side chain: MP (125.2 °C) > EP (115.2 °C) > PP (95.7 °C) > BP (69.0 °C). As previously shown by Caira et al. the formation of paraben/CD complexes leads to the disappearance of the endothermic melting peak, as expected for guest/CD complexes in general [13]. The CDs themselves do not display any melting peak but decomposition above 300 °C [31,32,33]. The temperature range in this study was 0 to 200 °C. The solid phases from the solubility experiments of the different parabens in pure aqueous solution (described as SE) were used as references during interpretation of the results. Due to the extensive amount of data and in order to provide better understanding of the results, Fig. 8 is shown as a representative example of the solid phases obtained from the phase-solubility profiles of the native CDs: AL-type (EP/βCD), BS-type (PP/αCD) and finally Bi-type (BP/γCD).
In the first depicted system (EP/βCD) one endothermic peak in both samples is observed (114.6 and 115.4 °C for EP/βCD 0.2% and EP/βCD 1.5%, respectively) that represents the melting peak of pure EP. This confirms the results obtained with previous techniques where for AL-type profiles, highly soluble inclusion complexes are formed without any precipitation of the complex (e.g., the initial and final CD concentration are close to identical) regardless of the CD concentration. For this reason, solid phase content exclusively consists of the pure paraben with similar melting temperature as the reference paraben (SE). PP/αCD was selected as a representative of BS-type phase-solubility diagram where two CD concentration points in the diagram are analyzed, one in the linear increasing region (PP/αCD at 1% αCD) and the other after the plateau region (PP/αCD at 10% αCD). In this case, thermograms of the solid phases have some distinctive features depending on the region of phase-solubility diagram. For the solid phase from PP/αCD 1% (taken from the part of the phase-solubility diagram that is similar to AL-type profiles), only one melting peak can be observed with a peak temperature lowered by 5 °C. This shift might be related to differences in the crystalline state (e.g. size and homogeneity) between the reference solid PP (PPSE) and the treated samples. This interpretation was supported by performing a second scan after cooling of the sample where the melting peak of pure PP 95.6 °C (data not shown) was observed. Increasing the αCD concentration led to formation of poorly soluble PP/αCD inclusion complexes that precipitated from the solution. After all non-complexed pure PP from the solid phase has been converted to either complex in solution or solid precipitated complex (i.e. after plateau region) the composition of the solid phase changes and only the insoluble complex could be found. That is why thermograms for added CD concentrations until the plateau region showed a gradual decrease of PP peak melting temperature with increasing CD concentration up to complete disappearing of it past the plateau region. Solid phase obtained at 10% αCD showed no melting peak that could be ascribed to PPSE, since it is already situated in phase-solubility diagram descendent part and so all PP in solid phase will be exclusively present as a new solid entity, the PP/αCD inclusion complex. Only one broad endothermic peak starting around 100 °C is observed which could be associated with the CD dehydration process [31,32,33].
Last example shown in Fig. 8 is the BP/γCD system (Bi-type). Similar explanations as described above for BS-type solid phases can be given, although with a notable difference. The formed inclusion complex is so insoluble that it starts to precipitate as soon as the solid paraben interacts with CD medium. This suggests that pure paraben will never be present in the solid phase of Bi-type systems. Instead a mixture of paraben and its CD complex is detected throughout the whole CD concentration range. The presence of pure paraben in the descendent part will only depend on CD availability in the liquid phase as well as on the amount of paraben added. Despite that in the BP/γCD at 1% γCD thermogram only the BP peak is visible also inclusion complex must be present. The complex is probably not present in high enough amount to be detected or has a melting temperature above the range of study/decomposition temperature of the CD. Regarding the solid sample collected beyond the plateau region (BP/γCD at 10% γCD) inclusion complex in the solid phase was shown to be present by a broad endothermic peak (approximately at 110 °C) due to the dehydration process of CD [31,32,33] together with absence of BP melting peak.
Hydroxypropylated cyclodextrins
Representative examples of DSC diagrams of the solid phases of the paraben/HP-CD systems obtained at 25% CD concentration (MP/HPαCD 25%; EP/HPγCD 25%; BP/HPβCD 25%) are shown in Fig. 9. No significant difference between onset and peak temperatures for the melting peaks of parabens and representative solid phases was observed, suggesting that solid parabens (present in the solid phases) maintained their original crystal structures. This was valid for all paraben/HP-CDs combinations tested. These results demonstrate that paraben/HP-CD complexes exist only in the liquid phase and therefore increasing CD concentration will only promote dissolution of the solid paraben present in the system.
