Abstract
In the present study, simultaneous improvement in dissolution profile and content uniformity of Lafutidine (LAF) has been achieved through co-inclusion in urea. LAF, a recently reported histamine H2 receptor antagonist used in the treatment of peptic ulcer, exhibits low aqueous solubility and dissolution rate limited absorption leading to its poor bioavailability. LAF is a potent and normally non-complexing substituted cyclic organic compound. Urea, a well known host for forming inclusion complexes with linear organic compounds was successfully employed for co-inclusion of LAF in the presence of readily complexing guest (RCG). The modified Zimmerschied calorimetric method was employed for the estimation of the minimum amount of RCG required for co-inclusion of lafutidine in urea. The formation of lafutidine urea co-inclusion complexes (LUCIC) was confirmed by DSC, FTIR, XRD and 1H-NMR studies. LUCIC complexes containing varying proportions of guests were prepared and subjected to thermal analysis using DSC. The thermal data was subjected to the regression analysis to study the influence of the relative molar proportion of RCG on the heat of decomposition of LUCIC. Study revealed good content uniformity of lafutidine in LUCIC. In vitro dissolution rate studies demonstrated steep improvement in dissolution profile of LAF—a BCS class II drug. The resulting dissolution data was subjected to various release kinetic models. Studies reveal that urea co-inclusion complex formation may be a promising technique for the formulation of potent poorly soluble drugs into immediate release products with improved content uniformity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcer is caused by imbalance between digestive fluids in the region of stomach and duodenum. Ulcer is the result of excessive use of painkillers i.e. anti inflammatory drugs in muscular pains and arthritis. It is also caused by infection with bacterium Helicobacter pylori leading to painful sores and inflammation in stomach [1, 2]. H2 receptor antagonists are the category of drugs used in treatment of peptic ulcer and gastro-esophageal reflux disease by blocking the action of histamine on parietal cells in the stomach and suppressing acid production [3, 4]. Numerous drugs such as cimetidine, famotidine, nizatidine, ranitidine, roxatidine and lafutidine (LAF) are currently available in the market for treatment of ulcer and for preventing acid related gastrointestinal diseases.
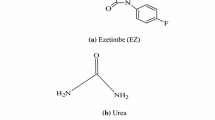
Lafutidine, 2[2(furyl) sulfinyl]-N-[(2Z)-4-{(4-(piperidin-1-yl methyl) pyridine-2yl) oxy} but-2-en-l-yl) acetamide (Fig. 1a) is a newly developed second generation histamine H2-receptor antagonist having antiulcer and gastro protective activity [5]. LAF is used in treatment of duodenal ulcers, peptic ulcers and gastro-esophageal reflux disease, gastric mucosal lesions associated with acute gastritis and before general anaesthesia to reduce the occurrence of aspiration pneumonia [6–8]. LAF is mainly absorbed from the small intestine and it reaches gastric cells through systemic circulation [9]. LAF ameliorates peptic ulcer disease simply by blocking the attachment of H. pylori with gastric cells [10]. LAF displays antisecretory action through direct binding with the gastric cell histamine H2 receptors, hindering the stimulation of cAMP and suppressing daytime (post prandial) as well as night time gastric acid production [11–13]. LAF exhibits gastro protective action by inducing calcitonin gene related peptide release which stimulates nitric oxide (NO) production in endothelial cells [14–17]. Resulting NO diminishes mucosal inflammation by promoting/regulating gastric mucosal blood flow through vasodilation in gastric vasculature and stimulates mucin biosynthesis resulting in gastro protection [18–20].
LAF is a yellowish white, slightly bitter crystalline powder that is practically insoluble in water and poorly absorbed from the gastro-intestinal tract. Therefore, it is naturally of utmost significance to attempt an effective approach for enhancing the dissolution profile of LAF so as to improve its bioavailability. LAF is a potent BCS class II histamine H2 receptor antagonist drug and its dissolution is considered to be a rate-determining step in its absorption from gastrointestinal fluids. Improvement of dissolution profile of LAF has been recently attempted through inclusion in cyclodextrins [21] or by employing solid dispersion in water soluble carriers [22, 23]. In current study, another attempt has been made for steep improvement of dissolution profile of LAF through co-inclusion of LAF in urea using a modified technique [24–26].
In supramolecular chemistry, host guest chemistry describes the complexes formed by two or more molecules bonded together in structural relationship by forces other than covalent bonds. Host–guest chemistry has been observed in clathrates, intercalation compounds and inclusion compounds. Inclusion compounds are defined as complexes formed by inclusion of one kind of molecules, called guest molecules into cavities of crystalline framework of another kind called host molecules without forming any specific chemical bond between guest and host [27]. Common host molecules reported in literature are urea, cyclodextrins, thiourea, porphyrins, deoxycholic acid, hydroquinone, zeolites, calixarenes, tri-o-thymotide, cryptophanes, crown ethers etc. for forming inclusion complexes [28].
Bengen, in 1940, while working on behaviour of milk proteins in presence of urea found by chance that urea can form an inclusion complex with n-octanol. Ever since that accidental discovery of urea complexes, the chemistry of urea inclusion complexes has attracted much attention and is a subject of continuing interest [29]. This small molecule is quite versatile, biologically important and has gained commercial and industrial utility. However, considerable research involving urea has been undertaken during past few decades due to capacity of urea to form inclusion complexes with a large variety of organic materials. Urea is commercially used as a component of fertilizer providing a relatively cheap source of fixed nitrogen to promote growth of plants [30]. Urea is included in beef cattle diets to economically replace a portion of protein content and is used as an inexpensive protein source in ruminants feed [31]. Urea occurs physiologically in nature as the major nitrogen containing end product of protein in mammals which excrete urea in urine. It is synthesized in the liver by urea cycle and is then secreted into blood stream (normal blood urea level: 3.3–6.7 mmol/L) and is taken up by the kidneys for excretion in urine. The adult human body discharges about 25–35 g of urea daily in urine. [32]. Thus, amongst numerous host molecules, urea being highly soluble, safe, non-toxic, stable, biocompatible, cheap and easily available was chosen as a host in the current study.
Urea (Fig. 1b) is a white crystalline material revealing a tetragonal structure with extensive hydrogen bonding at room temperature. The urea lattice consists of ribbons of molecules linked in a head-to-tail fashion along the tetragonal axis. Urea is reported to form hexagonal helical structure surrounding a canal like void space in the presence of straight chain hydrocarbons. Urea host structure comprises of a hexagonal structure with non-intersecting, linear and parallel tunnels at ambient temperature. The diameter of the urea tunnel varies between 0.55 and 0.58 nm. Urea inclusion complexes are characterized by urea molecules bonded to one another by extensive hydrogen bonds between the nitrogen and oxygen atoms. The interlinked urea molecules are orderly arranged in a distinct pattern of honey-comb network forming long tunnel cavities in which guest molecules are incorporated. [33–37].
