Abstract
Carvedilol is one of the most effective antihypertensive drugs used in the treatment of congestive heart failure. A major disadvantage of this pharmaceutical active substance is its limited solubility in water, gastric and intestinal fluids. One way to overcome this problem is the preparation of inclusion complexes. The aim of this study was to prepare the inclusion complexes of carvedilol with β-cyclodextrin (β-CD) and (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD) and to investigate their physical properties. The formation of inclusion complexes with β-CD and HP-β-CD was confirmed using FTIR, 1H-NMR and XRD methods. Phase solubility studies indicate the formation of inclusion complexes in 1:2 molar ratio and the increase of carvedilol solubility. The stability constant (β 2) was found to be 3.4 × 104 and 5.1 × 104 M−2 for inclusion complexes of carvedilol:β-CD and carvedilol:HP-β-CD, respectively. Photostability of carvedilol was increased after preparation of inclusion complexes with β-CD and HP-β-CD. Based on the results of this study, it can be concluded that the prepared complexes of carvedilol improve the solubility and stability of carvedilol and give it an advantage to be applied for the design of new pharmaceutical formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
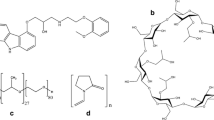
Carvedilol is one of a bioactive compound that belongs to alpha and beta blockers [1, 2], with multiple therapeutic potential. It is applied in treatment of hypertension, coronary heart disease, and in congestive heart failure. It showed a clear reduction in mortality in compared with other drugs from the same group [3]. Carvedilol and its metabolites are powerful antioxidants [4]. Recent studies were shown that long term use of carvedilol is associated with a reduction in the risk of cancer in the upper part of gastrointestinal tract and in lung [5]. Carvedilol is a racemic mixture [R(+) and S(−) enantiomers] with the chemical structure presented in Fig. 1 [6].
It is known that carvedilol is colourless crystalline solid soluble in dimethylsulfoxide, methylene chloride, methanol, but slightly soluble in ethanol, isopropanol and ethyl ether. However, carvedilol is practically insoluble in water (10.42 mg dm−3 at 25 °C) and simulated gastric and intestinal fluids at pH 1.1 and 7.5, respectively [7]. Because of these properties, carvedilol belongs to class II of the biopharmaceutical classification system [8, 9]. So, carvedilol has a very poor oral bioavailability (about 25–30 %) and shows extensive first-pass metabolism in the liver [10]. Also, it is known that carvedilol is the photosensitive substance [11]. This fact impacts on the reduction of its therapeutic effect.
In the literature, there are many attempts to overcome these disadvantages, such as: preparation of solid dispersions based on water-soluble polymers [12–15], microemulsion [16], the self-emulsifying and self-microemulsifying systems [17, 18] carboxylate carbon microparticles [19]. Complexation with cyclodextrins (CD) is an important approach to improve the physico-chemical properties of hydrophobic drugs [20–27]. Based on chemical composition, CD are cyclic oligosaccharides composed of glucopyranose units, connected by α-1,4-glucoside bonds (Fig. 1) [28]. Commercially available and significant for pharmaceutical application are the α-, β- and γ-cyclodextrins [29]. β-CD is one of the most widely used CDs. However, this CD has relatively poor water solubility. Due to these reasons, its derivatives with better water solubility and low toxicity such as methyl-β-cyclodextrin (M-β-CD), hydroxyethyl-β-cyclodextrin (HE-β-CD), (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD) and glucosyl-β-cyclodextrin (G-β-CD) have a significant application today [30]. The complexation of carvedilol with cyclodextrins was subject of many studies [12, 31–39]. Yuvaraja et al. [32] were used experimental design for preparation of a solid dispersion of ionized carvedilol with HP-β-CD and tartaric acid by “kneading technique”. The amounts of HP-β-CD (mg) and tartaric acid (mg), as well as kneading time (min) were used as the independent variables, while solubility (mg cm−3) and drug release (%) from the solid dispersion were defined as dependent variables. The optimal formulation was obtained under following conditions: the amounts of carvedilol, HP-β-CD and tartaric acid were 200, 689.6 and 227.6 mg, respectively, while a kneading time was 45 min. This complex showed complete drug release (~99 %) within 15 min and enhanced solubility of 1.89 mg cm−3. Sharma and Jain [33] described preparation of inclusion complex of carvedilol with β-CD by physical mixing, kneading and co-precipitation method. By application of various instrumental methods, they performed the structural characterization of prepared inclusion complexes. After investigation of dissolution rate, it was confirmed that the complex prepared by co-precipitation method showed better release among all other carvedilol-CD complexes. Unlike them, Pamudji et