Abstract
Formation of inclusion complexes between α- and β-cyclodextrins and three antipyrine type compounds (antipyrine, 4-amino-antipyrine, 4-nitroso-antipyrine), in solution and in solid state, has been investigated. UV–Vis measurements indicate that in solution antipyrine derivatives do not form inclusion complexes and oxidative process of amino group is not influenced by the presence of cyclodextrins. The oxidation of 4-amino-antipyrine to 4-nitroso-antipyrine using DPPH type radicals was investigated using spin trapping experiments in organic solvents and in water. These results were explained by betainic configuration of antipyrine derivatives in aqueous solutions. Formation of inclusion complexes in solid states was evidenced by DSC, as peaks associated with various thermodynamic events (dehydration, melting, thermal decomposition) are shifted towards higher temperature or disappear in case of inclusion complexes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compounds containing antipyrine moiety have analgesic and antipyretic properties and for this reason physico-chemical studies can be connected with their biomedical applications. In a previously study, the lipophilicity of some antipyrine derivatives and their interaction with amino acids were analysed by reversed phase thin layer chromatography [1]. Lipophilicity of a biochemical active compound is related with the ability to pass through membrane cell [2] and one way to determine this parameter for organic compounds is to use reverse phase thin layer chromatography [3]. Using this method we observed the influence of the fragment attached to antipyrine moiety on molecular lipophylicity for a series of antipyrine derivatives [1].
Physico-chemical properties of physiological active compounds can be changed by interaction with other species used in drug formulation. A class of excipients, which can control drugs release or improve their solubility, is represented by cyclodextrins (CDs). CDs contain a number of glycopyranosic units (≥6) linked by α-(1,4)-glycosidic bonds which determine a rigid, well define ring structure, with a hydrophobic inner cavity and a hydrophilic outer surface [4]. These features are responsible for CDs ability to form inclusion complexes in aqueous solution and in solid state with small organic molecules or parts of large polymeric molecules resulting supramolecular complexes [4–6]. Stability of CDs inclusion complexes is determined by geometric properties of each CD cavity (which are related with the number of glycosidic units) and by the size of guest molecules [4, 5].
Naturally CDs (especially β-CD which contain 7 glycosidic units) have found various technological applications (pharmaceutics, catalysis, separation processes) but were also subjects of fundamental studies regarding molecular recognition [1, 4, 5]. In the absence of a guest, CD cavity contains some hydration water molecules, which are removed during complexation process. Changes accompanying an inclusion complex formation in solid state, including dehydration process, can be evidenced by differential scanning calorimetry (DSC). There are numerous studies reporting changes in DSC curves: shifting in melting, boiling or sublimating points for the guest molecules or even disappearance of respective peaks [7–9]. An indication of host-guest interaction is the disappearance of the melting endotherm peak of the potential guest in the DSC curves of the mixtures. Measurements of the melting enthalpy are an indication of the amount of guest that has not interacted [10]. Inclusion of a guest in a CD cavity may displace water, so a change in the endotherm peak associated with dehydration of the CDs may be an additional indication of inclusion phenomena.
In this paper we report on the formation in solid state of inclusion complexes between two CDs and antipyrine derivatives (Fig. 1) evidenced by DSC measurements and analysis of antipyrine/cyclodextrins systems in solution using UV-vis measurements. In addition, was analyzed the oxidation reaction of 4-amino-antipyrine with diphenyl-picryl-hydrazyl type radicals. 4-Nitroso-antipyrine can be an oxidation product of 4-amino-antipyrine in aqueous media, following probably a radical mechanism. In order to evidence this mechanism, oxidation of 4-amino-antipyrine was carried out with DPPH radicals (Fig. 1) in various solvents, and radical intermediates were trapped using DMPO (5,5-dimethylpyrroline-N-oxide).