X-ray powder diffraction (XRPD) studies
XRPD analysis was used to identify the crystalline nature of the solid phase precipitates. Only the paraben/native CD solid phases were studied since both DSC and FTIR had shown that the solid phases of the parabens/HP-CDs systems did not contain solid paraben/CD complexes. All the analyzed diffractograms presented high quality diffraction patterns.
It should be noted that pure EP and BP (as received) have somewhat different XRPD pattern in comparison to EPSE and BPSE (i.e. solid phases from the phase-solubility experiments). This is contrarily to the XRPD patterns of MP and PP that look identical (Fig. 10). The XRPD results suggest that pure EP and BP (as received) do not have the same crystal maturity (e.g. size and homogeneity) as the ones that have been subjected to the solubility studies. There are several explanations for this observation. First, the production methods and purification of commercial parabens can affect the crystal structure. Different processes will most probably result in varying qualities of the crystals produced. Second, the solubility study method may be considered to be a slow recrystallization process for the parabens with a constant dissolution and crystallization of the parabens resulting in more homogeneous crystals. This underlines that XRDP of the SE samples, in contrast to the “as received” samples, are needed in order to provide appropriate diffractograms for comparison with the ones containing CDs.
In Fig. 11, XRPD patterns of parabens SE, the native CDs and four paraben/CD systems are presented. Although only a selection of the obtained data is shown, it is illustrative of the trends obtained for the various phase-solubility profiles of the paraben/native CD systems studied (AL, BS and Bi-type). The results show that all parabens precipitate as CD complexes at high αCD and γCD concentrations, but just PP and BP are able to form solid complexes at lower CD concentrations and with βCD. These complexes can be identified by diffraction peaks at low diffraction angle as seen from the crystal structures of the CDs. This signifies (relatively) long range order in the crystals.
The EP/αCD and BP/βCD systems represent solid phases from Bs-type profiles (Fig. 11). The EP/αCD 2% solid phase contains EP crystals but no signal from the complex is observed. This is in accordance with linear part of phase-solubility diagram of this system. No solid complexes are formed since it is still under complex saturation concentration (moreover no notable changes of the αCD concentration were detected in the liquid phase).
The XRPD spectrum of EP/αCD 10% concentration point shows crystals that are neither pure EP nor αCD. At this point of the phase-solubility diagram, that is post the plateau region, the poorly soluble EP/αCD inclusion complex starts to precipitate and the new crystalline entities present in solid phase are most probably one or more type of EP/αCD inclusion complex crystals. Quantification of EP/αCD 10% liquid phase showed that 65% of the initial αCD concentration added was still present, so if some solid EP would still be in the system it would be complexed by this remaining amount of αCD (form insoluble complex). This explains why tendentially no pure EP (or just residual amount) can be found in solid phase. The same explanation can be given for the BP/βCD 1.5% system, where the XRPD data suggests the mainly the presence of BP/βCD complex but also residual traces from pure BP.
Figure 11 also shows that for the MP/βCD 1.5% system, even at the highest βCD concentration possible, the MP/βCD complex formed will always stay in the liquid phase (does not precipitate) and, consequently, only crystals identical to MPSE are detected in the solid phase. This behavior is a trend observed for all systems that follow the AL-type phase-solubility profile. Previously Caira et al. published XRPD spectra showing that βCD can form inclusion complexes with this series of parabens which is in accordance with presented results [13].
Representative XRPD spectra for systems following the Bi-profiles are also illustrated in Fig. 11 by the PP/γCD system. Similarly to the description of the EP/αCD system, the diffractogram obtained in the plateau region (PP/γCD 0.5%) was almost identical to PPSE. The presence of PP crystals in the solid phase was confirmed and γCD concentration in the liquid phase not affected. The solid phase from PP/γCD 10% (in the post plateau region) showed a pattern quite different from crystalline PP and γCD. It would be a mixture of pure PP and complex if solid PP would be added in quantity large enough to deplete all the available γCD which is still present in the liquid phase at this stage. Approximately 90% of initial γCD was detected in this supernatant, which suggests that insoluble PP/γCD complex is the main constituent of the solid phase. The complexation with native CDs may promote recrystallization of parabens giving them different paraben crystal dimensions compared to SE.