al. [34] prepared the same complex by freeze drying method in the molar ratio of 1:3. The obtained inclusion complex revealed the highest percentage of the dissolved carvedilol within 120 min (87.68 %). Drug solubilization can be obtained by combining salt formation and CD complexation. Due to Loftsson et al. [35] investigated the effects of salt formation on CD solubilization of carvedilol. The obtained results have indicated that the addition of acetic acid at pH below 5 significantly increases the aqueous solubility of carvedilol. The effect of methyl-β-cyclodextrin (M-β-CD) on the solubility and dissolution rate of carvedilol was investigated by Hirlekar and Kadam [36–38]. After complexation with M-β-CD, the dissolution rate of carvedilol was significantly increased. Wen et al. [40] prepared the inclusion complex of carvedilol with β-CD in a molar ratio of 1:2. Based on the phase solubility study, they showed that the complexed carvedilol has a higher solubility in water in compared with the pure carvedilol. Their constant of complex formation was 8.5 × 105 M−2, determined by fluorescence spectroscopy. The other attempts to improve the solubility of carvedilol such as the formation of ternary complexes with β-CD and citric acid in a molar ratio of 1:2:2 can be found in the literature [41]. They determined that the solubility of carvedilol is increased about 110 times. The influence of pH value and HP-β-CD concentration on the solubility of carvedilol was examined by Shewale et al. [42]. The results of phase solubility study showed that the carvedilol solubility increases with increasing HP-β-CD concentration and that the greatest value of solubility is achieved at pH of 7.4. The value of the stability constant K1:1 = 4.19 × 104 M−1 indicates that the prepared complex in a molar ratio of 1:1 is stable. Based on phase solubility study, Bhutani et al. [43] proved that the complex between carvedilol and HP-β-CD formed in a molar ratio of 1:1 with the stability constant of 582.78 M−1.
The aim of study was to prepare and structural characterize the inclusion complex of carvedilol with β-CD and HP-β-CD using FTIR, 1H-NMR and XRD methods. The phase-solubility of carvedilol was determined at room temperature using UV–vis method in accordance with standard procedure. The photostability of free and complexed carvedilol was also investigated using UV–vis method in order to achieve the high quality and safety use of this drug.
Materials and methods
Materials
Carvedilol was purchased from NOSCH Labs Private Limited (India). β-CD (purity 98 %, average Mr ~ 1135) and HP-β-CD (purity 98 %, average Mr ~ 1540) were purchased from Sigma Aldrich (Steinheim, Germany). Potassium bromide FT-IR grade was supplied by Merck (Darmstadt, Germany). Ethanol (96 %, v/v) was supplied by Zorka Pharma (Sabac, Serbia). All other reagents and solvents were of analytical grade.
Preparation of inclusion complexes
Carvedilol:β-CD inclusion complex was prepared by suspending 101.6 mg of carvedilol and 567.5 mg of β-CD in a mixture of 100 cm3 water and 50 cm3 of 96 % (v/v) ethanol. For the preparation of carvedilol:HP-β-CD inclusion complex, 50.8 mg of carvedilol and 385 mg HP-β-CD was weighed accurately and suspended in a mixture of 50 cm3 water and 25 cm3 of 96 % (v/v) ethanol. These suspensions were homogenized on a magnetic stirrer (HANNA, HI300) at a stirring speed of 600 rpm. After a time, the suspended carvedilol in the solution of β-CD, and HP-β-CD were dissolved and equilibrated at room temperature for 24 h. A flask was covered with aluminum foil to prevent a photodegradation reaction of carvedilol. After preparation of the complex, the solution was evaporated up to 5 cm3 at 60 °C using a rotary vacuum evaporator. The obtained complex was stored at 25 °C in a desiccator until further analysis.
Physical mixture
The physical mixture of carvedilol and β-CD or carvedilol and HP-β-CD were prepared by weighing in a molar ratio of 1:2 and homogenized in glass vessel with a spatula. The initial compounds were mixed in this ratio in order to obtain the relevant and comparable spectra with those prepared inclusion complexes as described in the previous section. The prepared physical mixtures were stored in a desiccator until further analysis.
Structural characterization of complexes
Carvedilol:β-CD and carvedilol:HP-β-CD complexes were structurally characterized using infrared, nuclear magnetic resonance and X-ray diffraction methods. The obtained spectra of complexes were compared with the spectra of carvedilol, β-CD, HP-β-CD and physical mixtures.
Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared spectroscopy was performed on a Fourier Transform Infrared Spectrometer (Bomem Hartmann & Braun MB-series). The adequate amounts of samples (carvedilol, β-CD, HP-β-CD, inclusion complexes and physical mixtures) were mixed with KBr and then compressed in the press to obtain pellets. The spectra were scanned in the wavenumber range of 4000–400 cm−1 at the resolution of 4 cm−1. The spectra were processed using the software Win–Bomem Easy.