Experimental
Materials
Antipyrine and 4-amino-antipyrine were purchased from Alfa Aesar; DMPO and cyclodextrines (α-, β-CD) were purchased from Aldrich. 4-Nitroso-antipyrine was prepared by reaction with sodium nitrite and hydrochloric acid. Radicals 2-(p-phenylsulphonic acid)-2-phenyl-1-picrylhydrazyl (NaSO3DPPH) and 2,2-(p-nitro-phenyl)-1 pycrylhydrazyl were prepared following the procedure described in literature [11, 12].
Methods and samples preparation
The DSC analysis was carried out for pure antipyrine, 4-amino-antipyrine, 4-nitroso-antipyrine, pure α-CD and β-CD, and their inclusion complexes and physical mixtures. The DSC curves were recorded on a Perkin Elmer DSC under a heating rate of 10 K/min over a temperature range (323–673 K), with an instrument sensitivity of 5 mcal/s. The apparatus was calibrated for temperature and enthalpy by melting high purity indium. The instrument was flushed with argon. Sample of 3–6 mg were transferred into aluminium crucibles which were sealed and weighed with the Partner XA balance, with a precision of 10 μg.
Physical mixtures of 4-nitroso-antipyrine and CDs were prepared by mixing weighed amounts corresponding to 1:1 ratio in a mortar. In case of mixtures between CDs and the other two antipyrine derivatives was considered a weigh ratio 1:1.
The solid complexes between antipyrine derivatives and each CD were prepared by evaporating 50% (v/v) aqueous solution of antipyrine derivative and CD at a 1:1 molar ratio at room temperature.
UV–Vis spectra where recorded on Lamda 35 spectrometer (Perkin-Elmer) at room temperature with 0.2 × 1 × 4 cm3 quartz cells.
The EPR spectra were recorded at room temperature on a JEOL FA 100 spectrometer with 100 kHz modulation frequency, 0.998 mW microwave power, 120 s sweep time, 1 G modulation amplitude, time constant 0.3 s. Solutions of DMPO 10−1 M were freshly prepared. For each spin trapping experiment 0.2 mL of DMPO solution was added at 0.5 mL 4-amino-antipyrine solution and then DPPH radical solution was added. DPPH radicals were prepared by oxidizing the corresponding hydrazine with lead dioxide.
Results and discussion
UV–Vis measurements
In aqueous solutions, antipyrine type compounds used in this study adopt a betainic form (Fig. 2), which is responsible by their solubility in this solvent. However, the nature of functional moiety R—determines water solubility of these compounds. The solubility of 4-nitroso-antipyrine (3) in water is significantly lower comparatively with compounds 1 and 2. Due to the betainic conformation adopted by these compounds in water we expect the complexes with CDs to be less stable, as in formation of such systems hydrophobic forces are important, and not the electrostatic ones. Some of our previous experiments using ESR spectroscopy, regarding complexation of amphiphilic spin probes with CDs in solution, showed that the interaction with CDs are preferentially with hydrophobic part of the guest molecule and not with the ionic part [13].
Indeed, UV–Vis spectra of 4-amino-antipyrine and antipyrine did not report changes in the presence of CDs. In case of 4-nitroso-antipyrine, UV–Vis spectral changes in the presence of α-CD (Fig. 3) have been used to quantify the association constant using Benesi-Hildebrand equation [14] for absorbance at 385 nm. The calculated value for association constant between α-CD and 4-nitroso-antipyrine was 194 mol−1 × l. Concentration of 4-nitroso-antipyrine was 4×10−5 M, while α-CD concentration varied in the range of 2×10−3–2×10−2 M. In the presence of β-CD, changes in UV–Vis spectra of 4-nitroso-antipyrine where in the limit of experimental errors, therefore formation in solution of inclusion complexes was not considered.
The UV–Vis experiments in case of 4-nitroso-antipyrine suggest that complexation with β-CDs do not occur via heterocyclic part of the molecule, but the interaction between phenyl moiety from antipyrine compounds structure and CDs can be considered.