Urea is well known host for forming complexes with linear long chain organic compounds. In general, molecules having benzene and cyclohexane rings do not form inclusion complexes because these structural components are too bulky to engulf inside the narrow urea tunnels. However, urea forms inclusion complexes with guest molecules having a sufficient long alkyl chain and only a limited degree of substitution of this chain is allowed [38–40]. The guest molecules interact partially with the tunnel walls and show vibrational and translational motion along tunnel axis. Also, 3-methyl heptane, a normally non-complexing guest (NNCG) exhibits complex formation with urea only in the presence of a slender hydrocarbon e.g. n-paraffin, a readily complexing guest (RCG) which functions as a “pathfinder” [41, 42]. Cyclic guests possessing a sufficiently long anchor length (i.e. n-alkane chain) are rapidly included in urea channels and known as RCG while sufficiently substituted cyclic guests with bulky aromatic rings are named as NNCG [43–45].
Recently, the steep improvement in dissolution profile of amiloride hydrochloride, glipizide, enalapril maleate, cis-retinoic acid and simvastatin—potent NNAG drugs through co- inclusion in urea has been achieved [46–50]. In the current study, an attempt has been made for the enhancement of dissolution profile of substituted potent organic compound, LAF (NNCG) by the inclusion of the drug in urea lattice in the presence of a suitable RCG [25, 26]. A variety of long straight chain linear compounds such as fatty acids, alkanes, alkenes, amino acids, alcohols, monoesters and diesters can be utilized as RCG. However, dimerization of fatty acid complexes in hexagonal urea network leads to improved stability of fatty acid–urea inclusion complex as compared to n-aliphatic compounds. Moreover, oleic acid as a formulation ingredient is already included in Generally recognized as safe list notified by FDA and is reported to increase the bioavailability of hydrophobic drugs [51]. Hence, oleic acid (Fig. 1c) was used as the RCG for co-inclusion of LAF in urea. LAF is significantly substituted cyclic organic compound and is not known to form complex with urea under any known conditions. However, using a modified technique LAF can be readily co-included in urea in the presence of oleic acid or any other suitable RCG.
Materials and methods
Materials
Lafutidine, manufactured by Emcure Pharmaceuticals Ltd, India, was supplied as a gift sample by Akums Drugs and Pharmaceuticals Ltd, New Delhi, India. Urea crystals (CDH, India), methanol AR grade (Rankem, India) and oleic acid (CDH, India) were utilized. All other chemicals used in the study were of analytical grade.
Methods
Preparation of urea co-inclusion complexes of LAF with RCG
LAF of 0.5 g was dissolved in 25 ml methanol containing 5 g urea by gentle heating. Subsequently, 0.5 g of oleic acid was incorporated as RCG to the above solution leading to the immediate precipitation of the crystals of urea co-inclusion complex. The solution was allowed to stand for 2–3 h at room temperature. The crystals were separated from the mother liquor by filtration, dried and suitably packed [24–26].
Characterization of urea co-inclusion complexes
FTIR analysis
The FTIR spectra of urea, urea/oleic acid complex (UOAC), LAF and lafutidine urea co-inclusion complex (LUCIC) were obtained using IR Affinity-1 Fourier transform infrared spectrophotometer (Shimadzu 02205 model) using KBr disc technique (KBr Press Model M-15, Techno Search Instruments) and all samples were scanned over a range of 400–4000 cm−1.
Differential scanning calorimetry
Thermal analysis of urea, UOAC, LAF and LUCIC was conducted using Differential scanning calorimeter (Perkin Elmer, Germany Model—DSC 4000 serial no. 520A2031904). The DSC analysis of each sample was carried out by heating the sample (~5 mg) in a crimped aluminium pan at a scanning rate of 10 °C/min from ambient temperature to 350 °C in an atmosphere of nitrogen gas by passing gas at a flow rate of 20 ml/min. An empty aluminium pan was employed as the reference pan.
Powder X-ray diffraction studies
X-ray diffractograms of the urea, LAF and LUCIC crystals were obtained using Philips X-ray diffractometer type PW1710 having Cu based tube anode and the UOAC using Xpert-Pro X-ray diffractometer type PW3050/60. Experimental settings employed were: Nickel filtered Cu-Kα radiations [wavelength (λ) α1 = 1.54056 A and wavelength (λ) α2 = 1.54439 A], current 20 mA, generator tension 40 kV, divergence slit 1ϕ, intensity ratio 0.500, and scanning rate 2°/min over a 2ϴ range of 0°–50°.
Proton nuclear magnetic resonance studies
The 1H-NMR spectra of urea and UOAC were recorded in Bruker FT-NMR spectrometer (400 MHz) using DMSO (δH = 2.5 ppm) as the reference compound. The 1H-NMR spectra of LAF, oleic acid and LUCIC were conducted in Bruker Avance II 400 NMR spectrometer (400 MHz) using DMSO (δH = 2.5 ppm) as the reference compound.
Determination of minimum ratio of RCG and LAF for formation of co-inclusion complexes with urea
Phase I
Determination of stoichiometric ratio between urea and RCG
A calorimetric method reported by Zimmerschied et al. was employed using oleic acid as RCG in urea for the determination of stoichiometric ratio between urea and RCG [52]. A small mouthed silvered Dewar flask with rubber stopper bearing a thermocouple system, capable of reading up to 0.01 °C, served as the calorimeter. Hence, 12 g of urea dissolved in 70 ml methanol was shaken gently in the calorimeter until an equilibrium temperature was attained. The increments of 1.6 mmol (0.5 ml) of oleic acid were successively incorporated into the calorimeter using thistle funnel. The calorimeter was shaken after each addition to facilitate an early attainment of the equilibrium temperature. A plot showing an increase in temperature following incremental addition of oleic acid in millimoles to methanolic solution of urea was obtained to calculate the minimum amount of RCG needed for the inclusion in the urea lattice. The initial and final curves of rise in temperature were extrapolated for obtaining a point of intersection for determining the amount of oleic acid required for inclusion with a given quantity of urea.