Nuclear magnetic resonance spectroscopy (NMR)
1H-NMR spectra of the prepared inclusion complexes were recorded on a Bruker Avance III NMR spectrometer equipped with a glass cuvette of 5 mm diameter with an operating frequency of 250 MHz at room temperature by the pulse method with multiple pulse repetitions. The heavy water (D2O) was used as a solvent.
X-ray diffraction (XRD)
X-ray diffraction patterns were recorded on an Phillips PW1030 automatic powder diffractometer. The samples were irradiated with monochromatized Cu-kα radiation and analyzed at the angle 2θ between 3° and 60° with 0.05° increments and recording time of 2 s. The used voltage and current were 40 kV and 12 mA, respectively.
Analysis of carvedilol using UV–vis method
The stock solution of carvedilol (50 μg cm−3) was prepared in ethanol and further diluted to obtain a series of solutions for construction of the calibration curve. The calibration curve was constructed based on the values of absorbance for the analyzed solutions. The absorbance of the samples was measured at 285 nm and at room temperature. As a blank was used 96 % (v/v) ethanol. The absorbance of samples was recorded on a Varian Cary-100 Conc. UV spectrophotometer using the quartz cells (1 × 1×4.5 cm).
Phase solubility
The investigation of phase solubility was carried out in accordance with the method of Higuchi and Connors [44]. This method based on the addition of excess amount of carvedilol (50 mg) to the aqueous solution containing the increased concentrations of β-CD or HP-β-CD. A series of the aqueous solutions of cyclodextrins (CDs) was prepared in the volumetric flask (10 cm3). The total volume of samples was 5 cm3. The concentration of β-CD or HP-β-CD was varied in the range of 0.5–2.5 mmol dm−3. The samples were stirred in dark at room temperature for 24 h, and then filtered through the cellulose membrane filters with 0.45 μm pore size (Econofilters, Agilent Technologies, Germany) in order to remove the undissolved carvedilol. After that, the amount of dissolved carvedilol was determined spectrophotometrically at 285 nm. The distilled water was used as a blank solution. The presence of β-CD and HP-β-CD in the aqueous solutions do not interfere spectrophotometric monitoring the carvedilol concentration.
Conventionally, the stability constants are the equilibrium constants for the complex formation reactions:
and for the second step:
where G means the guest, D means the cyclodextrin, while the square brackets denote the equilibrium concentrations. The overall stability constant of the 1:2 complex (usually denoted as β 2) is then for the reaction:
The apparent stability constants (K 1:1 and K 1:2) of inclusion complexes were calculated by solving Eq. (4) [49]:
where S t —the total amount of carvedilol in the system, S o —the solubility of carvedilol at 25 °C without the presence of β-CD or HP-β-CD.
Photostability of inclusion complexes
In order to investigate the effect of UVB irradiation, the ethanol solution of carvedilol:β-CD (3.8 mg) and carvedilol:HP-β-CD (4.8 mg) inclusion complexes, as well as carvedilol standard (2.5 mg) were prepared into the flask (25 cm3). The samples were sonicated for 15 min and then exposed to the effect of UVB light at different time intervals (0, 5, 30, 150, 600, 1500 and 3600 s). The irradiation of the samples was performed in a cylindrical photochemical reactor “Rayonet” with ten symmetrically placed UVB lamps with maximum emission at 300 nm and total flux of energy 14.5 W m−2. During radiation, the samples were kept in the quartz cells (1 × 1×4.5 cm) mounted on a circular rotating bracket. The irradiated samples were further analyzed using UV–vis method.
Results and discussion
Structural characterization of inclusion complexes
FTIR analysis
Fourier transform infrared spectra of pure carvedilol, inclusion complexes and physical mixtures are presented in Figs. 2 and 3. The characteristic peaks of pure carvedilol are shown in Fig. 2a. Bands at 3411 and 3345 cm−1 originate from O–H and N–H stretching vibrations, while those at 2922 and 2852 cm−1 are due to aliphatic C–H stretching vibrations. The presence of low intensity bands at higher wavenumbers than 3000 cm−1 indicates the aromatic C–H stretching vibrations. The aromatic C = C stretching vibrations in the aromatic ring have appeared at 1608, 1503 and 1454 cm−1. Another band at 1591 cm−1 is attributed to the N–H bending vibrations. Band at 1253 cm−1 is due to the aromatic C–N stretching vibration, while C–O the stretching vibration is indicated by the band at 1215 cm−1.