Spin trapping experiment
The oxidation of 4-amino-antipyrine to 4-nitroso-antipyrine with DPPH type radicals was studied by spin trapping method. Reaction was performed in organic solvents (DCM, toluene, and ethanol) or in water (in this case the presence of CDs was also taken into account). For experiments in DCM and toluene was used as oxidizing agent 2,2-(p-nitro phenyl)-1-picrylhydrazyl (NO2)2DPPH while for the experiments in water and ethanol was used the free radical 2-(p-phenylsulphonic acid)-2-phenyl-1-picrylhydrazyl (NaSO3DPPH).
DPPH type radicals abstract one hydrogen atom from the amino moiety of 4-amino-antipyrine leading to the corresponding short-lived radical centered to the nitrogen atom (Fig. 4).
This unstable radical was trapped by DMPO in solvents such DCM and toluene. The ESR spectra recorded for DMPO adduct of intermediate radical in DCM is shown in Fig. 5. Simulation of this spectra using Bruker WINSIM software gave the following parameters for the spin adduct: aN1 = 14.15 G, aH = 15.00 G, aN2 = 3.15 G. A good fit with the experimental spectra was obtain only considering the presence of another free radical in solution (which can be an oxidation product of DMPO or of DMPO adduct), with aN = 13.89 G. The initial ratio between these two radicals is about 65, respectively 35%. The DMPO adduct of intermediate radical was not stable enough to obtain ESR spectra in polar solvents as water or ethanol.
We noticed that in aqueous solution the ESR spectra shown only three lines attributed to degradation of the initial spin adduct and the presence of CDs did not have any influence.
DSC measurements
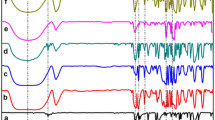
DSC curves were recorded for pure compounds (CDs and antipyrine derivatives), physical binary mixture of CD/antipyrine derivative and for solids containing corresponding inclusion complexes. In Fig. 6 are presented DSC profiles for systems referring to antipyrine derivatives and β-CD.
I. DSC diagram of antipyrine-β-CD systems a βCD, b antipyrine, c inclusion complex, d physical mixture; II. DSC diagram of 4-amino-antipyrine-β-CD systems a β-CD, b 4-amino-antipyrine, c inclusion complex, d physical mixture; III. DSC diagram of 4-nitroso-antipyrine-β-CD systems a β-CD, b 4-nitroso-antipyrine, c inclusion complex, d physical mixture
The DSC curves of β-CD (presented in all Fig. 6 and marked with (a)) shown the broad endothermic peak characteristic to water lost between 337 and 392 K [15, 16]. The small peak around 490 K correspond to an irreversible solid–solid phase transition [15] and is followed finally by a degradation process taking place around 563 K. The DSC traces corresponding to antipyrine derivatives presented in Fig. 6 and marked with (b) shown sharp peaks. In case of antipyrine and 4-amino-antipyrine the event associated is melting process occurring at 388 K and 385.5 K respectively. Melting enthalpies obtained from DSC measurements for antipyrine and 4-amino-antipyrine are 25.1 kJ mol−1 respectively 22.94 kJ mol−1. The value calculated for melting enthalpy of antipyrine is comparable with literature data [17]. In case of pure 4-nitroso-antipyrine the DSC curve showed an exothermic event with a peak at 456 K. The enthalpy variation associated with this peak is 105.06 kJ mol−1. In DSC curve corresponding to the inclusion complex of antipyrine (Fig. 6I(c)) the peak attributed to melting process in Fig 6I(b) is not present. In the same time, the peak attributed in DSC curve for β-CD (Fig. 6I(a)) to dehydration process is smaller, which sustain the replacing of water molecules due to encapsulation of antipyrine in β-CD cavity. The possibility to have a mixture of inclusion complex and free β-CD can be taken into account as the peak corresponding to dehydration process is still present. The thermal stability of inclusion complex is higher, as it decomposition starts at 584 K. The melting enthalpy for pure antipyrine obtained from DSC curve was 25.1 kJ mol−1 while for the physical mixture antipyrine/β-CD was determined a less melting enthalpy (22.1 kJ mol−1) suggesting a partial complexation and thus less free antipyrine.