Phase II
Determination of minimum ratio of RCG and LAF for formation of urea co-inclusion complexes
The minimum amount of RCG required for the formation of co-inclusion complexes of LAF and RCG in urea was determined using modified Zimmerschied calorimetric method based on measurement of temperature increase following addition of RCG to methanolic solution of urea containing excess of normally non-complexing guest (NNCG) [24–26]. Hence, 12 g urea and 3 g LAF were dissolved in 70 ml methanol and shaken gently in the calorimeter until an equilibrium temperature was obtained. The initial increments of 0.63 mmol (0.2 ml) and subsequently of 1.6 mmol (0.5 ml) of oleic acid (RCG) were successively incorporated into the calorimeter. The calorimeter was gently shaken after each addition and the equilibrium temperature was noted after each addition. The plot of increase in temperature versus the amount of RCG added in millimoles to methanolic solution of urea and LAF was obtained showing the events in the following sequence i.e., an initial increase in temperature, followed by an intermediate transition phase, subsequent second temperature rise curve leading to attainment of a final temperature. The initial and intermediate transition phase curves of temperature were extrapolated for obtaining a point of intersection for determining the minimum amount of oleic acid needed for co-inclusion with a given amount of LAF in urea [46–50].
Preparation of urea co- inclusion complexes containing varying proportions of LAF and RCG
A variety of urea LAF–RCG co- inclusion complexes containing varying proportions of LAF and RCG (Table 1) were prepared by adding 2 g of guests to 30 ml methanol containing 10 g urea. The solutions were kept for 2–3 h at room temperature and resulting crystals were separated from the mother liquor by filtration, dried and packed suitably. All these urea inclusion complexes were subjected to DSC analysis from ambient temperature to 350 °C.
Assay procedure
Lafutidine was estimated by ultraviolet–visible (UV–Vis) spectrophotometric method using Labindia UV/Vis spectrophotometer UV 3000. Both urea and oleic acid did not show interference in the estimation of LAF at 268 nm in methanol and at 274 nm in phosphate buffer. The absorbance was recorded on UV/Vis spectrophotometer of various dilutions of LAF at λ max of 268 nm in methanol for content uniformity studies and at λ max of 274 nm in phosphate buffer (pH 6.8) for performing dissolution rate studies [53, 54].
Content uniformity studies
For the determination of content uniformity and for checking compliance with the JP requirements, exactly weighed quantities of ten randomly drawn samples of LAF–RCG-urea co-inclusion complexes (LUCIC) containing quantity equivalent to 10 mg of LAF were dissolved in methanol and suitably diluted. The drug contents were determined at 268 nm in methanol spectrophotometrically [55].
Dissolution rate studies
The in vitro dissolution rate studies were performed in 900 ml of phosphate buffer (pH 6.8), maintained at 37 ± 0.5 °C at a speed of 50 rpm using USP dissolution apparatus II (Lab India Dissolution Test paddle apparatus—DS 8000).The quantity equivalent to 10 mg of LAF in co-inclusion complex (LUCIC-2) was added to dissolution medium. 5 ml of the samples were withdrawn at predetermined time intervals (2, 5, 10, 20, 30, 45 and 60 min) with volume replacement. The samples were filtered and assayed spectrophotometrically at 274 nm. Other co-inclusion complexes, LUCIC-3, LUCIC-4 and LUCIC-5 were similarly analyzed for dissolution profile. All the experiments were performed in triplicate. The in vitro dissolution drug release data of LUCIC-2 and LUCIC-4 was analyzed using dissolution model software (KinetDS version 3.0, 2010) for various release kinetics model i.e. Zero-order, First-order, Korsmeyer Peppas and Hickson Crowell model [55].
Results and discussion
In the present study, an attempt was made for enhancement of dissolution profile through formation of co-inclusion complexes of LAF in urea in the presence of a suitable RCG. LAF is normally non complexing highly substituted cyclic organic compound and is presumably too wide to accommodate inside the narrow urea tunnels. However, using a modified technique, LAF was co-included in urea in the presence of oleic acid acting as RCG. Hence, the addition of small amount of oleic acid as RCG to a methanolic solution of urea and LAF led to an immediate precipitation of crystals of LAF–RCG-urea co-inclusion complex (LUCIC). The formation of inclusion complexes of LUCIC was confirmed by FTIR, DSC, XRD and 1H-NMR.
FTIR spectrometry
The FTIR spectra of UOAC, pure drug LAF and LUCIC crystals are depicted in Fig. 2. The FTIR spectrum of UOAC exhibits skeletal out-of-plane bending vibrations at 795 cm−1, symmetric C–N frequency at 1015 cm−1, NH2 rocking vibrations at 1157 cm−1, C–N vibration frequency at 1481 cm−1, absorption bands due to combination of NH2 bending and C = O stretching vibrations at 1597 and 1659 cm−1, out-of-phase NH2 vibrations at 3383 cm−1and in-phase NH2 vibrations at 3225 cm−1, which are characteristic peaks of the hexagonal form of urea [50]. The similar vibrations in FTIR spectrum of urea indicative of the characteristic peaks of tetragonal form of urea are obtained at 787, 1003, 1150, 1458, 1620, 1674, 3429 and 3325 cm−1. These results indicate conversion of uncomplexed tetragonal urea to hexagonal structure of urea in UOAC IN presence of oleic acid [50]. The FTIR spectrum of LAF shows the bands of LAF, the important one at 1034 cm−1 (S = O sulfoxides stretching vibration); 1007 cm−1 (=C–H vinyl vibration); 1296 and 1354 cm−1 (C–O ether stretching vibration); 1477 cm−1 (CH2 methylene bending vibration); 1400 cm−1(C–N stretching vibration); 1549 cm−1(N–H secondary amine bending vibration); 1609 cm−1 (C = C alkene vibration); 1659 cm−1 (C = O-NH amide stretching vibration); 2847 and 2931 cm−1(C–H alkane stretching vibration); 3283 cm−1(N–H secondary amide stretching vibration) [22]. The FTIR spectrum of LUCIC crystals indicates out-of-phase vibrations at 3383 cm−1and in-phase vibrations at 3229 cm−1, which are important peaks of the hexagonal structure of urea [56]. Similarly, the existence of bands at 1628 and 1597 cm−1 (due to NH2 bending and C = O stretching vibrations), slight raising of the skeletal out-of-phase bending frequency at 791 cm−1, NH2 rocking vibrations at 1165 cm−1, symmetric C–N frequency at 1015 cm−1 indicate characteristic peaks of hexagonal form of urea [57]. These results indicate the conversion of tetragonal urea to channel like hexagonal form of urea in LUCIC. Moreover, the FTIR spectrum of LUCIC crystals also exhibit certain absorption bands of LAF at 1069 cm−1 (sulfoxides S = O stretching vibrations); 1292 and 1346 cm−1 (ether C–O stretching vibrations); 1485 cm −1 (methylene -CH2bending vibration); 1628 cm−1 (amide C = O-NH stretching vibrations); 2855 and 2928 cm−1(alkane C–H stretching vibrations) [22] indicating the inclusion of the guest moiety (LAF) in LUCIC complex.