The spectra of β-CD (Fig. 2b) and HP-β-CD (Fig. 3a) have the intense band at 3395 cm−1 for β-CD, and 3411 cm−1 for HP-β-CD due to O–H stretching vibration in the primary and secondary hydroxyl groups. The band with maximum at 2926 cm−1 was also observed in these spectra and it belongs to the valence vibrations of the aliphatic C–H bonds in the CH and CH2 groups. The O–H deformation vibrations of two different types of water molecules existing in the cavities of β-CD and HP-β-CD give the band at 1635 cm−1. The vibrations bands at 1412 and 1334 cm−1 for β-CD (Fig. 2b), and 1414 and 1378 for HP-β-CD (Fig. 3a) originate from the deformation vibrations of the C–H bonds in the primary and secondary hydroxyl groups. The C–C stretching vibration causes the occurrence of band at 1156 cm−1. In the range of 1100–1030 cm−1, the absorption bands of the valence vibrations of the C–O bonds in the ether and hydroxyl groups of β-CD (1079 and 1030 cm−1), and of HP-β-CD (1082 and 1035 cm−1) were noticed. The absorption bands in the wavenumber range of 950-700 cm−1 belong to the deformation vibrations of the C–H bonds.
The FTIR spectra of the inclusion complexes (Figs. 2c and 3b) did not have the significant differences in compared with the spectra of carvedilol, β-CD and HP-β-CD. However, the spectra of the complex have some changes in the intensity of bands. The increase in the frequency was caused probably due to the inclusion of benzene ring into the electron rich cavity of β-CD, after what increase the density of electron cloud [40]. The decrease in the frequency is the result of the formation of hydrogen bonds and the presence of van der Waals forces during formation of the inclusion complexes [45].
The differences in frequency (Δν) between β-CD, and HP-β-CD and inclusion complexes are presented in Table 1.
It can be noticed that the band of the valence vibration of O–H bond in the inclusion complexes was shifted to the higher or lower wavenumbers. Its presence was confirmed at 3402 cm−1 in the spectrum of carvedilol:β-CD inclusion complex, and at 3406 cm−1 in the spectrum of carvedilol:HP-β-CD inclusion complex (Table 1). In accordance with previous studies for preparation of the inclusion complexes [46, 47], the suppression of –OH vibrational modes in the 3000–3700 cm−1 region was related to evidence of host–guest interaction as a consequence of complete water release upon inclusion [48]. The absorption band of the valence vibrations of the C–H bond in both complexes was shifted for two units to the lower wavenumbers. The same vibration band at both complexes was shifted to the higher wavenumbers in compared with carvedilol. The bands at 1635 and 1636 cm−1 in the spectra of β-CD and HP-β-CD, respectively, which belongs to the deformation vibrations of the O–H bonds, was shifted to 1632, and 1631 cm−1 in both complexes. The differences were found in the wavenumber of 1100–1030 cm−1 at the complexes, which were attributed to valence vibrations of C–O in the ether and hydroxyl groups of β-CD and HP-β-CD. In the low frequency region (900–750 cm−1) of the inclusion complexes (Figs. 2c and 3b), the characteristic bands of glucopyranose unit are assigned to 857, 756 and 707 cm−1 for carvedilol:β-CD inclusion complex and at 851, 757 and 710 cm−1 for carvedilol:HP-β-CD inclusion complex. On the other hand, the FTIR spectra of physical mixtures have the characteristic peaks of β-CD or HP-β-CD and carvedilol. The peaks of carvedilol at 1503, 1454 and 1253 cm−1 are missing in the spectra of the inclusion complexes, but appear in the spectrum of physical mixtures. The corresponding vibrations are probably significantly modified due to inclusion of carvedilol into the cavities of CDs. Thus, it is a good indication of the formation of the inclusion complexes between CDs and carvedilol. This hypothesis was also confirmed by many researchers in synthesizing the inclusion complex between CDs (host) and guest molecules published elsewhere [47].
1H-NMR analysis
The positions of atoms in the chemical structures of carvedilol and CDs are shown in Fig. 1. Chemical shifts in the spectrum of carvedilol and the differences in their chemical shifts after formation of inclusion complexes are given in Table 2. The greatest changes in the chemical shifts (Δδ) of carvedilol protons have been reported in the case of H-1′, H-2′, H-2″, H-5″, H-6″ and H-7″ after the complexation with β-CD. Δδ for phenyl proton H-6″ which belongs to the B-ring (Fig. 1a) has the highest value of +0.051. The chemical shift of H-2″ proton in the complexed carvedilol was +0.031 greater in compared with pure carvedilol. Slightly lower changes in the chemical shifts can be noticed for H-5″ and H-7″ phenyl protons from ring B. Also, the minor changes in the chemical shifts were observed for H-1′ and H-2′ protons from A ring. Based on the changes of chemical shifts, it can be concluded that the most likely occurred the inclusion of B ring of carvedilol molecule. After complexation of carvedilol with HP-β-CD, the changes in chemical shifts are almost identical as in the case of complex with β-CD. The highest Δδ of +0.059 has been recorded for H-6″ phenyl proton in the B ring. Also, the changes in chemical shifts in descending order were observed for H-5″ and H-7″ protons of the B ring.