Conclusions
Based on the phase-solubility study we can conclude that all parabens form complexes with the native CDs and that the formed complexes have limited water solubility resulting in B-type phase-solubility profiles. Exceptions are the MP/βCD and EP/βCD profiles that are of AL-type probably due to the poor solubility of βCD and, thus, high enough βCD concentrations cannot be obtained to observe BS-type diagrams.
Hydroxypropylated CDs increase substantially the paraben solubility in comparison to the native CDs. The hydroxypropylated CD derivatives consist of isomeric mixtures that lead to enhanced aqueous solubility and, thus, their aqueous solubility, as well as those of their complexes, is much greater that of their parent CDs. In case of the HP-CDs increased CD concentration will result in increased paraben solubilization (positive effect) but for the native CDs it will enhance precipitation of the paraben/CD complex and decreased apparent solubility of both paraben and CD. The IS values show on one hand that γCD was the best native CD to increase solubility of parabens, except in the case of BP (which was βCD). On the other hand, HPγCD was shown to be the best solubilizer of the HP-CDs tested. Difference in IS values between native CD and its correspondent HP-derivative is not significant, although HP-CDs permit solubilization of greater amounts of parabens (due to their greater water solubility). Slope values higher than unity show that some parabens can form higher order complexes (i.e. paraben2/CD, paraben3/CD, etc.). These higher order complexes with respect to the paraben were detected for all MP systems (except for HPαCD), for EP with γCD/HPβCD/HPγCD and for PP/BP with HPγCD. The remaining complexes were described using model assuming a simple paraben/CD 1:1 stoichiometry.
In general, the analysis of the solid phases by FTIR, DSC and XRPD lead to similar conclusions, supporting results obtained from phase-solubility experiments. It was shown that parabens and HP-CDs can interact and form inclusion complexes that are highly water-soluble and, consequently, their complexes will never be present in the solid phase. On the other hand, native CDs form complexes with limited water solubility that start to precipitate as solid phase after the solubility of the complex has been reached.
In Fig. 2, a schematic representation of the two types of phase-solubility profiles (A- and B-type) is presented. After analysis and combination of data it can be concluded that for A-type phase solubility diagrams only the excess of pure paraben can be found in the solid phase. On the other hand, for the B-type phase-solubility profiles composition of solid phase will vary depending on the CD concentration. Before the plateau region, only solid paraben can be detected but beyond the plateau region, mainly solid paraben/CD complex can be found. In the plateau region, a mixture of pure solid paraben and solid complex will be present.
Finally, it can be concluded that the composition of solid phases is dependent on the type of phase-solubility profile. Also that CD binding of parabens and, consequently, the solubilization of parabens in aqueous CD solutions increases with increasing size of the alkyl side chain (i.e. MP > EP > PP > BP) as well as with increasing diameter of the CD cavity (αCD < βCD < γCD). Overall saturation of test solutions containing the native (i.e. unsubstituted) CD will partially or fully transform solid parabens into insoluble paraben/CD complexes.