In case of DSC curves corresponding to 4-amino-antipyrine (Fig. 6II) were observed the endotherm peak at 385.65 K corresponding to melting and the exotherm peak corresponding to decomposition starting at 477.45 K (Fig 6II(b)); in case of 4-amino-antipyrine/β-CD inclusion complexes also dehydration peak decreases and decomposition starts at 575 K. DSC curve for the physical mixture of β-CD and 4-amino-antipyrine presents all peaks attributed to pure compounds, suggesting no interaction between components.
DSC curve for pure 4-nitroso-antipyrine shown that decomposition of this compound occur before the melting (Fig. 6III(b)). In the DSC curve corresponding to inclusion complex of β-CD with 4-nitroso-antipyrine (Fig. 6III(c)) the peak of decomposition around 456 K is missing, but the peak corresponding to β-CD dehydration is still present. Similar to the systems referring to antipyrine derivatives 1 and 2, DSC curve presented in Fig. 6III(d), corresponding to the physical mixture of 4-nitroso-antipyrine (3) with β-CD, shown all peaks founded for pure components.
For the systems containing α-CD, DSC curves are presented in Fig. 7. In each case, DSC traces marked with (a) correspond to α-CD and show a broad peak between 350 and 400 K corresponding to dehydration and reorganization of α-CD molecule and another peak with maximum at 560 K attributed to it decomposition. This behavior for α-CD was also reported before in literature, [18].
I DSC diagram for antipyrine/α-CD systems (a) α-CD, (b) antipyrine, (c) inclusion complex, (d) physical mixture. II. DSC diagram of 4-amino-antipyrine-α-CD systems (a) α-CD, (b) 4-amino-antipyrine, (c) inclusion complex, (d) physical mixture; III. DSC diagram of 4-nitroso-antipyrine-α-CD systems (a) α-CD, (b) 4-nitroso-antipyrine, (c) inclusion complex, (d) physical mixture
DSC curves for physical mixture (Fig. 7I(d)) and inclusion complexes (Fig. 7I(c)) of α-CD and antipyrine are similar with those corresponding to β-CD/antipyrine systems.
DSC curve for 4-amino-antipyrine/α-CD complex does not exhibit the peak for CD dehydration and for the guest melting. Thermal decomposition in complex occurs around 584 K (Fig. 7II(c)) comparatively with 560 K in case of pure α-CD. DSC curve of physical mixture all peaks of pure compounds (Fig. 7II(d)). Results for the systems 4-amino-antipyrine/α-CD are similar with the other systems: disappearance of peaks corresponding to dehydration and melting process in case of inclusion complexes and their presence in case of physical mixture.
For the systems regarding 4-nitroso-antipyrine, DSC curves presented in Fig. 7III(b) (pure compound) and 7III(d) (physical mixture) have shown the peak corresponding to thermal decomposition of this compounds, while the DSC curve recorded for inclusion complex (Fig. 7III(c)) exhibit a broader peak corresponding to the dehydration of α-CD present in excess and the peak corresponding to decomposition of the complex.
Conclusions
In summary, this study shown that in aqueous solution the complexation between antipyrine derivatives and CDs is weak or does not occur due to the existence of these compounds in betainic form. Spin trapping experiments indicated a radical mechanism for oxidation of 4-amino-antipyrine with DPPH type radicals. The radical intermediate formed in water solution is not stabilized by the presence of CDs and was evidenced as a DMPO adduct only in experiments performed in DCM and toluene. In solid state, formation of inclusion complexes between these antipyrine derivatives and CDs is possible. From DSC traces of pure compounds were calculated enthalpies associated with thermodynamic events. The melting enthalpies calculated for antipyrine derivatives in physical mixtures with CDs were lower comparatively with pure compounds as partial inclusion occurs. Thermal decomposition of inclusion complexes are shifted in all cases towards higher temperature comparatively with pure CDs.
