Abstract
A new chemically modified carbon paste electrode for Cd(II) ions based on 3,5-dinitro-N-(tri-2-pyridyl methyl) benzamide (DNTPMBA) as an ionophore was prepared. The electrode exhibits a Nernstian response for Cd(II) ions over a wide concentration range (2.16 × 10−7–1.00 × 10−1 mol L−1) with a slope of 30 ± 1 mV per decade. It has a response time of about 50 s and can be used for a period of 3 months with good reproducibility. Detection limit obtained in the optimal conditions was 1.82 × 10−7 mol L−1. The electrode was successfully used for potentiometric determination of Cd(II) in well water. The pH influence and interference of some cations were also studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium represents one of the important toxic metals and the effect of its acute poisoning is manifested in a variety of symptoms, including high blood pressure, kidney damage, anemia, hypertension, bone marrow disorders, cancer and toxicity to aquatic biota [1]. In its ionic form, Cd(II) (ionic radius 95 pm) shows great chemical similarity with two biologically very important metal ions, viz., the lighter homologue Zn(II) (ionic radius 74 pm), and Ca(II) (ionic radius 100 pm) which comes very close in size. Accordingly, cadmium as a softer and more thio philic metal may displace cysteinate- coordinated zinc from its enzymes and even replace it in special cases, while it can also substitute for calcium, e.g., in bone tissue [2]. Even though its toxicity is well recognized this metal has important industrial uses. So, controlling the levels of this pollutant in natural waterways, potable waters, soils, air, and industrial wastes is important.
Several analytical methods, including atomic absorption spectrometry (AAS), cold vapor-AAS [3, 4], graphite furnace-AAS [5], inductively coupled plasma-mass spectroscopy (ICP-MS) [6], anodic stripping voltammetry [7], and electrothermal-AAS [8] have been utilized for Cd(II) detection at low concentration levels. These methods give accurate results, but are not much convenient for large scale monitoring and require good infrastructure back up [9]. So there is critical need for the development of selective, portable, and inexpensive diagnostic tools for the detection of cadmium. Analytical methods based on potentiometric detection with ion-selective electrodes (ISEs) can be considered as an advantageous alternative, because they are eco-friendly techniques, they provide easy construction and manipulation, present good selectivity in a wide concentration range of operatability, have a relatively low detection limit, and show a fast response and non-destructive analysis. Carbon pastes are well known as useful materials for the fabrication of various electrometric sensors for analytical purposes [10–12]. The operation mechanism of such chemically modified carbon paste electrodes (CMCPEs) depends on the properties of the modifier materials used to impart selectivity to the target species [13]. In comparison with ion-selective electrodes based on polymeric membranes, the CMCPEs possess advantages of much lower ohmic resistance, very stable response, and easy renewal of their surface [14].
The present work describes the construction, potentiometric characterization, and analytical application of a carbon paste modified electrode based on a new tripodal receptor (DNTPMBA) as a potentiometric sensor in cadmium detection (Fig. 1).
DNTPMBA was chosen as a selective agent for Cd(II), because the tripodal receptors constitute a special class of acyclic ionophers, which consist of multi-armed ligands with each arm bearing a functional group that can coordinate with the target ion. The selectivity of a tripodal receptor relates greatly to the rigidity of its arms and its cavity size [15–17].Therefore, tripodal-based receptors have been reported to be used successfully as recognition components in ion selective electrode membranes [18–21]. The principal analytical parameters of the electrode including linear response range, pH effect, response time, detection limit, and selectivity to other ions are evaluated. The electrode is successfully used for cadmium detection in well water.
Experimental
Reagents
Solutions were prepared from a stock solution of 0.1 mol L−1 Cd(II), prepared from a sufficient quantity of Cadmium(II) nitrate(Merck) in distilled deionized water. The working solutions were prepared daily by suitable dilution of stock solution. Potassium nitrate (1 mol L−1) solution was prepared and used as supporting electrolyte, to maintain constant ionic strength. All other solutions used in interference studies were prepared from analytical grade nitrate salts (all from Merck Company). Pure graphite powder (Merck) and paraffin oil (Fluka) was used for the preparation of carbon paste electrode. DNTPMBA as an ionophore was synthesized and purified according to the literature [22].