Differential scanning calorimetry
Figure 3 exhibits the thermograms of UOAC, pure LAF and crystals of co-inclusion complexes of LAF (LUCIC). The thermogram of urea alone displays melting onset temperature at 134.31 °C with a sharp melting endotherm at 141.0 °C. The UOAC thermogram shows that urea melts in two steps, a characteristic of hexagonal form of complexed urea. The first step (117.34 °C) is attributed to the collapse of the hexagonal form of the UOAC to yield the tetragonal solid urea, while the second step (134.83 °C) involves melting of tetragonal urea [50]. Pure LAF reveals a melting endotherm in the temperature range of 99.94–107.66 °C with a sharp melting endothermic peak at 104.95 °C [22]. LUCIC thermogram shows that the urea crystal melts incongruently in two steps. The first step (115.70 °C) involves the decomposition of the hexagonal form of urea inclusion complex to yield the guest moiety (LAF) and the tetragonal solid urea while the second step (134.34 °C) is attributed to the melting of tetragonal urea. The disappearance of a sharp melting endotherm of LAF at 104.95 °C indicates the inclusion of the drug (LAF) into the urea lattice and the presence of LAF in an amorphous state in LUCIC complex [58–61].
Powder X-ray diffraction studies
The powder X-ray diffractograms of UOAC, pure LAF and urea co-inclusion complex of LAF with RCG (LUCIC) are illustrated in Fig. 4. The X-ray powder diffractograms of urea reveals characteristic interplanar spacings corresponding to tetragonal form of urea at 2.4368, 2.5419, 2.8294, 3.0528, 3.6144 and 4.0198 A [62, 63]. The XRD diffractogram of UOAC exhibits interplanar spacings indicative of hexagonal form of urea at 3.2637, 3.3858, 3.4089, 3.5920, 3.8481, 4.1031 and 7.0769 A. These results indicate the transformation of uncomplexed tetragonal urea to hexagonal structure of urea in UAOC [50]. Pure LAF exhibits a highly crystalline nature indicated by the sharp and intense diffraction peaks, the important ones at interplanar spacings 15.6151, 10.4677, 8.3864, 5.2205, 4.5369, 4.2318, 4.0533, 3.9191, 3.5484, 3.3262 and 3.1862 A [22]. However, the diffractograms of crystals of LUCIC co-inclusion complexes reveals the important peaks at interplanar spacings 3.2823, 3.4088, 3.5814, 3.8661, 4.1738 and 7.2725 A, which are characteristic peaks of the hexagonal form of urea. The complete absence of major peaks of LAF indicates the fact that guest molecules are trapped and isolated from one another in the honey-comb network of urea and do not contribute towards crystallinity of LAF. These results clearly reveal conversion of LAF from crystalline nature to amorphous state following the formation of inclusion complex with urea in the presence of RCG [64, 65].
Proton nuclear magnetic resonance studies
The 1H-NMR spectra of UOAC, oleic acid, pure drug LAF and LUCIC crystals are depicted in Fig. 5.The 1H-NMR spectrum of pure urea exhibits a peak of N–H proton (singlet) at δH = 5.51 ppm. The conversion from tetragonal urea to hexagonal structure of urea in UOAC is represented by shift of N–H proton from tetragonal urea δH = 5.51 to δH = 5.32–5.51 ppm in UOAC indicating H-bonding between urea and oleic acid (RCG) [50]. The complete engulfing of LAF and oleic acid in LUCIC inclusion complex is exhibited by many changes in the1H-NMR spectrum. The transformation of tetragonal urea to hexagonal form of urea in LUCIC is shown by shift of N–H proton from 5.51 to δ H = 5.43–5.56 ppm with high prominence due to interaction between (–NH2) group of urea and (–COOH) group of oleic acid. Moreover, O–H proton from carboxylic function of oleic acid at δH= 11.82 ppm nearly deplete in LUCIC complex indicating that oleic acid is included as a guest in LUCIC complex. Also, C9H = C10H protons of oleic acid showing peak at δH= 5.27–5.35 appeared at 5.27–5.37 ppm, C3H2 protons at 1.51–1.54 appeared at δH = 1.51–1.53 ppm in LUCIC indicating inclusion of oleic acid in LUCIC. Moreover, 20 protons of oleic acid at C3–C7 and C12–C16 at 1.26–1.28 appeared at δH = 1.25–1.27 ppm in LUCIC; C8H2 and C11H2 protons of oleic acid at 1.95–2.05 appeared at 1.96–2.04 ppm and C18H3 at 0.85–0.89 appeared at δH = 0.84-0.88 ppm in LUCIC indicating complete incorporation of oleic acid in LUCIC complex [66–68]. Most of the exposed protons of LAF e.g. C1H2 at 1.39–1.42 appeared at 1.41–1.42 ppm in LUCIC; C2H2, C2′H2 at 1.49–1.54 appeared at 1.49–1.53 ppm in LUCIC; C3H2, C3′H2 at 2.32, 2.51–2.53 appeared at 2.37 and 2.68–2.75 ppm; C4H2 at 3.88–3.91 appeared at 3.89–3.92 ppm in LUCIC; C6H2 at 6.70 appeared at 6.71 ppm; C8H2 at 7.63–7.64 appeared at 7.62–7.63 ppm; C9H at 6.89–6.90 appeared at 6.91–6.92 ppm; C10H2 at 4.88–4.90 appeared at 4.89–4.90 ppm; C11H = C12H protons of LAF at 5.55–5.61 and 5.73–5.79 appeared at 5.59–5.61 and 5.72–5.81 ppm in LUCIC; C13H2 and C15H2 at 4.33–4.37 and 3.71–3.74 appeared at 4.35–4.39 and 3.71–3.77 ppm; C16H2 at 4.15–4.19 appeared at 4.18–4.22 ppm; C18H, C19H at 6.44–6.46 appeared at 6.46–6.47 ppm; C20H at 8.04–8.06 appeared at 8.05–8.07 ppm and NH proton of LAF at 8.49–8.51 appeared at δH = 8.59–8.62 ppm in LUCIC. The integration of NMR data was considered to ascertain the composition of protons in LUCIC. The digitized integral values for integrals printed below the peaks in NMR spectrum of LUCIC complex were observed to calculate the total area (602.92 mm) and dividing the total area by total number of hydrogen atoms to determine the actual number of protons in the complex. Thus, 28 protons of LAF, 33 protons of oleic acid and 4 protons of urea appeared in LUCIC complex indicating the complete incorporation of guest moieties (oleic acid and LAF) in LUCIC complex. [22].