XRD analysis
X-ray diffraction diffractograms of carvedilol, physical mixtures and prepared inclusion complexes are different from each other (Fig. 4). On the diffraction pattern of carvedilol, the major peaks at the 2θ values of 11.8°, 12.8°, 14.9°, 17.3°, 18.5°, 24.3° and 26.1° indicate its crystalline nature (Fig. 4a). β-CD also exhibited a typical crystalline diffraction pattern with major peaks at the 2θ values of 6.2°, 8.9°, 10.7°, 11.8°, 12.6°, 15.5°, 17.2°, 19.6°, 20.9° and 22.8° (Fig. 4b). On the diffraction pattern of carvedilol:β-CD inclusion complex (Fig. 4c), the characteristic peaks of carvedilol is not possible to identify. This phenomenon is probably the result of “guest” molecule incorporation into the inner cavity of the “host” compound. On the other hand, XRD-scanning of physical mixture showed the several peaks, which can be attributed to carvedilol and β-CD.
In contrast, HP-β-CD showed an amorphous structure where lacking the crystalline peaks (Fig. 4e). Unlike diffractogram of physical mixture, where is identified the presence of peaks at 2θ values of 13.2°, 14.8°, 18.7° and 24.9° (Fig. 4g), the peaks which would indicate the crystalline nature of the molecules have not been identified in the diffractogram of carvedilol:HP-β-CD complex (Fig. 4f). These facts suggested that carvedilol was molecularly dispersed in the HP-β-CD matrix.
Actually, XRD study was used as a quantitative measure of the estimation of crystallinity of the formed carvedilol inclusion complexes. Based on the change in the crystallinity of the carvedilol, the formation of inclusion complexes with CDs was established. XRD analysis showed that the complex with HP-β-CD has an amorphous structure. It points to the fact that during the experiment occurred a dispersion of carvedilol in the molecule of CDs [38].
Analysis of carvedilol using UV–vis method
The calibration curve for determination of carvedilol content using UV–vis method can be presented as A285 = 0.0298 (S.E. = 0.0086) × C (μg cm−3) + 0.063 (S.E. = 4.22·10−4), R2 = 0.9986. The coefficient of determination indicates that 99.86 % variation in the absorbance at 285 nm could be explained by this linear model. The linearity of this calibration curve was confirmed in the concentration range of 2–40 μg cm−3. The values of LOD and LOQ are 0.95 and 2.89 μg cm−3, respectively. This proposed regression model can be used for determination the amount of dissolved carvedilol in phase solubility study and for monitoring the concentration of carvedilol in the samples after irradiation. The absorption spectra of non-dissociating guests are usually not significantly changed by the complex formation. The UV–vis spectra of carvedilol and inclusion complexes are presented in Fig. 5. Based on these spectra, it can be concluded that the presence of the CDs does not interfere monitoring of carvedilol.
Phase solubility study
According to the Higuchi and Connors classification, the phase solubility diagrams belong to Ap type for the formation of complexes between carvedilol and β-CD, and HP-β-CD in the aqueous solution at 25 °C (Fig. 6) [44, 49, 50]. They illustrated the enhancement of carvedilol solubility in the solutions with increasing concentrations of CDs. These isotherms are curves that deviate in a positive direction from linearity, what indicates the formation of the inclusion complexes between carvedilol and CDs in the molar ratio 1:2. The solubility of carvedilol in water was found to be 36.637 µM based on the slope of phase solubility diagram. In order to confirm the obtained solubility of carvedilol, the saturated solution was prepared. The concentration of this solution was determined using UV method.
The solubility of carvedilol was increased to 62.9 µM in the presence of 2.5 mmol dm−3 β-CD, and to 57.7 µM in the presence of 2.5 mmol dm−3 HP-β-CD. The calculated values of stability constants were K1:1 = 204.7 M−1 and K1:2 = 165.3 M−1 or β 2 = 3.4 × 104 M−2 for carvedilol:β-CD inclusion complex. For carvedilol:HP-β-CD inclusion complex, the calculated values were K1:1 = 104 M−1 and K1:2 = 490.8 M−1 or β 2 = 5.1 × 104 M−2. The correlation between the solubility of carvedilol and concentration of CDs suggested that the water soluble complexes were formed. This prepared complex could be used as an antihypertensive agent to develop new oral formulation with improved bioavailability.
Photostability study of inclusion complexes
In order to assure the high quality of drug products, it is necessary to examine the stability of active substances in accordance with ICH guidelines [51]. For these reasons, in this study was investigated the photostability of prepared complexes and compared with photostability of pure carvedilol. The effect of the exposure time on degradation of carvedilol and their complexes is presented in Fig. 7.