Preparation of CMCPE
About 0.047 g of pure graphite powder, 0.018 g of DNTPMBA, and 7–10 mL tetrahydrofuran (THF) were mixed together in a 25 mL beaker. The mixture was stirred by sonication method or sometimes by a spatula for 20–25 min. After the complete evaporation of THF, 0.035 g of liquid paraffin was added to the mixture and then mixed well into a uniform paste. The electrode bodies were made by pressing the mixture into (2.5 mm diameter) insulin syringes the tips of which had been cut off with a razor blade. Smooth surfaces were obtained by applying manual pressure to the piston while holding the electrode surface against a smooth solid support. A fresh electrode surface was obtained by squeezing out a small amount of paste, scrapping off the excess against a conventional paper and polishing the electrode on a smooth paper to obtain a shiny appearance. The electrical connection was made with a copper wire. Unmodified carbon paste electrode was prepared in a similar fashion, without the addition of DNTPMBA in graphite powder.
Apparatus
A digital potentiometer (HIOKI 3256.50) was used for the potentiometric measurement. The reference electrode was a double junction saturated Ag/AgCl reference electrode. A Metrohm pH meter (CRISON GLP 22) was used for pH controlling, and a Heidolph type of MR 2,000 stirrer was used for stirring the solutions.
Procedure
The electrodes were immersed directly in a test solution. The pH of this solution was adjusted to 6. The solution was stirred (150 rpm) until the response of the potentiometer became stable (20–25 min). Then by means of a micropipette (5–100 μL) the solutions of 0.001, 0.01 and 0.1 mol L−1 Cd(NO3)2 in 0.6 mol L−1KNO3 (with a pH adjusted to 6) were added to the test solution in different portions. Potential readings were recorded after each addition, when stable values had been obtained (usually after 50–70 s).
Results and discussion
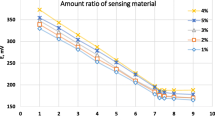
The potential responses of chemically modified CPEs based on DNTPMBA for various elements are shown in Fig. 2. The cadmium selective electrode exhibited linear response to the logarithm of the activity of Cd(II) ions within the concentration range of 2.16 × 10−7–1.00 × 10−1 mol L−1of Cd(II) with a Nernstian slope of 30 ± 1 (mV) per decade and correlation coefficient of 0.991. The sensitivity of a potentiometric CMCPE depends on the carbon paste composition [23]. The influence of the modifier amount in the carbon paste was studied. The obtained results are presented in Table 1. These results show that the electrode with 35% paraffin oil, 47% graphite and 18% DNTPMBA has a good Nernstian slope.
The effect of ionic strength (0.1–1 mol L−1 KNO3) on the calibration curve of cadmium electrodes was investigated. The electrode response slightly changes within the 0.1–1 mol L−1 KNO3 electrolyte solution. However, we chose an added amount of 0.6 mol L−1as an optimal value, since in this ionic strength the linear range was wider than for the other concentrations.
The influence of pH on the potential response of the Cd(II)-selective electrode was tested at 1.0 × 10−3 mol L−1 Cd(II) concentration over the pH range 2–10, and the results are shown in Fig. 3.
As seen, the potential remained constant from pH 5–7 and the carbon paste electrode can be suitably used in this range of pH. However, the observed potential decrease at higher pH (≥7) values could be due to the formation of some hydroxy complexes of Cd(II) ion in the solution. On the other hand, at pH values lower than 5, the electrode potential rises sharply. This is probably due to the simultaneous response of the electrode to H3O+ and Cd(II) ions [24]. The contribution of H3O+ to the potential adds that of Cd(II) ion.
The average response time is defined [25] as the time required for the electrode to reach a stable potential within ±1 mV of the final equilibrium value, after successive immersion of the electrode in different cadmium solutions each having a 10-fold difference in concentration or after rapid 10-fold increase in concentration by addition of cadmium nitrate. In this work, the response time in the variation of concentration from 1 × 10−5 to 1 × 10−4 mol L−1 Cd(II) is measured. The measured response time was 50(s).