Minimum ratio of RCG and LAF for inclusion of LAF in urea
The calorimetric method involving the addition of successive small increments of RCG (oleic acid) to a mixture of urea and methanol until the temperature ceases to rise was employed [52]. The initial rise curve and final curve in Fig. 6a were extrapolated for obtaining the intersection point to calculate the minimum amount of RCG needed for inclusion in urea. The minimum amount of oleic acid calculated in terms of millimoles (mM) required for inclusion in 0.20 mol of urea was found to be 10.55 mmol of oleic acid. The amount expressed in terms of mole fraction was 0.05 mol fractions of oleic acid for 0.95 mol fraction of urea.
The Fig. 6b exhibited temperature rise on addition of successive small increments of oleic acid to a methanolic solution of urea and LAF. The initial rate of temperature rise curve and intermediate transition phase curve of temperature rise were extrapolated to obtain the intersection point for obtaining the minimum amount of RCG required for inclusion of LAF in urea [25, 26]. The minimum amount of oleic acid calculated in terms of mmol (mM) required for co-inclusion in 6.95 mmol of LAF was found to be 7.68 mmol of oleic acid. The minimum ratio of RCG: LAF calculated in terms of mmol (mM) required per 2.32 mmol of LAF for co-inclusion in urea was 2.54 mmol of oleic acid. The amount expressed in terms of mole fraction was 0.52 mol fractions of oleic acid for 0.48 mol fractions of LAF [24, 46–50].
The curve plotted for addition of oleic acid to methanolic solution of urea had a smooth sigmoid shape (Fig. 6a) but the same curve in the presence of the drug LAF revealed the following sequence of events, that is, an initial temperature rise, followed by an intermediate transition phase, subsequently second temperature rise curve and then attainment of a final temperature. The second stage of temperature rise is due to the displacement of LAF (NNCG) with RCG as demonstrated by the fact that the overall temperature rise is almost similar to that of RCG alone. This phenomenon is in consonance with earlier studies [46–50]. The optimum formulation ratio of RCG: LAF calculated in terms of molar ratio required per mole of LAF for co-inclusion in urea was found to be 1.10:1.0 (0.72:1 g/g). After determining the minimum ratio of RCG: LAF, the urea co-inclusion complexes containing varying molar proportions of RCG in guests (listed in Table 1) were prepared and utilized for further investigations.
Thermal analysis of urea co-inclusion complexes
Thermal analysis of urea co-inclusion complexes containing varying proportions of RCG and LAF was performed using DSC. Figure 7 presents the overlay of DSC thermograms of co-inclusion complexes containing varying proportions of RCG and LAF. In all these thermograms, a low temperature endotherm was observed corresponding to the collapse of the complexed urea and to the release of guest molecule and the solid tetragonal form of urea. The disappearance of the melting endotherm at 104.95 °C indicates the absence of crystalline form of LAF and the inclusion of the drug into the host lattice.
The Fig. 8 shows the plot of heat of decomposition against the molar proportion of RCG in guests in LUCIC co-inclusion complexes. The results for heat of decomposition of urea co-inclusion complexes of LAF (LUCIC) and UOAC are presented in Table 1. Thermal data pertaining to the influence of the relative proportion of RCG on the heat of decomposition was subjected to regression. Resulting relationship is as per the following:
where ΔH is the heat of decomposition of LAF urea co-inclusion complex (LUCIC) and R is the relative molar proportion of RCG in guests.
The plot indicates that an increase in molar proportion of RCG in the complex leads to corresponding enhancement in the heat of decomposition of the resulting urea co-inclusion complex and makes it more stable. Since, urea inclusion complex formation is exothermic in nature; therefore, its stability is directly linked to the heat of decomposition [49]. This indicates that an increase in the molar proportion of RCG in the urea co-inclusion complex leads to increase in heat of decomposition with consequent improved physical stability of the co-inclusion complex. The heat decomposition curve shows an increase in the heat of decomposition value (J/G) in various LUCIC complexes containing increasing molar proportions of RCG and clearly exhibits signs of saturation from LUCIC-7 onwards to UOAC complex without LAF. The saturation level in curve reveals that heat of decomposition value of the complex LUCIC-7 is similar to the value of UOAC complex without LAF. Since LAF is an aromatic bulky molecule with substitutions, it is presumably too wide to accommodate inside the hexagonal tunnel formed by urea lattice. However, in the presence of the RCG, LAF is co-included along with RCG in urea. This inclusion of bulky guest moiety would in turn, lead to distortion of the urea tunnel structure in the vicinity of cyclic ring, the extent of steric strain on the host lattice corresponding to the proportion of NNCG incorporated in the urea co-inclusion complex [61].
Content uniformity analysis
Content uniformity test listed in JP [55] is performed to establish homogeneity and uniformity of low dose drug(s) in a batch. If the batch fails to meet with content uniformity test, it results in under-dosage or over-dosage of potent drug in the formulated products which is highly objectionable. In order to meet the JP criterion of content uniformity, the formulation must contain 85–115 % drug of the label claim. The data of content uniformity analysis for different LUCIC urea co- inclusion complexes containing varying RCG to drug ratios are listed in Table 2. From the experimental data, it is apparent that the mean drug content of LAF in various co-inclusion complexes is not significantly different and the low dose drug is uniformly distributed in the complexes [55]. Hence, co-inclusion complexes of drug in urea lattice reveal good content uniformity and can be utilized for the development of potent drug formulations.
Dissolution rate studies
Figure 9 exhibits the dissolution profiles obtained from the experimental values of pure LAF and its urea co-inclusion complexes LUCIC-2, LUCIC-3, LUCIC-4 and LUCIC-5 in phosphate buffer (pH 6.8). The dissolution profiles were calculated by the dissolution percentage (DP) at 2 and 5 min. The dissolution percentage reveals the percentage of drug dissolved in the dissolution medium. The extent of the pure LAF released was found to be quite low showing drug release as DP2~24 % and DP5~32.5 %. However, co-inclusion of LAF in urea resulted in an immediate and almost complete release of drug as represented in Table 3 by release of contents DP2~91 % and DP5~100 % (LUCIC-2 and LUCIC-3); DP~87 % and DP5~95 % (LUCIC-4); DP2~81.5 % and DP5~90 % (LUCIC-5). The dissolution behaviour of various urea co-inclusion complexes (LUCIC-2, LUCIC-3 and LUCIC-4) was found to be almost similar. The release data of LUCIC-2 and LUCIC-4 were subjected to kinetic treatment to know the pattern of drug release. The regression (R2 value) for various models was calculated for LUCIC-2 and LUCIC-4 and found in the order Korsmeyer Peppas > Hickson Crowell > first order > zero order represented in Fig. 9. The Korsmeyer Peppas model explained the drug release kinetics of LAF in the most fitting manner [50, 55].