References
Giordano, F., Bettini, R., Donini, C., Gazzaniga, A., Caira, M.R., Zhang, G.G.Z., Grant, D.J.W.: Physical properties of parabens and their mixtures: solubility in water, thermal behavior, and crystal structures. J. Pharm. Sci. 88(11), 1210–1216 (1999). https://doi.org/10.1021/js9900452
Ma, M., Lee, T., Kwong, E.: Interaction of methylparaben preservative with selected sugars and sugar alcohols. J. Pharm. Sci. 91(7), 1715–1723 (2002). https://doi.org/10.1002/jps.10167
Jude Jenita, M., Thulasidhasan, J., Rajendiran, N.: Encapsulation of alkylparabens with natural and modified α- and β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 79(3), 365–381 (2014). https://doi.org/10.1007/s10847-013-0360-8
Stappaerts, J., Do Thi, T., Dominguez-Vega, E., Somsen, G.W., Van den Mooter, G., Augustijns, P.: The impact of guest compounds on cyclodextrin aggregation behavior: a series of structurally related parabens. Int. J. Pharm. 529(1), 442–450 (2017). https://doi.org/10.1016/j.ijpharm.2017.07.026
Saokham, P., Do, T.T., Van den Mooter, G., Loftsson, T.: Inclusion complexes of p-hydroxybenzoic acid esters and γ-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 90(1), 111–122 (2017). https://doi.org/10.1007/s10847-017-0776-7
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59(7), 645–666 (2007). https://doi.org/10.1016/j.addr.2007.05.012
Crini, G.: Review: a history of cyclodextrins. Chem. Rev. 114(21), 10940–10975 (2014). https://doi.org/10.1021/cr500081p
Loftsson, T., Duchene, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329(1–2), 1–11 (2007). https://doi.org/10.1016/j.ijpharm.2006.10.044
Loftsson, T., Masson, M., Brewster, M.E.: Self-association of cyclodextrins and cyclodextrin complexes. J. Pharm. Sci. 93(5), 1091–1099 (2004). https://doi.org/10.1002/jps.20047
Sá Couto, A.R., Salústio, P., Cabral-Marques, H.: Cyclodextrins. In: Kishan Gopal Ramawat, J.-M.M. (ed.) Polysaccharides, Bioactivity and Biotechnology, vol. 1. pp. 247–288. Springer International Publishing, Berlin (2015)
Schonbeck, C., Madsen, T.L., Peters, G.H., Holm, R., Loftsson, T.: Soluble 1:1 complexes and insoluble 3:2 complexes—understanding the phase-solubility diagram of hydrocortisone and gamma-cyclodextrin. Int. J. Pharm. 531(2), 504–511 (2017). https://doi.org/10.1016/j.ijpharm.2017.05.024
Cohen, J., Lach, J.L.: Interaction of pharmaceuticals with Schardinger dextrins. I. Interaction with hydroxybenzoic acids and p-hydroxybenzoates. J. Pharm. Sci. 52, 132–136 (1963)
Caira, M.R., de Vries, E.J.C., Nassimbeni, L.R.: Cyclodextrin inclusion of p-hydroxybenzoic acid esters. J. Therm. Anal. Calorim. 73(2), 647–651 (2003). https://doi.org/10.1023/a:1025446617121
Chan, L.W., Kurup, T.R.R., Muthaiah, A., Thenmozhiyal, J.C.: Interaction of p-hydroxybenzoic esters with beta-cyclodextrin. Int. J. Pharm. 195(1), 71–79 (2000). https://doi.org/10.1016/S0378-5173(99)00393-2
Lach, J.L., Cohen, J.: Interaction of pharmaceuticals with schardinger dextrins II: interaction with selected compounds. J. Pharm. Sci. 52(2), 137–142 (1963). https://doi.org/10.1002/jps.2600520207
de Vries, E.J.C., Caira, M.R., Bogdan, M., Farcas, S.I., Bogdan, D.: Inclusion of parabens in β-cyclodextrin: a solution NMR and X-ray structural investigation. Supramol. Sci. 21(5), 358–366 (2009). https://doi.org/10.1080/10610270801956202
Holm, R., Olesen, N.E., Alexandersen, S.D., Dahlgaard, B.N., Westh, P., Mu, H.: Thermodynamic investigation of the interaction between cyclodextrins and preservatives—application and verification in a mathematical model to determine the needed preservative surplus in aqueous cyclodextrin formulations. Eur. J. Pharm. Sci. 87, 22–29 (2016). https://doi.org/10.1016/j.ejps.2015.09.011
Lehner, S.J., Müller, B.W., Seydel, J.K.: Interactions between p-hydroxybenzoic acid esters and hydroxypropyl-β-cyclodextrin and their antimicrobial effect against Candida albicans. Int. J. Pharm. 93(1), 201–208 (1993). https://doi.org/10.1016/0378-5173(93)90178-I
