Abstract
The solubility of α-lipoic acid (LA) with the addition of modified cyclodextrins was investigated using the solubility method. The solubility of LA in the presence of β-cyclodextrin (β-CD), hydroxypropyl-β-cyclodextrin (HP-β-CD), mono-6-O-glucopyranosyl-β-cyclodextrin (mono-G1-β-CD), methyl-β-cyclodextrin (Me-β-CD), 2,6-di-O-methyl-β-cyclodextrin (DM-β-CD), and sulfobutylether-β-cyclodextrin (SBE-β-CD) was higher than that of LA itself. In particular, the solubility of LA in the presence of SBE-β-CD was 20 times higher than that of LA alone. The structure of the inclusion complex of SBE-β-CD and LA in aqueous solution was examined by 1H-1H ROESY NMR spectroscopy. The 1,2-dithiolane moiety of LA was included from the secondary hydroxyl face of SBE-β-CD. The solid complexes of LA and SBE-β-CD were prepared by the kneading and freeze-drying methods. Formation of the solid complexes was confirmed by X-ray diffraction patterns (XRD), differential scanning calorimetry (DSC), and infrared spectroscopy (IR). The kneading and freeze-drying methods were successful for obtaining the solid inclusion complexes with improved thermal stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Lipoic acid (LA) is a potent anti-oxidant [1–4]. LA administration has also been reported to ameliorate some of the symptoms in diabetic patients [5–7]. However, the amount of LA in the human body decreases with aging. Normally LA is taken up orally from food such as liver, spinach, and tomatoes. It is difficult to obtain a large amount of LA since its concentration in these foods is very low, e. g., ca. 0.3 mg of LA in 100 g spinach. Recently, it has become popular to take LA as a supplement. Since LA is hard to dissolve in water and is unstable to light and heat, the development of a product with higher solubility and stability is required.
Natural cyclodextrins (α-, β-, and γ-cyclodextrin) are widely used in many fields and form inclusion complexes with a variety of guest compounds [8, 9]. However, natural cyclodextrins have relatively low solubilities in water, and this fact limits their use in pharmaceutical formulations. Recently, various kinds of cyclodextrin derivatives have been synthesized to extend their applications [10–13].
Junquera et al. have previously reported study on the LA and β-CD inclusion complex by spectroscopic and thermodynamic analysis [14] although the improvement of solubility of LA is limited due to low solubility of β-CD. More recently, Trentin and Carofiglio carried out capillary zone electrophoresis studies of the interaction of LA by natural cyclodextrins, and alkylated β-cyclodextrins [15]. Binding constants of lipotate anion with CDs were obtained in an alkaline solution such as pH 9 although there was no solubility data and structural study. For structural study a bimodal complexation of LA inside the β-CD cavity has been discussed in the literature [16] by ROESY NMR. And there is no structural data for LA and modify CD complex with improved solubility.
In the present work, we studied the inclusion complexes of LA and modified cyclodextrins in order to increase solubility under acidic condition and improve thermal stability of LA. We first investigated natural cyclodextrins and then examined sulfobutylether-β-cyclodextrin (SBE-β-CD), hydroxypropyl-α-cyclodextrin (HP-α-CD), hydroxypropyl-β-cyclodextrin (HP-β-CD), hydroxypropyl-γ-cyclodextrin (HP-γ-CD), mono-6-O-glucopyranosyl-β-cyclodextrin (mono-G1-β-CD), methyl-β-cyclodextrin (Me-β-CD), and 2,6-di-O-methyl-β-cyclodextrin (DM-β-CD).
Materials and methods
Materials
LΑ, HP-α-CD, HP-β-CD, HP-γ-CD and DM-β-CD were purchased from Sigma–Aldrich (St. Louis, USA). α-Cyclodextrin (α-CD), β-cyclodextrin (β-CD) and γ-cyclodextrin (γ-CD) were purchased from Wako Chemical (Tokyo, Japan). Mono-G1-β-CD, SBE-β-CD, and Me-β-CD were purchased from BioResearch of Yokohama (Yokohama, Japan), Cydex (Kansas, USA), and Junsei Chemical Co., Ltd. (Tokyo, Japan), respectively.
Phase solubility studies
Various concentrations of CD in acetic acid buffer (40 mM, pH 4) were added to an excess of LA, and the solutions were shaken at 25 ºC (modified CDs) or 40 ºC (natural CDs) for 2 weeks. The solutions were then passed through a 0.2 μm membrane filter. The amount of LA in solution was determined using the absorption at 333 nm measured by a V-530 spectrophotometer.