Validity of the proposed method
Linearity
Linear curve fitting using IUPAC method has been used for the determination of ISE characteristics. The electrode shows a linear response (y = −30.598x + 605.39, R 2 = 0.991) to the activity of Cd(II) ion in the range of 2.16 × 10−7–1.00 × 10−1 mol L−1.
The Limit of detection (LOD)
The LOD was measured as the lowest amount of the analyte that may be detected to produce a response which is significantly different from that of a blank. The Limit of detection was approved by calculations based on the standard deviation of the response (δ) and the slope (s) of the calibration curve. The LOD for cadmium electrode was 1.82 × 10−7 mol L−1.
Precision and accuracy of the method
The precision of the method was checked by the analysis of four replicates of the sample, expressed by R.S.D. % at the limit of quantification range, which was <1%. Also, the accuracy was expressed in terms of percentage deviation of the measured concentration from the actual concentration. The obtained results are within the acceptance range of <1%.
Ruggedness
The ruggedness of the potentiometric method was evaluated by caring out an analysis using a standard working solution, the same electrodes, and the same conditions on the different days. The R.S.D. of <1% was observed for repetitive experiments in 3 day time periods. The result indicates that the method is capable of producing results with high precision on different days.
Selectivity and interference
The selectivity coefficients of the modified carbon paste electrode were evaluated by the fixed interference concentration method [26]. According this method, the potentiometric selectivity coefficient, \( K_{\text{Cd,M}}^{\text{pot}} \), can be evaluated from the potential measerments of solutions containing a fix concentration of interfering ion, Mn+ (10−3 mol L−1 for all cations except for K+ for which the concentration was 0.6 mol L−1) and titration with Cd2+ solution. The potential values obtained were then plotted against the activity of the cadmium ion. The intersecation of the extrapolated linear portions of this curve will indicate the activity of Cd(II), which is to be used to calculate \( K_{\text{Cd,M}}^{\text{pot}} \) values from the equation:
The selectivity coefficients and analytical properties of the present electrode are compared with some recently reported cadmium electrodes in Table 2. This table shows that all 14 cations would not affect the selectivity of the present cadmium electrode, and have a very small value of selectivity coefficient in most cases compared with the previously reported cadmium electrodes.
Also and in a wider linear range, the Nernstian slope and the lower detection limit of the proposed electrode are somewhat similar to, or even better than, the recently published papers in some cases.
Practical application
Cadmium concentrations in unpolluted natural waters are usually below 1 μg/L [31]. Median concentrations of dissolved cadmium measured at 110 stations around the world were <1 μg/L, the maximum value recorded being 100 μg/L in the Rio Rimao in Peru [32]. Contamination of drinking-water may occur as a result of the presence of cadmium as an impurity in the zinc of galvanized pipes or cadmium-containing solders in fittings, water heaters, water coolers and taps.
The new cadmium selective electrode was successfully applied to obtain recoveries of cadmium in well water. The analysis was performed by using the standard addition technique. The results are given in Table 3. Good recoveries were obtained in all samples. The electrode was also successfully applied to the direct determination of cadmium in waste water sample from a near electroplating factory in Tehran and the results were compared with those obtained using inductively coupled plasma (ICP). Table 4 shows that the results obtained using the proposed electrode are in fair agreement with those obtained by ICP.
Conclusion
The sensitivity and stability offered by this simple electrode configuration are high enough to allow accurate determination of low levels of cadmium. The selectivity coefficient data for cadmium relative to most of the interfering ions are negligibly small. In conclusion, the developed 3,5-dinitro-N-(tri-2-pyridyl methyl) benzamide modified carbon paste electrode described in this work offers a reliable, sensitive, and selective tool for the quantitative determination of cadmium in some important matrices.