References
Sahini, V.Em., Caproiu, M.T., Ionita, G.: Lipophilicity study of some antipyrine derivatives and their interaction with amino acids. Rev. Roum. Chim. 44(9), 817–822 (1999)
Hansch, C., Leo, A.: Substituent Constants for Correlation Analysis in Chemistry and Biology. Wiley-Interscience, New York (1979)
Kaliszan, R.: Quantitative Structure—Chromatographic Retention Relationships. Wiley, New York (1987)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97, 1325–1357 (1997)
Harada, A.: Cyclodextrin-based molecular machines. Acc. Chem. Res. 34, 456–464 (2001)
Liu, L., Zhu, S.: A study on the supramolecular structure of inclusion complex of β-cyclodextrin with prazosin hydrochloride. Carbohydr. Polym. 68, 472–476 (2007)
Marques, H.C., Hadgraft, J., Kellaway, I.: Studies of cyclodextrin inclusion complexes. I. The salbutamol-cyclodextrin complex as studied by phase solubility and DSC. Int. J. Pharm. 63, 259–266 (1990)
Giordano, F., Novak, C., Moyano, J.R.: Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta 380(1), 123–151 (2001)
Rotich, M.K., Brown, M.E., Glass, B.D.: Thermal Studies on mixtures of aminosalicylic acids with cyclodextrins. J. Therm. Anal. Calorim. 73, 687–706 (2003)
Ionita, G., Caragheorgheopol, A., Caldararu, H., Jones, L., Chechik, V.: Inclusion complexes of cyclodextrins with nitroxide-based spin probes in aqueous solutions. Org. Biomol. Chem. 7, 598–602 (2009)
Putirskaja, G.V., Siladi, T.: Acta Chim. (Hung.) 72, 134–139 (1972)
Ionita, P., Caproiu, M.T., Balaban, A.T.: New sulfonyl derivatives of 2, 2-diphenyl-1-pycryl hydrazyl and their supramolecular complexes with crown ethers of cryptands. Rev. Roum. Chim. 45, 935–941 (2000)
Benesi, H.A., Hildebrand, J.H.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71, 2703–2707 (1949)
Morin, N., Crini, G., Cosentino, C., Millet, J., Vebrel, J.: Formation of two particular structures between β-cyclodextrine and bifonazole. J. Chem. Soc., Perkin Trans. 2, 2647–2651 (1999)
Gines, J.M., Arias, M.J., Perez-Martinez, J.I., Moyano, J.R., Morillo, E., Sanchez-Soto, P.: Determination of the stoichiometry of 2, 4-dichlorophenoxyacetic acid-β-cyclodextrin complexes in solution and in solid state. J. Thermochim. Acta 321, 53–58 (1998)
Schnitzler, E., Lencone, K., Kobelnik, M.: Exact and soil science, characterization of pharmaceuticals by thermal analysis. Agrarian S. Eng. 8(1), 91–100 (2002)
Teixeira, L.R., Sinisterra, R.D., Vieira, R.P., Scarlatelli-Lima, A., Moraes, M.F.D., Doretto, M.C., Denadai, A.M., Beraldo, H.: An inclusion compound of the anticonvulsant sodium valproate into α-cyclodextrin: physico-chemical characterization. J. Incl. Phenom. Macrocyclic Chem. 54, 133–138 (2006)
Acknowledgements
This work was supported by CNCSIS (Romania) grant 729/2006-2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meltzer, V., Pincu, E., Rogozea, A. et al. Inclusion complexes of some antipyrine derivatives with cyclodextrins: influence of guest configuration. J Incl Phenom Macrocycl Chem 65, 385–390 (2009). https://doi.org/10.1007/s10847-009-9596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9596-8