Sample preparation
The solid complexes of LA and CD were prepared by the following methods. A solid was also prepared by physical mixing for comparison.
Physical mixture
CD and LA were mixed in a molar ratio of 1:1, 2:1, and 5:1. An aliquot of the mixture was shaken in a nylon bag for 30 min.
Kneading
CD and LA with molar ratios of 1:1, 2:1, and 5:1 were mixed with a small amount of ethanol for 30 min.
Freeze-drying
CD and LA with molar ratios of 1:1, 2:1, and 5:1 were mixed. An aliquot of the mixture was dissolved in 10 mL of milliQ water. The solutions were shaken at 25 ºC for 2 weeks and then passed through a 0.2 μm membrane filter. The filtered solution was concentrated at −40 ºC in vacuo.
Analytical methods
X-ray diffraction
Powder X-ray diffraction patterns (XRD) were measured by a Rigaku Denki (Tokyo, Japan) Rint 2000 diffractometer using Ni-filtered Cu Kα radiation.
Differential scanning calorimetry (DSC)
Characterization of the solid complexes was carried out by DSC 3100SA (Bruker AXS, Berlin, Germany) at a scanning speed of 10 ºC/min from 25 to 500 ºC.
Infrared spectroscopy
Characterization of the solid complexes was carried out by a Nicolet IR 200 spectrometer (Thermo Fisher Scientific Inc., Waltham, USA) according to the KBr disk method.
Thermal stability test
10 mg of each solid complex was added to six test tubes and heated in a dry block bath (MG-2200, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) at 100 ºC. The temperature control accuracy was ±0.1–0.2 ºC. LA was extracted with 50% methanol and measured by HPLC at 1, 2, 4, 6, 8, and 10 h. HPLC analysis was carried out with a JASCO HPLC-800 system (Tokyo, Japan) using a Capcell Pack (C18, UG80, 5 μm, Shiseido, Tokyo, Japan) column (250 × 4.6 mm i. d.). The column temperature was maintained at 25 ºC. An isocratic elution technique using water including 0.04% acetic acid:methanol (1:1) solution was employed. The flow rate of the eluent was 1.0 mL/min. The UV absorbance of the effluent was monitored continuously at 333 nm. The remaining amount of LA (%) was calculated by the ratio of the HPLC peak area before heating to the HPLC peak area after 1, 2, 4, 6, 8, or 10 h.
Results and discussion
Phase solubility
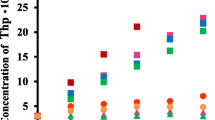
The solubility of LA in the presence of α-, β-, and γ-CD was examined using the Solubility Method [17]. In the case of β-CD, the solubility was only two times higher than that of LA itself. In order to increase solubility of LA various β-CD derivatives was examined. Figure 1 shows the phase solubility diagram of various CDs and LA at pH 4 and 25 ºC. An enhancement of LA solubility was observed for SBE-β -CD, Me-β-CD, and DM-β-CD. In the case of SBE-β-CD, the solubility was about 20 times higher than that of LA itself. According to the Higuchi-Connors theory [17], Me-β-CD, DM-β-CD, HP-β-CD and mono-G1-β-CD show AN and SBE-β-CD shows AL type solubility curves, respectively.
Table 1 shows values of the apparent stability constant (K′), estimated from Eq. 1:
Although the complexation ability of Me-β-CD appears to be highest among the CDs used in this study, Me-β-CD has toxicity with an injection [18]. Recently, SBE-β-CD has gained considerable attention because of its improved safety profiles [18, 19]. Therefore, SBE-β-CD was examined in detail.
Structure of the inclusion complex
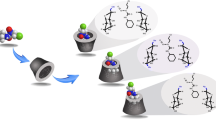
SBE-β-CD (1 mM) and LA (1 mM) was dissolved in acetic acid buffer (pH 4.4) for 1H-1H COSY and 1H-1H ROESY NMR measurements. Figure 2 shows the 1H-1H ROESY NMR spectrum of the LA and SBE-β-CD complex. It shows a correlation between the signal at 3.15 ppm (H-8 of LA) and that at 3.78 ppm (H-3 of SBE-β-CD). A correlation between the signal at 2.40 ppm (H-7 of LA) and that at 3.51 ppm (H-2 of SBE-β-CD) was also observed. The signals at 2.40 and 3.15 ppm were assigned to H-7, H-8 of LA, respectively, by the 1H-1H COSY NMR experiment. There was no cross peak between protons of LA and H-5 of SBE-β-CD. Also, no cross peaks between protons of alkylchain of LA (H-2, H-3, H-4, and H-5) and H-3 of SBE-β-CD showed no inclusion of alkylchain of LA in the cavity of β-CD. On the other hand, cross peaks between protons of alkylchain of LA (H-2, H-3, H-4, and H-5) and 2.84 ppm, which was assigned to the sulfobutylether groups of SBE-β-CD, showed the mutual interaction. Therefore, the dithiolane ring of LA was included in the cavity of SBE-β-CD and alkylchain of LA was surrounded by the chains of sulfobutylether of SBE-β-CD as shown in Fig. 3.
