Abstract
Orophilous species are often unable to escape the consequences of climate change because mountains are surrounded by unsuitable habitats. Among them, several endemic species belonging to the genus Erebia Dalman (Lepidoptera, Nymphalidae, Satyrinae) can be considered as key species to assess the risk of biodiversity loss of mountain habitats. The aim of this paper is to measure changes that have occurred in the altitudinal distribution of Erebia cassioides on the Pollino Massif (Southern Italy) during the last 37 years. Sixteen sites sampled in 1975 have been resampled after about three decades (2004, 2012). In 1975 56 % of the sampled population inhabited sites above and 44 % sites below the treeline, while in 2004 and 2012 99 % of the population were observed above the treeline. Furthermore, we observed an uphill shift of 180 m in the barycentre altitude of the species distribution and an unexpected increased density of the population above the treeline which led to a range reduction coupled to population increase of E. cassioides. This pattern contrasts with the usually observed one that couples habitat reduction to population decreasing. The reason for the observed pattern is unclear, but the implication for conservation strategies could be important if confirmed for other species. In fact, during coming decades local extinctions as a consequence of climate change might be fewer and more delayed than expected, and relict populations of cold adapted species could be preserved for a longer time span within optimal habitat refugia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several evidences suggest that animals respond to climate changes with altitudinal and latitudinal shifts of their ranges (Parmesan and Yohe 2003; Wilson et al. 2005; Hickling et al. 2006). Orophilous cold adapted species are often unable to escape climate warming northward because mountains are surrounded by unsuitable habitats. Uphill shifts are necessarily associated with the reduction and fragmentation of the suitable habitat and the extinction risk of such species increases due to fitness lowering and genetic impoverishment (Schmitt and Hewitt 2004). The loss of suitable habitat is related to population size reduction, but the relationship is not linear. Evidences from birds demonstrated that population size usually decreases more rapidly than habitat area (Shoo et al. 2005), enhancing the extinction risks of a given species.
The genus Erebia Dalman (Lepidoptera, Nymphalidae, Satyrinae) is one of the most diverse groups of butterflies, several endemic species are strictly alpine or arctic and distributed on the mountains of the Holarctic region (Brandmayr et al. 2003). It can be considered a key group to assess the risk of biodiversity loss of Mediterranean mountain habitats and some species are the objective of conservation initiatives (Cizek et al. 2003; Schmitt et al. 2005; De Groot et al. 2009). A very isolated population of Erebia cassioides (Reiner and Hohenwarth 1792) was discovered some decades ago on the Pollino Massif, the southernmost massif in continental Italy with alpine habitats (Balletto et al. 1977). According to recent paleobiogeographic reconstructions, during the Riss period (200,000–130,000 Y b.p.) the Apennines were one of the isolated refugial zones for E. cassioides. The subsequent gene flow disruption induced genetic divergence between isolated populations (Albre et al. 2008). The taxonomic identity of the Apennine populations of E. cassioides is not unequivocally defined, while their genetic divergence from E. cassioides sensu stricto is confirmed by several authors. Lattes et al. (1994) stated that western and southern populations of the E. cassioides complex are genetically well differentiated at specific or subspecific level, attributing to them the name Erebia (cassioides) carmenta Fruhstorfer 1909. Albre et al. (2008) confirmed the genetic identity of Erebia carmenta populations suggesting a subspecific taxonomic level for them, but these authors have not analysed the Apennine populations. In this paper we considered the population under study as belonging to the species E. cassioides, considering its genetic divergence from E. cassioides sensu stricto as a parameter increasing its conservation value.
Erebia cassioides inhabits the grassy areas of the sub-alpine and alpine zones, it is univoltine, and needs only 1 year for a complete biological cycle. Adults are active in July–August, the eggs are laid singly, close to the ground and usually on dead grasses. It usually hatches less than 1 month later, and the larvae can be found from August to May. The larval food plants are grasses like Nardus, Festuca and Sesleria. After an overwintering period, the caterpillars resume their activity in spring and pupate on the ground in May–July (Higgins and Riley 1983; Tolman and Lewington 1999). Van Swaay et al. (2010) reported for this species a decline in distribution or population size for Romania and Austria, with a general decreasing population trend. Settele et al. (2008) evaluated this species as at extinction risk due to climate changes, estimating the reduction of the 28–64 % of areas with suitable climate in 2,050–5,080. Under the worst climatic scenario E. cassioides will strongly shrink its Alpine range and will disappear from all the interglacial refugia in 2,080, while under the best climatic scenario it will survive far from the Alps only in a small area of Pyrenean and Cantabrian mountains.