Based on the comparison of photodegradation profiles of the samples, it can be concluded that the CDs had the significant effect on carvedilol photodegradation. This especially observed after 1500 s of irradiation, where the content of carvedilol in the complex with β-CD and HP-β-CD decreased for 6.56 and 9.17 %, respectively. The decrease of pure carvedilol was around 12 %. The decrease of carvedilol content is most likely due to the formation of photodegradation products. Jouyban et al. [52] identified the presence of 1-(2-(2-methoxyphenoxy)ethylamine)-3-(9H-carbazol-8-yloxy)propan-2-ol in the samples exposed to the effect of daylight for 4 weeks at room temperature using capillary electrophoresis method. This compound has described as an impurity of carvedilol in pharmacopoeia [53]. However, the second degradation products was an isomer of N-(2-(2-methoxyphenoxy) ethyl)-3-(9H-carbazol-8-yloxy)prop-2-en-1-amin which has not described in pharmacopeia. The potential photodegradation products of carvedilol are presented in Fig. 8 [52].
Conclusion
The results of FTIR, NMR and XRD analysis clearly indicate that the carvedilol was efficiently participated in complexation with β-CD and HP-β-CD. The preparation of inclusion complexes increases the water solubility of carvedilol, which results in the increase of its bioavailability. Also, the increase in photostability of carvedilol indicates that the complexation approach may be especially useful in improving the shelf life of this drug. These satisfactory physical and chemical properties of the prepared complexes of carvedilol with CDs can be used for the design of new oral antihypertensive formulations with the corresponding advantages.
References
Packer, M., Lukas, M., Tenero, D., Baidoo, C., Greenberg, B.: Pharmacokinetic profile of controlled-release carvedilol in patients with left ventricular dysfunction associated with chronic heart failure or after myocardial infarction. Am. J. Cardiol. 98, 39–45 (2006)
Weber, A., Sica, A., Tarka, A., Iyengar, M., Fleck, R., Bakris, L.: Controlled-release carvedilol in the treatment of essential hypertension. Am. J. Cardiol. 98, 32–38 (2006)
Palazzuoli, A., Calabria, P., Verzuri, M.S., Auteri, A.: Carvedilol: something else than a simple betablocker? Eur. Rev. Med. Pharmacol. Sci. 6, 115–126 (2002)
Ouyang, Y., Chen, Z., Tan, M., Liu, A., Chen, M., Liu, J., Fang, J.: Carvedilol, a third-generation β-blocker prevents oxidative stress-induced neuronal death and activates Nrf2/ARE pathway in HT22 cells. Biochem. Bioph. Res. Co. 441(4), 917–922 (2013)
Lin, C.S., Lin, W.S., Lin, C.L., Kao, C.H.: Carvedilol use is associated with reduced cancer risk: a nationwide population-based cohort study. Int. J. Cardiol. 184, 9–13 (2015)
Takekuma, Y., Yagisawa, K., Sugawara, M.: Mutual inhibition between carvedilol enantiomers during racemate glucuronidation mediated by human liver and intestinal microsomes. Biol. Pharm. Bull. 35(2), 151–163 (2012)
Shete, A.S., Yadav, A.V., Murthy, S.M.: Chitosan and chitosan chlorhydrate based various approaches for enhancement of dissolution rate of carvedilol. DARU J. Pharm. Sci. 20(1), 93–97 (2012)
Kasim, N., Whitehouse, M., Ramchandran, C., Bermejo, M., Lennernas, H., Hussain, A.S., Junginger, H.E., Stavchansky, S.A., Midha, K.K., Shah, V.P., Amidon, G.L.: Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 1, 85–96 (2004)
Khadra, I., Zhou, Z., Dunn, C., Wilson, C.G., Halbert, G.: Statistical investigation of simulated intestinal fluid composition on the equilibrium solubility of biopharmaceutics classification system class II drugs. Eur. J. Pharm. Sci. 67, 65–75 (2015)
Möllendorff, E.V., Reiff, K., Neugebauer, G.: Pharmacokinetics and bioavailability of carvedilol, a vasodilating beta-blocker. Eur. J. Clin. Pharmacol. 33(5), 511–513 (1987)
Savic, I., Marinkovc, V., Savic, I., Sibinovic, P., Cekic, N.: Application of the experimental design method to photostability studies of Karvileks tablet. Ind. J. Pharm. Edu. Res. 46(3), 275–282 (2012)