The inclusion structure of LA and SBE-β-CD was thus verified in the solution state. Next, the solid complex of LA and SBE-β-CD was synthesized and characterized.
Characterization of solid complex of LA and SBE-β-CD
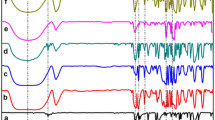
Figure 4 shows X-ray diffraction patterns of LA, SBE-β-CD, and LA–SBE-β-CD (1:1) solids obtained by physically mixing, kneading, and freeze-drying. As can be seen in Fig. 4a, two characteristic diffraction peaks due to the LA crystal appeared at 2θ = 17.9 and 23.5º. On the other hand, the XRD pattern of SBE-β-CD (Fig. 4b) shows a broad diffraction profile due to an amorphous state caused by the random substitution pattern of sulfobutylether groups in SBE-β-CD.
The solid obtained by physical mixing (Fig. 4c) showed two peaks at 2θ = 17.9 and 23.5º from the LA crystal. In the solid complexes obtained by kneading (Fig. 4d) and freeze-drying (Fig. 4e), these peaks disappeared completely and the diffraction pattern showed an amorphous state. This suggests that LA was included in SBE-β-CD and the solid complex was formed. LA–SBE-β-CD (1:2) and LA–SBE-β-CD (1:5) complexes obtained by physical mixing, kneading, and freeze-drying were also studied and found to exhibit diffraction patterns similar to that of the LA–SBE-β-CD (1:1) complex.
Figure 5 shows the DSC curves of LA, SBE-β-CD, and LA–SBE-β-CD (1:1) solids obtained by physical mixing, kneading, and freeze-drying. The exothermic peak around 60 ºC, which is the melting point of LA, was observed in the LA (Fig. 5a) and physical mixture (Fig. 5c). The melting points of LA and the physical mixture were 62 and 58 ºC, respectively. This depression of the melting point of the physical mixture was attributed to the depression of the freezing point of LA due to addition of SBE-β-CD. SBE-β-CD (Fig. 5b) alone showed a broad exothermic peak around 50–150 ºC.
In the solid complexes prepared by kneading (Fig. 5d) and freeze-drying (Fig. 5e), the exothermic peak corresponding to the melting of LA disappeared. These results suggest that LA interacts with SBE-β-CD in the solid state to form an inclusion complex. LA–SBE-β-CD (1:2) and LA–SBE-β-CD (1:5) complexes obtained by physical mixing, kneading, and freeze-drying were also studied. A similar behavior was observed.
Figure 6 shows the IR spectra of the LA, SBE-β-CD, and LA–SBE-β-CD (1:1) solids obtained by physical mixing, kneading, and freeze-drying. The absorption of the OH group appeared at 3400 cm−1. The band at 1690 cm−1 was assigned to the C=O stretching of LA and also appeared in the physical mixture. However, in the kneading and freeze-drying samples, these bands were shifted to a higher wavenumber, whereas that of the physical mixture showed no shift. This suggests that some interaction between LA and SBE-β-CD existed in the solid complex obtained by kneading and freeze-drying.
These results suggested that the solid products prepared by kneading and freeze-drying were completely different from that by physical mixing. Thus, LA interacts with SBE-β-CD in the solid complexes prepared by kneading and freeze-drying to form inclusion complexes.
The thermal stability of the LA–SBE-β-CD complex (1:1) was tested next. The degradation profiles of the LA alone and its complexes with SBE-β-CD at 100 ºC are shown in Fig. 7. For LA, degradation began within 1 h, and more than 40% of LA decomposed after 2 h. On the other hand, the remaining amount of LA after kneading and freeze-drying was 79 and 86%, respectively after 10 h. Compared with the LA alone, improved thermal stability by complexation with SBE-β-CD was verified similar to the case of LA–β-CD complex reported in the literature [20]. As shown in the NMR results, the dithiolane ring of LA is included in the CD, which enhances the stability of LA.