In South Italy climate has changed over the last decades. Brunetti et al. (2006) found that all investigated Italian regions became warmer and drier during the period 1865–2003. More evident changes occurred in southern Italy, where precipitation decreased by 10 % relative to the mean of the standard period 1961–1990 and the mean minimum temperatures increased by 1.3 °C. On the other hand, spring precipitation decreased by 20 %, more than the summer (−13 %) ones, while winter and autumn did not show significant decreases.
The aim of this paper is to measure changes of the altitudinal distribution of E. cassioides on the Pollino Massif during last 37 years, assessing the extinction risk of this very isolated population. Mediterranean mountains host several species at low-latitude range boundaries as consequences of glacial and interglacial periods occurring from the Pleistocene until recently, representing a true open air laboratory for studying consequences of climate changes on mountain habitats (Pizzolotto et al. 2010).
The study area
Pollino National Park extends over about 190,000 ha and includes the highest mountain ranges at the southern end of the calcareous Apennine range, with three peaks over 2,000 m height (Serra Dolcedorme, Mt. Pollino and Serra del Prete). There are three major biomes in the area: the sclerophyll belt from 0 to 1,200 m, mostly with evergreen Quercus ilex forests or shrubs (macchia), widely transformed in garigues and xeric pastures; the sub-Mediterranean-temperate broad leaved summer green vegetation belt from 600 to 1,400 m, with several types of oakforests (Q. pubescens, Q. cerris, Q. petraea) and a widespread Fagus zone (1,200–2,100 m); the alpine zone, with remnants of high mountain (above treeline) grass mats of the Mediterranean area such as for instance, by Festuca bosniaca and Carex kitaibeliana natural grasslands. Pasturelands are well represented at any elevation, inside the beech belt large clearings are covered by Festuca violacea-Meum athamanticum grasslands, the past land use led to an irregular zooanthropogenous treeline (1,800–1,900 m) that is surrounded by a wide variety of xeric or mesic herbaceous biotopes. In karstic depressions around 2,000–2,100 m scattered patches of acidophilic Nardus grass mats are found, (Nardo-Luzuletum pindicae, known in the Corine classification as: 36.381: Subalpine southern Italian mat-grass swards). For further details see Brandmayr et al. (2002) and Pizzolotto et al. (2010).
Climate
The climate of the Pollino National Park varies from summer-arid true Mediterranean to mountain Mediterranean, the heaviest rains concentrated from November to February. Climate data for the area are fragmentary and available for sites at lower altitude than that of the study area. Temperatures are available for the Castrovillari meteorological station (400 m a.s.l.), while rainfall data are available for Castrovillari and Campo Tenese (1,400 m a.s.l.) meteorological stations. At elevations of 400 m the annual mean temperature lies around 16 °C (average for the years 1925–2009), the annual mean rainfall is 835 mm (years 1919–2009), with marked minimum values in June, July and August (23.1–23.2–25.0). At major elevations, around 1,400 m, the annual mean temperature drops to 10.0 °C (2002–2009), and rainfall increases up to 1,478 mm (years 1922–2009). Temperature and precipitation trends of the last decades (Fig. 1) are consistent with those found by Brunetti et al. (2006) in South Italy. The Castrovillari temperatures show an oscillating pattern, with an evident increase of maxima after 1985, and of minima after 2000. The rainfall strongly drops starting from the 70’s, but at higher elevations (Campo Tenese) the relative decrease seems more pronounced (about 50 % in 35 years), that means that also above 1,400 m a.s.l. increasing dryness and soil water shortage may affect habitats and insect populations.
Temperatures and precipitation in two key localities of the Pollino National Park; a annual mean temperatures in Castrovillari (Calabria); b 10 years running mean of Tmax and Tmin anomalies, the value 0 represents the average conditions of the period 1925–2012; c annual precipitation of Castrovillari and Campotenese stations; d relative annual rain surplus (>1) or deficit (<1) of Castrovillari and Campotenese stations. Original data courtesy of “ARPACAL”, Agenzia Regionale per l’Ambiente, Calabria
Materials and methods
Data on butterflies were recorded by visually determining in the field each specimen at the species level, with the aid of a butterfly net in cases of fast-flying species. Butterfly communities were sampled four times from the beginning of July to the end of August every 2 weeks in order to cover the entire E. cassioides flight period.