Yuvaraja, K., Khanam, J.: Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acid. J. Pharm. Biomed. 96, 10–20 (2014)
Lee, S.N., Poudel, B.K., Tran, T.H., Marasini, N., Pradhan, R., Im Lee, Y., Kim, J.O.: A novel surface-attached carvedilol solid dispersion with enhanced solubility and dissolution. Arch. Pharm. Res. 36(1), 79–85 (2013)
Planinšek, O., Kovačič, B., Vrečer, F.: Carvedilol dissolution improvement by preparation of solid dispersions with porous silica. Int. J. Pharma. 406(1), 41–48 (2011)
Tapas, A., Kawtikwar, P., Sakarkar, D.: An improvement in physicochemical properties of carvedilol through spherically agglomerated solid dispersions with PVP K30. Acta Pol. Pharm. 69(2), 299–308 (2012)
Sanjula, B., Shah, F.M., Javed, A., Alka, A.: Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J. Drug Target. 17(3), 249–256 (2009)
Wei, L., Sun, P., Nie, S., Pan, W.: Preparation and evaluation of SEDDS and SMEDDS containing carvedilol. Drug Dev. Ind. Pharm. 31(8), 785–794 (2005)
Salimi, A., Zadeh, B.S.M., Hemati, A., Birgani, S.A.: Design and evaluation of self-emulsifying drug delivery system (SEDDS) of carvedilol to improve the oral absorption. Jundishapur J. Nat. Pharm. Prod. 9(3), e16125 (2014)
Zhang, Y., Zhi, Z., Li, X., Gao, J., Song, Y.: Carboxylated mesoporous carbon microparticles as new approach to improve the oral bioavailability of poorly water-soluble carvedilol. Int. J. Pharm. 454(1), 403–411 (2013)
Nikolic, V., Kapor, A.J., Nikolic, L.B., Savic, I.M., Savic-Gajic, I.M.: The importance of inclusion complexes with cyclodextrins in pharmacy. In: Ramirez, F.G. (ed.) Cyclodextrins: Synthesis, Chemical Applications and Role in Drug Delivery, pp. 225–240. Nova Science Publishers Inc, New York (2015)
Savic, I.M., Nikolic, V.D., Savic-Gajic, I., Nikolic, L.B., Radovanovic, B.C., Mladenovic, J.D.: Investigation of properties and structural characterization of the quercetin inclusion complex with (2-hydroxypropyl)-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 82(3), 383–394 (2015)
Tačić, A., Savić, I., Nikolić, V., Savić, I., Ilić-Stojanović, S., Ilić, D., Petrović, S., Popsavin, M., Kapor, A.: Inclusion complexes of sulfanilamide with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 80(1–2), 113–124 (2014)
Dawoud, A.A., Al-Rawashdeh, N.: Spectrofluorometric, thermal, and molecular mechanics studies of the inclusion complexation of selected imidazoline-derived drugs with β-cyclodextrin in aqueous media. J. Incl. Phenom. Macrocycl. Chem. 60(3–4), 293–301 (2008)
Al-Rawashdeh, N.A., Al-Sadeh, K.S., Al-Bitar, M.B.: Physicochemical study on microencapsulation of hydroxypropyl-β-cyclodextrin in dermal preparations. Drug Dev. Ind. Pharm. 36(6), 688–697 (2010)
Bani-Yaseen, A.D., Al-Rawashdeh, N.F., Al-Momani, I.: Influence of inclusion complexation with β-cyclodextrin on the photostability of selected imidazoline-derived drugs. J. Incl. Phenom. Macrocycl. Chem. 63(1–2), 109–115 (2009)
Al-Rawashdeh, N.A., Al-Sadeh, K.S., Al-Bitar, M.B.: Inclusion complexes of sunscreen agents with β-cyclodextrin: spectroscopic and molecular modeling studies. J. Spectrosc. 2013, 11 (2013)
Al-Rawashdeh, N.A.: Interactions of nabumetone with γ-cyclodextrin studied by fluorescence measurements. J. Incl. Phenom. Macrocycl. Chem. 51(1–2), 27–32 (2005)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98(5), 1743–1754 (1998)
Lombardo, D., Longo, A., Darcy, R., Mazzaglia, A.: Structural properties of nonionic cyclodextrin colloids in water. Langmuir 20(4), 1057–1064 (2004)
Del Valle, E.M.: Cyclodextrins and their uses: a review. Process Biochem. 39(9), 1033–1046 (2004)
Zoghbi, A., Wang, B.: Carvedilol solubility enhancement by inclusion complexation and solid dispersion: review. J. Drug Deliv. Ther. 5(2), 1–8 (2015)
Yuvaraja, K., Das, S.K., Khanam, J.: Process optimization and characterization of carvedilol solid dispersion with hydroxypropyl-β-cyclodextrin and tartaric acid. Korean J. Chem. Eng. 32, 1–9 (2015)
Sharma, A., Jain, C.P.: Carvedilol-β-cyclodextrin systems: preparation, characterization and in vitro evaluation. Dhaka Univ. J. Pharm. Sci. 12(1), 51–58 (2013)