The drug release from urea co-inclusion complexes of LAF (LUCIC) was very rapid and exhibited steep rise in dissolution rate as compared to pure drug LAF. The urea co-inclusion complexes of LAF presented superior dissolution profile showing an almost instantaneous release of drug in comparison to the pure drug. Urea co-inclusion complexes of LAF when come in contact with an aqueous dissolution medium, the urea lattice dissolves rapidly and results in an instantaneous release of the incorporated drug present at the molecular level. The co-inclusion of a NNCG drug along with RCG in urea hexagonal tunnels leads to weakening of urea host lattice and facilitates further dissolution enhancement. However, this immediate release of the drug was followed by a subsequent decrease in drug contents in solution and DP60 was found to be ~83 %. Moreover, as the amount of RCG in the inclusion complex is increased, heat of decomposition value gets increased showing improved stability of the co-inclusion complex and subsequent decrease in the drug release rate (LUCIC-5) due to improved stability of distorted urea lattice. However, as LAF has limited aqueous solubility, the initially released drug molecules subsequently tend to crystallize out in excess of the solubility. This may be caused by non-sink conditions of the dissolution medium. Since, LAF is a BCS class II drug having low aqueous solubility and rapid permeability, therefore, the drug molecules released may rapidly permeate through biological barriers in vivo and the built- up of concentration at the site of dissolution may not actually occur [47, 50]. Thus, urea co-inclusion complex formation can be exploited as a valuable technique for the development of immediate release pharmaceutical formulation of LAF.
Conclusion
In the current study, Lafutidine, a BCS class II potent substituted cyclic organic compound, was successfully incorporated in urea in the presence of RCG. The formation of lafutidine urea co-inclusion complexes was confirmed by DSC, FTIR, XRD and 1H-NMR studies. The regression studies depicted that an increase in the molar proportion of RCG in the urea co-inclusion complex leads to increase in heat of decomposition with consequent improved physical stability of the co-inclusion complexes. Content uniformity studies revealed that LAF being a low dose drug was uniformly distributed and co-inclusion complexes of LAF were found to show good content uniformity. In vitro dissolution rate studies demonstrated steep enhancement in dissolution profile of LAF having poor aqueous solubility. The urea co-inclusion complexes of LAF (LUCIC) exhibited far superior dissolution profile as compared to that of pure drug. The Korsmeyer Peppas model exhibited drug release kinetics in the most fitting manner while analyzing the dissolution data by various release kinetic models. Simultaneous improvement in content uniformity and dissolution rate offer urea co-inclusion complexes a vast potential for formulation of low dose poorly soluble drug candidates into immediate release products with reduced processing time and minimal cost.
References
Kurata, J.H., Nogawa, A.: Meta-analysis of risk factors for peptic ulcer: nonsteroidal anti-inflammatory drugs, Helicobacter pylori, and smoking”. J. Clin. Gastroenterol. (1997). doi:10.1097/00004836-199701000-00002
Onodera, S., Tanaka, M., Aoyama, M., Arai, Y., Inaba, N., Suzuki, T., Nishizawa, A., Shibata, M., Sekine, Y.: Antiulcer effect of lafutidine on indomethacin-induced gastric antral ulcers in refed rats. Jpn. J. Pharmacol. 80(3), 229–235 (1999)
Higuchi, K., Watanabe, T., Tominaga, K., Shiba, M., Nakagawa, K., Uno, H., Kitada, K., Satoh, H., Chono, S., Oshitani, N., Fujiwara, Y., Arakawa, T.: Lafutidine can improve the quality of gastric ulcer healing in humans: a randomized, controlled, multicenter trial. Inflammopharmacol. 14(5–6), 226–230 (2006)
Devault, K.R., Castell, D.O.: Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The practice parameters committee of the American college of gastroenterology. Am. J. Gastroenterol. 94(6), 1434–1442 (1999)
Onodera, S., Nishida, K., Takeuchi, K.: Unique Profile of Lafutidine, a novel histamine H2-receptor antagonist—mucosal protection throughout gastrointestinal tract mediated by capsaicin-sensitive afferent neurons. Drug Design Rev.—Online 1(2), 133–144 (2004)
Yamagishi, H., Koike, T., Ohara, S., Horii, T., Kikuchi, R., Kobayashi, S., Abe, Y., Iijima, K., Imatani, A., Suzuki, K., Hishinuma, T., Goto, J., Shimosegawa, T.: Stronger inhibition of gastric acid secretion by lafutidine, a novel H2 receptor antagonist, than by the proton pump inhibitor lansoprazole. World J. Gastroenterol. (2008). doi:10.3748/wjg.14.2406
Uesugi, T., Mikawa, K., Nishina, K., Morikawa, O., Takao, Y., Obara, H.: The efficacy of lafutidine in improving preoperative gastric fluid property: a comparison with ranitidine and rabeprazole. Anesth. Analg. (2002). doi:10.1097/00000539-200207000-00025
Romero, C.M., Fass, R.: New and future drug development for gastroesophageal reflux disease. J. Neurogastroenterol. Motil. (2014). doi:10.5056/jnm.2014.20.1.6
Iida, H., Inamori, M., Nozaki, Y., Endo, H., Hosono, K., Akiyama, T., Sakamoto, Y., Takahashi, H., Koide, T., Tokoro, C., Abe, Y., Nakajima, A.: Early effects of oral administration of lafutidine with mosapride compared with lafutidine alone on intragastric pH values. BMC Gastroenterol. (2009). doi:10.1186/1471-230X-9-52
Nozawa, Y., Nishihara, K., Akizawa, Y., Orimoto, N., Nakano, M., Uji, T., Ajioka, H., Kanda, A., Matsuura, N., Kiniwa, M.: Lafutidine inhibits Helicobacter pylori-induced interleukin-8 production in human gastric epithelial cells. J. Gastroenterol. Hepatol. 19(5), 506–511 (2004)