References
Sherlock, J.C.: Cadmium in foods and the diet. Experientia 40, 152–156 (1984). doi:10.1007/BF01963578
Price, N.M., Morel, F.M.: Cadmium and cobalt. substitution for zinc in a marine diatom. Nature 344, 658–660 (1990). doi:10.1038/344658a0
Fries, J., Getrost, H.: Organic Reagents for Trace Analysis, p. 227. E. Merck, Darmestadt (1977)
Manzoori, J.L., Abdolmohammad-Zadeh, H., Amjadi, M.: Ultratrace determination of cadmium by cold vapor atomic absorption spectrometry after preconcentration with a simplified cloud point extraction methodology. Talanta 71, 582–587 (2007). doi:10.1016/j.talanta.2006.04.036
Burguera, E.A., Burguera, M.: Determination of cadmium in urine specimens by graphite furnace atomic absorption spectrometry using a fast atomization program. Talanta 63, 419–424 (2004). doi:10.1016/j.talanta.2003.11.010
Li, L., Hernández-Caraballo, B.H., Xia, L., Jiang, Z.: Determination of trace Cd and Pb in environmental and biological samples by ETV-ICP-MS after single-drop microextraction. Talanta 70, 468–473 (2006). doi:10.1016/j.talanta.2006.03.006
Suteerapataranon, S., Jakmunee, J., Vaneesorn, Y., Grudpan, K.: Exploiting flow injection and sequential injection anodic stripping voltammetric systems for simultaneous determination of some metals. Talanta 58, 1235–1242 (2002). doi:10.1016/S0039-9140(02)00445-9
Viitak, A., Volynsky, A.B.: Simple procedure for the determination of Cd, Pb, As and Se in biological samples by electrothermal atomic absorption spectrometry using colloidal Pd modifier. Talanta 70, 890–895 (2006). doi:10.1016/j.talanta.2006.02.006
Gupta, V.K., Jian, A.K., Ludwig, R., Maheshwari, G.: Electroanalytical studies on cadmium(II) selective potentiometric sensors based on t-butyl thiacalix[4]arene and thiacalix[4]arene in poly(vinyl chloride). Electrochim. Acta 53, 2362–2368 (2008). doi:10.1016/j.electacta.2007.10.001
Kalcher, K., Kauffmann, J.M., Wang, J., Svancara, I., Vytras, K., Neubold, C., Yang, Z.P.: Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period 1990–1993. Electroanalysis 7, 5–22 (1995). doi:10.1002/elan.1140070103
Lefevre, G., Bessiere, J., Walcarius, A.: Cuprite-modified electrode for the detection of iodide species. Sens. Actuators B Chem 59, 113–117 (1999). doi:10.1016/S0925-4005(99)00206-3
Yeom, J.S., Won, M.S., Shim, Y.B.: Voltammetric determination of the iodide ion with a quinine copper (II) complex modified carbon paste electrode. J. Electroanal. Chem 463, 16–23 (1999). doi:10.1016/S0022-0728(98)00427-6
Midgley, D., Mulcahy, D.E.: Carbon Substrate Ion-Selective Electrodes. Ion-Selective Electrode Rev 5, 165–241 (1983)
Gismera, M.J., Procopio, J.R., Sevilla, M.T., Hernan-Dez, L.: Copper(II) Ion-Selective Electrodes Based on Dithiosalicylic and Thiosalicylic Acids. Electroanalysis 15, 126–132 (2003). doi:10.1002/elan.200390013
Sato, K., Arail, S., Yamagishi, T.: A new tripodal anion receptor with C–H···X− hydrogen bonding. Tetrahedron Lett 40, 5219–5222 (1999). doi:10.1016/S0040-4039(99)00942-9
Ballester, P., Costa, A., Deyii, P.M., Morey, J.: Influence of remote intramolecular hydrogen bonds on the thermodynamics of molecular recognition of cis-1, 3, 5-cyclohexanetricarboxylic acid. Tetrahedron Lett 40, 171–174 (1999). doi:10.1016/S0040-4039(98)80050-6
Fan, A.L., Hong, H.K., Valiyaveettil, S., Vittal, J.J.: A Urea-Incorporated Receptor for Aromatic Carboxylate Anion Recognition. J. Supramol. Chem 2, 247–254 (2002). doi:10.1016/S1472-7862(03)00079-0