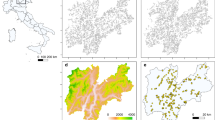
We repeated the sampling carried out in 1975 by Balletto et al. (1977) on butterfly communities of the Pollino Massif, in 2004 and 2012 (after 29 and 37 years respectively), in the same sites previously sampled (Table 1; Fig. 2), with the exclusion of the sites 6–9 because these sites were at lower altitudes than the field survey took place (750–1,100 m a.s.l.) and did not have historical records for E. cassioides, and of the sites 12, 13 and 18 because it was not possible to find the exact field location.
Study area and sampled sites (squares). Site number codes are the same of Balletto et al. (1977). Black squares extinct populations; grey squares species absent also in 1975; white squares species present. White arrows indicate the upward range shift, black lines indicate the 1,850 m contour line
The population density of each species was measured by Balletto et al. (1977) as the number of recorded individuals per hectare (D 1975, in the following), and they reported in their tables the maximum density recorded for any species during their study. We applied a time-constrained method (Pollard and Yates 1993), by sampling for 20 min along a transect within homogeneous habitats. The sites where E. cassioides was not found during the survey were revisited for 30 min the following day in order to reduce this source of error, but the absence was always confirmed. The sampling design we utilised produced data (number of individuals) not comparable per se with those collected in 1975 (individuals per hectare). Anyway, we measured that the surveyed surfaces varied from 0.6 ha during a sample session with a high butterfly abundance, to 1.7 ha during a sample session with no butterflies. In 2004 and 2012 the highest abundance of E. cassioides overlapped the highest (4 out of 6 sites) or the second (2 out of 6 sites) overall butterfly abundance registered in study sites leading us to utilise 0.6 ha as a standard surface to transform the number of individuals into density values. Then, we calculated the recent densities of E. cassioides as follows:
where n = maximum number of individuals sampled in a site during a sampling season.
The variation of the minimum altitude (Wilson et al. 2005; Chen et al. 2011) has been often utilized as a measure of uphill shifting of a given species. This measure could underestimate the real consistency of changes along the altitudinal gradient because favourable microclimatic conditions of small sites may conceal changes occurring along the whole altitudinal distribution of a given species. A more consistent evaluation of changes is given by the barycentre of altitudinal distribution, which is a quantitative value independent from climatic conditions at local scale and from vagrant individuals, so that it is useful for showing the optimal species distribution along ecological gradients. In our case the gradient is given by the altitude, and the barycentre (G alt) was computed as follows (see Blondel 1979):
where ni = abundance value at the altitude i; alti = metres above the sea level of the sampled site i; N = Σni. It is independent from the abundance value because it is a measure of the proportional abundance distribution.
Results
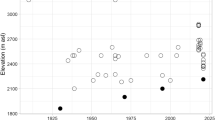
During 1975 E. cassioides was recorded from 10 sampled sites, whilst during 2004 and 2012 only from 6 and 5 sites respectively (Table 1). Thus, nowadays this butterfly disappeared from 50 % of sites. The lower margin of the altitude range shifted 20 m up in 2004 and 170 m up in 2012. The barycentre of altitudinal distribution was calculated at 1,812 m a.s.l. in 1975, while it was calculated at 2,027 m a.s.l. in 2004 and at 1,994 m in 2012, showing an uphill shift of 215 m and 182 m respectively.
Erebia cassioides showed D 1975 > 3.0 in five sites, whereas it went extinct from two of these sites after 29 years. It gradually decreased in the pasture of Colle Gaudolino (1,680 m of altitude), where it was the most abundant species in 1975, with a density of 7.2, then it decreased to 5.0 in 2004 and to 3.3 in 2012 (Table 1). Such a density decrease occurred beneath the 1,850 m of altitude, while the density was stable or strongly increased at higher altitude, where E. cassioides was exponentially more abundant than in 1975 within the two highest sites (Table 1).