Shimatani, T., Inoue, M., Kuroiwa, T., Xu, J., Nakamura, M., Tazuma, S., Ikawa, K., Morikawa, N.: Lafutidine, a newly developed antiulcer drug, elevates postprandial intragastric pH and increases plasma calcitonin gene-related peptide and somatostatin concentrations in humans: comparisons with famotidine. Dig. Dis. Sci. 51(1), 114–120 (2006)
Ikawa, K., Shimatani, T., Hayato, S., Morikawa, N., Tazuma, S.: Pharmacokinetic and pharmacodynamic properties of lafutidine after postprandial oral administration in healthy subjects: comparison with famotidine. Biol. Pharm. Bull. 30(5), 1003–1006 (2007)
Dewan, B., Shah, D.: A study to evaluate the symptomatic efficacy and safety of Lafaxid TM (lafutidine 10 mg) in patients with acid peptic disorders in India. Br. J. Med. Med. Res. 3(4), 821–831 (2013)
Sugiyama, T., Hatanaka, Y., Iwatani, Y., et al.: Lafutidine facilitates calcitonin gene-related peptide (CGRP) nerve-mediated vasodilation via vanilloid-1-receptors in rat mesenteric resistance arteries. J. Pharmacol. Sci. 106(3), 505–511 (2008)
Ichikawa, T., Ishihara, K., Saigenji, K., Hotta, K.: Lafutidine-induced stimulation of mucin biosynthesis mediated by nitric oxide is limited to the surface mucous cells of rat gastric oxyntic mucosa. Life Sci. 62, 259–264 (1998)
Onodera, S., Shibata, M., Tanaka, M., Inaba, N., Arai, Y., Aoyama, M., Lee, B., Yamaura, T.: Gastroprotective mechanism of lafutidine, a novel anti-ulcer drug with histamine H2-receptor antagonistic activity. Arzneimittelforschung 49(6), 519–526 (1999)
Tanaka, M., Banba, M., Joko, A., Moriyama, Y.: Pharmacological and therapeutic properties of lafutidine (stogar and protecadin), a novel histamine H2 receptor antagonist with gastroprotective activity. Nihon Yakurigaku Zasshi 117(6), 377–386 (2001)
Ichikawa, T., Ishihara, K., Saigenji, K., Hotta, K.: Effects of acid-inhibitory antiulcer drugs on mucin biosynthesis in the rat stomach. Eur. J. Pharmacol. 251, 107–111 (1994)
Onodera, S., Tanaka, M., Aoyama, M., Arai, Y., Shibata, M., Yamaura, T., Ohnishi, H.: Effect of lafutidine, a novel antiulcer agent, on healing and relapse of acetic acid-induced gastric ulcer in rats. Nihon Yakurigaku Zasshi 111(3), 167–175 (1998)
Akiba, Y., Kaunitz, J.D.: Lafutidine, a protective H2 receptor antagonist, enhances mucosal defense in rat esophagus. Dig. Dis. Sci. (2010). doi:10.1007/s10620-010-1379-y
Katsu, Y., Iguchi, S., Sone, H., Uchida, C., Kojima, T.: Benzimidazolone compounds having 5-HT4 receptor agonistic activity. US Patent 7776885 B2 published on Aug 17, (2010)
Fule, R., Amin, P.: Development and evaluation of lafutidine solid dispersion via hot melt extrusion: investigating drug-polymer miscibility with advanced characterization. Asian J. Pharm. Sci. (2014). doi:10.1016/j.ajps.2013.12.004
Kanugo, A.Y., Chavhan, P., Potnis, V.V.: Melt evaporation method: a solid dispersion strategy to enhance solubility and dissolution of lafutidine. World J. Pharm. Res. 2(6), 2284–2300 (2013)
Madan, A.K.: Microencapsulation of low dose drugs. Ph.D. Thesis, IIT Delhi (1994)
Madan, A.K., Grover, P.D.: A process for preparation of urea based inclusion compounds of vitamin A esters. Indian Patent 180627 filed on 20/01/1993 (1993)
Madan, A.K., Bajaj, V.: A process for preparation of urea based inclusion compounds of vitamin E and its esters. Indian Patent 182620 filed on 24/10/1994 (1994)
Pineiro, A., Banquy, X., Pérez-Casas, S., Tovar, E., García, A., Villa, A., Amigo, A., Mark, A.E., Costas, M.: On the characterization of host-guest complexes: surface tension, calorimetry, and molecular dynamics of cyclodextrins with a non-ionic surfactant”. J. Phys. Chem. (2007). doi:10.1021/jp0688815
Frank, S.G.: Inclusion compounds. J. Pharm. Sci. 64, 1585–1604 (1975)
Bengen, M.F.: Urea channel inclusion compounds. German Patent Application OZ 123438 filed on 18/03/1940 (1940)
Marsh, K.L., Sims, G.K., Mulvaney, R.L.: Availability of urea to autotrophic ammonia-oxidizing bacteria as related to the fate of 14C- and 15N-labeled urea added to soil. Biol. Fert. Soil. 42, 137–145 (2005)
Clanton, D.C.: Non-protein nitrogen in range supplements. J. Anim. Sci. 47, 765–779 (1978)
U.S. EPA (Environmental Protection Agency). Toxicological review of urea. (CAS No. 57-13-6). In Support of Summary Information on the Integrated Risk Information System (IRIS). External peer review draft, Washington, DC; EPA/635/R-10/005A. Available online at http://www.epa.gov/iris/backgrd.html (Sept 2010). Accessed June 15, 2014
Harris, K.D.M.: Meldola lecture: understanding properties of urea and thiourea inclusion compounds. Chem. Soc. Rev. 26, 279–289 (1997)
Harris, K.D.M.: Fundamental and applied aspects of urea and thiourea inclusion compounds. Supramol. Chem. (2007). doi:10.1080/10610270600977706
Rujas, J.M., Harris, K.D.M., Desmedt, A., Guillaume, F.: In-situ monitoring of alkane-alkane guest exchange in urea inclusion compounds using confocal Raman microspectrometry. Mol. Cryst. Liq. Cryst. (2006). doi:10.1080/15421400600788633
Smith, A.E.: The crystal structure of urea-hydrocarbon complexes. Acta Crystallogr. 5, 224–235 (1952)
Takemoto, K., Sonada, N.: Inclusion compounds of urea, thiourea and selenourea. In: Atwood, J.L., Davis, J.E.D., MacNicol, D.D. (eds.) Inclusion compounds, vol. 2, pp. 47–67. Academic Press, London (1984)
Bishop, R., Dance, I.G.: New type of helical inclusion networks. Top. Curr. Chem. 149, 139–188 (1988)
Harris, K.D.M., Thomas, J.M.: Structure aspects of urea inclusion compounds and their investigation by X-ray diffraction: a general discussion. J. Chem. Soc., Faraday Trans. 86, 2985–2996 (1990)