Pamudji, J.S., Mauludin, R., Lestari, V.A.: Improvement of carvedilol dissolution rate through formation of inclusion complex with β-cyclodextrin. Int. J. Pharm. Pharm. Sci. 6(4), 228–233 (2014)
Loftsson, T., Vogensen, S.B., Desbos, C., Jansook, P.: Carvedilol: solubilization and cyclodextrin complexation: a technical note. AAPs Pharm. Sci. Tech. 9(2), 425–430 (2008)
Soleymanpour, A., Ghasemian, M.: Chemically modified carbon paste sensor for the potentiometric determination of carvedilol in pharmaceutical and biological media. Measurement 59, 14–20 (2015)
Cappello, B., De Rosa, G., Giannini, L., La Rotonda, M.I., Mensitieri, G., Miro, A., Russo, R.: Cyclodextrin-containing poly (ethyleneoxide) tablets for the delivery of poorly soluble drugs: potential as buccal delivery system. Int. J. Pharm. 319(1), 63–70 (2006)
Hirlekar, R., Kadam, V.: Preparation and characterization of inclusion complexes of carvedilol with methyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 63(3–4), 219–224 (2009)
Talvani, A., Bahia, M.T., de Sá-Barreto, L.C.L., Lima, E.M., da Cunha-Filho, M.S.S.: Carvedilol: decomposition kinetics and compatibility with pharmaceutical excipients. J. Therm. Anal. Calorim. 115(3), 2501–2506 (2014)
Wen, X., Tan, F., Jing, Z., Liu, Z.: Preparation and study the 1:2 inclusion complex of carvedilol with β-cyclodextrin. J. Pharm. Biomed. 34(3), 517–523 (2004)
Pokharkar, V., Khanna, A., Venkatpurwar, V., Dhar, S., Mandpe, L.: Ternary complexation of carvedilol, β-cyclodextrin and citric acid for mouth-dissolving tablet formulation. Acta Pharm. 59(2), 121–132 (2009)
Shewale, B.D., Sapkal, N.P., Raut, N.A., Gaikwad, N.J., Fursule, R.A.: Effect of hydroxylpropyl-β-cyclodextrin on solubility of carvedilol. Indian J. Pharm. Sci. 70(2), 255–257 (2008)
Bhutani, S., Hiremath, S.N., Swamy, P.V., Raju, S.A.: Preparation and evaluation of inclusion complexes of carvedilol. J. Sci. Ind. Res. 66(10), 830–834 (2007)
Higuchi, T., Connors, K.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 7, 117–122 (1965)
Hamidi, H., Abderrahim, R., Meganem, F.: Spectroscopic studies of inclusion complex of β-cyclodextrin and benzidine diammonium dipicrate. Spectrochim. Acta A. 75, 32–36 (2010)
Wen, X., Tan, F., Jing, Z., Liu, Z.: Preparation and study the 1:2 inclusion complex of carvedilol with β-cyclodextrin. J. Pharm. Biomed. 34(3), 517–523 (2004)
Sambasevam, K.P., Mohamad, S., Sarih, N.M., Ismail, N.A.: Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 14(2), 3671–3682 (2013)
Bocanegra-Diaz, A., Mohallem, N.D., Sinisterra, R.D.: Preparation of a ferrofluid using cyclodextrin and magnetite. J. Braz. Chem. Soc. 14(6), 936–941 (2003)
Loftsson, T., Magnusdottir, A., Masson, M., Sigurjonsdottir, J.F.: Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 91, 2307–2316 (2002)
Loftsson, T., Masson, M., Brewster, M.E.: Self-association of cyclodextrins and cyclodextrin complexes. J. Pharm. Sci. 93(5), 1091–1099 (2004)
Guideline, ICH Harmonised Tripartite.: Stability testing of new drug substances and products. Q1A (R2). Curr. Step 4, 1–22 (2003)
Jouyban, A., Hasanzadeh, M., Shadjou, N.: Non-aqueous electromigration analysis of some degradation products of carvedilol. Iran. J. Pharm. Res. 13(2), 471–486 (2014)
http://www.drugfuture.com/pharmacopoeia/usp35/data/v35300/usp35nf30s0_m2730.html
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia under the Project TRp-34012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal informed consent
This article does not contain any studies with human or animals subjects.
Rights and permissions
About this article
Cite this article
Savic-Gajic, I., Savic, I.M., Nikolic, V.D. et al. Study of the solubility, photostability and structure of inclusion complexes of carvedilol with β-cyclodextrin and (2-hydroxypropyl)-β-cyclodextrin. J Incl Phenom Macrocycl Chem 86, 7–17 (2016). https://doi.org/10.1007/s10847-016-0635-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-016-0635-y