Conclusion
We investigated the effect of β-CD derivatives on LA solubility using the solubility method. Compared to the solubility of LA alone, the solubility is about 20 times higher in the presence of SBE-β-CD. The kneading and freeze-drying methods were successful in obtaining solid inclusion complexes with improved thermal stability.
References
Sumathi, R., Jayanthi, S., Kalpanadevi, V., Varalakshmi, P.: Effect of DL α-lipoic acid on tissue lipid peroxidation and antioxidant systems in normal and glycollate treated rats. Pharmacol. Res. 27, 309–317 (1993)
Packer, L., Witt, E.H., Tritschler, H.J.: Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 19, 227–250 (1995)
Packer, L., Tritschler, H.J., Wessel, K.: Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radic. Biol. Med. 22, 359–378 (1997)
Jones, W., Li, X., Qu, Z.-C., Perriott, L., Whitesell, R.R., May, J.M.: Uptake, recycling, and antioxidant actions of α-lipoic acid in endothelial cells. Free Radic. Biol. Med. 33, 83–93 (2002)
Ziegler, D., Schatz, H., Conrad, F., Gries, F.A., Ulrich, H., Reichel, G.: Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care 20, 369–373 (1997)
Packer, L., Kraemer, K., Rimbach, G.: Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 17, 888–895 (2001)
Stevens, M.J., Obrosova, I., Cao, X., Huysen, C.V., Greene, D.A.: Effects of DL-α-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 49, 1006–1015 (2000)
Saenger, W.: Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Engl. 19, 344–362 (1980)
ÖZdemir, N., Ordu, Ş.: Improvement of dissolution properties of furosemide by complexation with β-cyclodextrin. Drug Dev. Ind. Pharm. 24, 19–25 (1998)
Uekama, K.: Pharmaceutical applications of methylated cyclodextrins. Pharm. Int. 6, 61–65 (1985)
Brewster, M.E., Estes, K.S., Loftsson, T., Perchalski, R., Derendorf, H., Mullersman, G., Bodor, N.: Improved delivery through biological membranes XXXI : solubilization and stabilization of an estradiol chemical delivery system by modified β-cyclodextrins. J. Pharm. Sci. 77, 981–985 (1988)
Szejtli, J.: The properties and potential uses of cyclodextrin derivatives. J. Incl. Phenom. Mol. Recognit. Chem. 14, 25–36 (1992)
Hirayama, F.: Development and pharmaceutical evaluation of hydrophobic cyclodextrin derivatives as modified-release drug carriers. Yakugaku Zasshi 113, 425–437 (1993)
Junquera, E., Alcart, E.: Thermodynamic analysis of the binding of a hepatoprotectant drug, thioctic acid, by β-cyclodextrin. J. Pharm. Sci. 88, 626–631 (1999)
Trentin, M., Carofiglio, T., Fornasier, R., Tonellato, U.: Capillary zone electrophoresis study of cyclodextrin–lipoic acid host–guest interaction. Electrophoresis 23, 4117–4122 (2002)
Carofiglio, T., Fornasier, R., Jicsinszky, L., Saielli, G., Tonellato, U., Vetta, R.: Capillary electrophoresis, ROESY NMR and molecular modelling study of the inclusion complex β-cyclodextrin/lipoic acid. Eur. J. Org. Chem. 2002, 1191–1196 (2002)
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Irie, T., Uekama, K.: Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 86, 147–162 (1997)
Thompson, D.O.: Cyclodextrins-enabling excipients: Their present and future use in pharmaceuticals. Crit. Rev. Ther. Drug Carr. Syst. 14, 1–104 (1997)
Tong, L.-H., Pang, Z.-Z., Yi, Y.: Inclusion complexes of α- and β-cyclodextrin with α-lipoic acid. J. Incl. Phenom. Mol. Recognit. Chem. 23, 119–126 (1995)
Acknowledgement
The authors thank Assistant Professor M. Sugiura of Kobe Pharmaceutical University for the 1H–1H COSY and ROESY NMR measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, H., Onodera, T. & Nakayama, H. Inclusion complex of α-lipoic acid and modified cyclodextrins. J Incl Phenom Macrocycl Chem 68, 201–206 (2010). https://doi.org/10.1007/s10847-010-9767-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9767-7



 ), Me-β-CD (
), Me-β-CD ( ), DM-β-CD (
), DM-β-CD ( ), HP-β-CD (
), HP-β-CD ( ), mono-G1-β-CD (
), mono-G1-β-CD ( )
)





 ), kneading (
), kneading ( ), freeze-drying (
), freeze-drying ( )
)