Smart, S.S., Baghdagi, A.E., Gullaume, F., Harris, K.D.M.: Conformational and vibrational properties of α, ω-dihalogenoalkane/urea inclusion compounds: a Raman scattering investigation. J. Chem. Soc., Faraday Trans. 90, 1313–1322 (1994)
Hollingsworth, M.D., Harris, K.D.M.: Urea inclusion compounds. In: Atwood, J.L., Davis, J.E.D., MacNicol, D.D., Vogtle, F. (eds.) Comprehensive Supramolecular Chemistry. Solid State Supramolecular Chemistry-Crystal Engineering, vol. 6, pp. 177–237. Pergamon Press, Oxford (1996)
Schiessler, R.W., Flitter, D.: Urea and thiourea adduction of C5-C42- Hydrocarbons. J. Am. Chem. Soc. 74, 1720–1723 (1954)
Harris, K.D.M.: Urea inclusion compounds. In: Atwood, J.L., Steed, J.W. (eds.) Encyclopedia of Supramolecular Chemistry, vol. 2, pp. 1538–1549. Marcel Dekker, New York (2004)
Findlay, R.A.: Adductive crystallization. In: Schoen, H.M., Mcketta, J.J. (eds.) New Chemical Engineering Separation Techniques, pp. 31–257. Interscience Publishers, New York (1962)
Schlenk, W.: Urea addition of aliphatic compounds. Justus Liebigs Ann. Chem. 565, 204–240 (1949)
Thakral, S., Madan, A.K.: Adduction of amiloride hydrochloride in urea through a modified technique for the dissolution enhancement. J. Pharm. Sci. 97(3), 1191–1201 (2008)
Thakral, S., Madan, A.K.: Urea co-inclusion compounds of glipizide for the improvement of dissolution profile. J. Incl. Phenom. Macrocycl. Chem. (2008). doi:10.1007/s10847-007-9368-2
Thakral, S., Madan, A.K.: Urea inclusion compounds of enalapril maleate for the improvement of pharmaceutical characteristics. J. Pharm. Pharmacol. 59(11), 1501–1507 (2007)
Thakral, S., Madan, A.K.: Urea co-inclusion compounds of 13 cis-retinoic acid for simultaneous improvement of dissolution profile, photostability and safe handling characteristics. J. Pharm. Pharmacol. 60(7), 823–832 (2008)
Dhall, M., Madan, A.K.: Studies on urea co-inclusion complexes of simvastatin for improvement of pharmaceutical characteristics. J. Incl. Phenom. Macrocycl. Chem. (2014). doi:10.1007/s10847-014-0439-x
Stella, V., Haslam, J., Yata, N., Okada, H., Lindenbaum, S., Higuchi, T.: Enhancement of bioavailability of a hydrophobic amine antimalarial by formulation with oleic acid in a soft gelatin capsule. J. Pharm. Sci. 67, 1375–1377 (1978)
Zimmerschied, W.J., Dinnerstein, R.A., Weitkamp, A.W., Marschner, R.F.: Crystalline adducts of urea with linear aliphatic compounds. Ind. Eng. Chem. 42, 1300–1306 (1950)
Jadhav, K., Dhamecha, D., Tate, A., Tambe, H., Patil, M.B.: Application of UV spectrophotometric method for easy and rapid estimation of lafutidine in bulk and pharmaceutical formulation. Pharm. Methods. (2011). doi:10.4103/2229-4708.93398
Parekh, R.R., Patel, P.H., Patel, C.D., Patel, K.S., Patel, H.N.: Development and validation of UV spectrophotometric method for estimation of lafutidine in bulk and pharmaceutical dosage form. Int. J. Drug Dis. Res. 4(1), 325–329 (2012)
Komiyama, Y.: The Japanese Pharmacopoeia-Supplement I (JP XVI), The Ministry of Health, Labour and Welfare (MHLW) Ministerial notification No. 519, pp. 2438–2440 (2012)
Fischer, P.H.H., McDowell, C.A.: The infrared absorption spectra of urea-hydrocarbon adduct. Can. J. Chem. 38, 187–193 (1960)
Durie, R.A., Harrison, R.J.: Effect of urea-adduct formation and physical state on the infrared spectra of n-paraffin hydrocarbons. Spectrochem. Acta. 18, 1505–1514 (1962)
Keller, W.E.: Evidence of planar structure of urea. J. Chem. Phys. 16, 1003–1004 (1948)
McAdie, M.G.: Thermal decomposition of molecular complexes. Can. J. Chem. 41, 2144–2153 (1963)
Thakral, S., Madan, A.K.: Topological models for prediction of heat of decomposition of urea inclusion compounds containing aliphatic endocytes. J. Incl. Phenom. Macrocycl. Chem. 60(1), 187–192 (2012)
White, M.A.: Origins of thermodynamic stability of urea; alkane inclusion compounds. Can. J. Chem. 76, 1695–1698 (1988)
Radell, J., Connolly, J.W.: Urea complexes of partially fluorinated esters. J. Org. Chem. 25, 1202–1206 (1960)
Brodman, B.W., Radell, J.: X-ray powder diffraction patterns of some—alkanone urea inclusion compounds. Seperation Sci. 2, 139–142 (1967)
Radell, J., Brodman, B.W.: Urea inclusion compounds of alkenoic acids and alkyl alkenoates. Can. J. Chem. 43, 304–305 (1965)
Radell, J., Connolly, J.W.: Determination of relative stability of urea complexes from X-ray powder diffraction data. In: Muller, W.M. (ed.) Advances in X-ray analysis, vol. 4, pp. 140–150. Plenum Press, New York (1961)
Harris, K.D.M., Jonsen, P.: 2H-NMR investigation of the dynamic behaviour of n-hexadecane in its urea inclusion compound. Chem. Phy. Lett. 154, 593–598 (1989)
Harris, K.D.M.: Solid state NMR. Nucl. Mag. Reson. 22, 230–260 (1993)
Ru-Sheng, Z., Ri-Fang, Y., Liu-Hong, Y., He, L.: Spectroscopic data derived structure of lafutidine. Acta Metall. Sin. 23(3), 313–319 (2006)
Acknowledgments
The authors are thankful to Akums Drugs and Pharmaceuticals Ltd, New Delhi, India, for providing gift sample of Lafutidine. Thanks are due to SAIF, Panjab University, Chandigarh, India for providing XRD and NMR facilities. Thanks are also due to JCDM College of Pharmacy, Sirsa, India for allowing them to conduct DSC studies. Thanks are due to Indian Agricultural Research Institute, Pusa, New Delhi, India for providing facilities to conduct XRD studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhall, M., Madan, A.K. Simultaneous improvement in dissolution profile and content uniformity of lafutidine through co-inclusion in urea. J Incl Phenom Macrocycl Chem 82, 335–350 (2015). https://doi.org/10.1007/s10847-015-0493-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0493-z