Malaekeh-Nikouei, B., Bazzaz, F., Soheili, B.S., Mohammadian, V.: K.: Problems in ophthalmic drug delivery: evaluation of the interaction between preservatives and cyclodextrins. Jundishapur J. Microbiol. 6(5), e6333 (2013). https://doi.org/10.5812/jjm.6333
Matsuda, H., Ito, K., Sato, Y., Yoshizawa, D., Tanaka, M., Taki, A., Sumiyoshi, H., Utsuki, T., Hirayama, F., Uekama, K.: Inclusion complexation of p-hydroxybenzoic acid esters with 2-hydroxypropyl-beta-cyclodextrins. On changes in solubility and antimicrobial activity. Chem. Pharm. Bull. 41(8), 1448–1452 (1993)
Loftsson, T., Stefánsdóttir, Ó, Friôriksdóttir, H., Guômundsson, Ö: Interactions between preservatives and 2-hydroxypropyl-β-cyclodextrin. Drug Dev. Ind. Pharm. 18(13), 1477–1484 (1992). https://doi.org/10.3109/03639049209040853
Tanaka, M., Iwata, Y., Kouzuki, Y., Taniguchi, K., Matsuda, H., Arima, H., Tsuchiya, S.: Effect of 2-hydroxypropyl-β-cyclodextrin on percutaneous absorption of methyl paraben. J. Pharm. Pharmacol. 47(11), 897–900 (1995). https://doi.org/10.1111/j.2042-7158.1995.tb03267.x
Saokham, P., Sa Couto, A., Ryzhakov, A., Loftsson, T.: The self-assemble of natural cyclodextrins in aqueous solutions: application of miniature permeation studies for critical aggregation concentration (cac) determinations. Int. J. Pharm. 505(1–2), 187–193 (2016). https://doi.org/10.1016/j.ijpharm.2016.03.049
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Loftsson, T., Hreinsdóttir, D., Másson, M.: Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 302(1), 18–28 (2005). https://doi.org/10.1016/j.ijpharm.2005.05.042
Coleman, A.W., Nicolis, I., Keller, N., Dalbiez, J.P.: Aggregation of cyclodextrins: an explanation of the abnormal solubility ofβ-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 13(2), 139–143 (1992). https://doi.org/10.1007/bf01053637
Wu, A., Shen, X., He, Y.: Investigation on gamma-cyclodextrin nanotube induced by N,N′-diphenylbenzidine molecule. J. Colloid Interface Sci. 297(2), 525–533 (2006). https://doi.org/10.1016/j.jcis.2005.11.014
McDonald, C., Palmer, L., Boddy, M.: The solubilities of esters of 4-hydroxybenzoic acid, determined separately and together, in aqueous solutions of 2-hydroxypropyl-β-cyclodextrin. Drug Dev. Ind. Pharm. 22(9–10), 1025–1029 (1996). https://doi.org/10.3109/03639049609065936
Narayanan, G., Boy, R., Gupta, B.S., Tonelli, A.E.: Analytical techniques for characterizing cyclodextrins and their inclusion complexes with large and small molecular weight guest molecules. Polym. Test. 62, 402–439 (2017). https://doi.org/10.1016/j.polymertesting.2017.07.023
Dibbern, H.W.: UV- and IR spectra of some important drugs: classified in therapeutic groups including tables of characteristic UV absorption data and examples for the UV spectrocognostic identification of drugs. vol. 2. Editio Cantor, (1978)
Muankaew, C., Jansook, P., Sigurđsson, H.H., Loftsson, T.: Cyclodextrin-based telmisartan ophthalmic suspension: formulation development for water-insoluble drugs. Int. J. Pharm. 507(1), 21–31 (2016). https://doi.org/10.1016/j.ijpharm.2016.04.071
Jansook, P., Ritthidej, G.C., Ueda, H., Stefansson, E., Loftsson, T.: yCD/HPyCD mixtures as solubilizer: solid-state characterization and sample dexamethasone eye drop suspension. J. Pharm. Pharm. Sci. 13(3), 336–350 (2010)
Cirri, M., Maestrelli, F., Furlanetto, S., Mura, P.: Solid-state characterization of glyburide-cyclodextrin co-ground products. J. Therm. Anal. Calorim. 77(2), 413–422 (2004). https://doi.org/10.1023/B:JTAN.0000038982.40315.8f
Acknowledgements
The financial support received from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT) (Grant No. 135040) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Couto, A.R.S., Aguiar, S., Ryzhakov, A. et al. Interaction of native cyclodextrins and their hydroxypropylated derivatives with parabens in aqueous solutions. Part 1: evaluation of inclusion complexes. J Incl Phenom Macrocycl Chem 93, 309–321 (2019). https://doi.org/10.1007/s10847-018-00876-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-00876-5