Kuswandi, B., Nuriman, Verboom, W., Reinhoudt, D.: Tripodal Receptors for Cation and Anion Sensors. Sensors 6, 978–1017 (2006). doi:10.3390/s6080978
Reinoso-Garcia, M.M., Dijkman, A., Verboom, W., Reinhoudt, D.N., Malinoswka, E., Wojciechowska, D., Pietrzak, M., Selucky, P.: Metal complexation by Tripodal N-Acyl(thio)urea and Picolin(thio)amide compounds: synthesis/extraction and potentiometric studies. Eur. J. Org. Chem. 2131–2138 (2005) doi:10.1002/ejoc.200500002
Kim, S.G., Kim, K.H., Jung, J., Shin, S.K., Ahn, K.H.: Unprecedented Chiral Molecular Recognition in a C3-Symmetric Environment. J. Am. Chem. Soc 124, 591–596 (2002). doi:10.1021/ja0119696
Reinso-Garcia, M.M., Janczewski, D., Reinhoudt, D.N., Verboom, W., Malinoswka, E., Pietrzak, M., Hill, D., Baca, J., Gruner, B., Selucky, P., Gruttner, C.: CMP(O) Tripodands: Synthesis, Potentiometric Studies and Extractions. N. J. Chem 30, 1480–1492 (2006). doi:10.1039/b600412a
Marjani, K., Abbastabar-Ahangar, H., Mohammadi, L., Mousavi, M., Attar, J.: 3, 5-Dinitro-N-(tri-2-pyridylmethyl)benzamide. Acta Crystallogr E63, o3345 (2007)
Abbas, M.N., Mostafa, G.A.: New triiodomercurate-modified carbon paste electrode for the potentiometric determination of mercury. Anal. Chim. Acta 478, 329–335 (2003). doi:10.1016/S0003-2670(02)01520-9
Sun, B., Fith, P.G.: Nitrate ion-selective sensor based on electrochemically prepared conducting polypyrrole films. Electroanalysis 9, 494–497 (1997). doi:10.1002/elan.1140090612
Analytical Chemistry Division, I.U.P.A.C., Commission on Analytical Nomenclature: Pure Appl. Chem 66, 2527 (1994)
Umezawa, Y.: umezawa, K., Sato, H.: Electrochemical Science and Technology Information Resource (ESTIR). pure Appl. Chem 67, 507 (1995)
Rezaei, B., Meghdadi, S., Fazel Zarandi, R.: A fast response cadmium-selective polymeric membrane electrode based on N, N′-(4-methyl-1, 2-phenylene)diquinoline-2-carboxamide as a new neutral carrier. J. Hazard. Mater 153, 179–186 (2008). doi:10.1016/j.jhazmat.2007.08.033
Javanbakht, M., Shabani-Kia, A., Darvich, M.R., Ganjali, M.R., Shamsipur, M.: Cadmium(II)-selective membrane electrode based on a synthesized tetrol compound. Anal. Chim. Acta 408, 75–81 (2000). doi:10.1016/S0003-2670(99)00771-0
Gupta, V.·K., Kumar, P.: Cadmium(II)-selective sensors based on Dibenzo-24-Crown-8 in PVC matrix. Anal. Chim. Acta 389, 205–212 (1999)
Lugtenberg, R.J.W., Egbrink, R.J.M., Engbersen, J.F.J., Reinhoudt, D.N.: Pb+2 and Cd+2 Selective Chemically Modified Field Effect Transistors based on Thioamide Functionalized 13-alternate Calix[4]Arenes. J. Chem. Soc 2, 1353–1357 (1997). doi:10.1039/a608063d. Perkin Trans
Friberg, L., Nordberg, G.F., Vouk, V.B.: Handbook of the toxicology of metals, pp. 130–184. Elsevier, Amsterdam (1986)
WHO/UNEP GEMS — Global fresh water quality: Published on behalf of the World Health Organization/United Nations Environment Programme. Blackwell Reference, Oxford (1989)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbastabar-Ahangar, H., Shirzadmehr, A., Marjani, K. et al. Ion-selective carbon paste electrode based on new tripodal ligand for determination of cadmium (II). J Incl Phenom Macrocycl Chem 63, 287–293 (2009). https://doi.org/10.1007/s10847-008-9519-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-008-9519-0