The pattern observed in 2004 over the treeline was generally confirmed in 2012, where E. cassioides decreased in the three more xeric areas (sites 5, 19, 20) and increased in the less xeric one (site 14).
In 2004 and 2012 the E. cassioides population had become increasingly restricted to higher altitudes, and all the most important sites for the species were located above the treeline. The retreat of E. cassioides from five sites below 1,850 m of altitude caused a reduction of 39 % of the distribution range, nowadays limited to the herbaceous habitats of mountain tops (Fig. 2). The presence of the species with low densities in the site 21 (below 1,850 m) is probably due to the position of this site between the two main sources of individuals, acting as a stepping stone for individuals exchange.
The overall abundance of E. cassioides was evaluated on the basis of density values and surfaces of the sampled grasslands. The density of the Monte Pollino prairie was computed using the mean density of sites 14, 19, 20, those of Piano di Ruggio pastures using the mean density of sites 1 and 2, and those of Piani del Pollino pastures using the mean of sites 10, 11 and 15. Only one site was sampled in Serra del Prete prairie, then its overall abundance could be different and probably higher than the evaluated one. The evaluated abundance provided a sufficiently realistic picture of the quantitative distribution of E. cassioides in the Pollino Massif.
In 1975 16.9 % of the E. cassioides population was present below the tree line, where nowadays it virtually disappeared decreasing to less than 1 % (Fig. 3). The overall abundance strongly increased in recent years. In 1975 we estimated a population of 2,520 individuals within sampled grasslands that increased to 31,701 individuals in 2004 and 21,977 in 2012.
Discussion
The altitudinal distribution changes of E. cassioides in the last decades on the Pollino Massif showed two apparently contrasting trends, i.e. a significant uphill shift, with a subsequent reduction of suitable habitat, is coupled to an increased population size above the treeline.
The barycentre of the altitudinal distribution of E. cassioides shifted upward of more than 180 metres, an altitudinal range shift consistent with those previously observed in other mountain habitats during similar timespans (Konvicka et al. 2003; Wilson et al. 2005; Chen et al. 2011), resulting in a strong reduction of its suitable habitat extension. The mountain habitats reduction is one of the expected consequences of climate changes (Haslett 1997), and it represents the main threat to Apennine populations of E. cassioides, causing large local extinctions as estimated by Settele et al. (2008). The extinction of the most isolated populations is a very severe consequence of climate change from a conservation point of view, considering the high level of genetic divergence, sometimes insufficiently explored, reached by individual populations (Albre et al. 2008).
Although a reduction of the E. cassioides range occurred during last decades, nowadays its abundance strongly increased at higher altitude. This pattern is in contrast with those usually observed, where range reduction is coupled to population size reduction, the last often of greater magnitude (Shoo et al. 2005). In the study area the climate was warmer and increasingly dryer than previous decades (Brunetti et al. 2006, 2012). These changes could give the limiting factor to the E. cassioides fitness at lower altitude, while at higher altitude the effects could be different and an improvement of the habitat quality cannot be excluded.
Between 2004 and 2012 the climate oscillated less than previous years showing temperatures increasingly higher and less precipitation. The more xeric climate may have caused the changes in the abundance of E. cassioides within the sites above the treeline between 2004 and 2012, where its density decreased in more sunny and dry sites and increased in a more humid site. This suggests a major role of topography and local habitat patchiness in affecting population size as suggested by studies on thermal preferences of some Erebia species (Kleckova et al. 2014).
Human induced changes sometimes assume a major role in driving modifications within insect communities (Franco et al. 2006), mainly in lowlands or in areas with a favourable topography. In the study area the human pressure is generally lower today than 37 years ago because during this period the Pollino National Park was designated. Hence, the extinction of E. cassioides from the sites at lower altitude within the study area cannot be attributable to human induced changes. The main land use change was the decline of stock raising that, thanks to new management rules, reduced the grazing intensity, an important threat for grassland diversity (Olff and Ritchie 1998). Anyway, the grazing intensity was reduced only, producing effects on plant associations just linked to the reduction of mechanical disturbances such as poaching. Grass cover in the region may now be less sparse, but we do not have evidence for succession to taller or denser vegetation. The change of land use does not seem to have affected short grasses such as Nardus, Festuca and Sesleria, on which develops E. cassioides larvae, so negative changes in the lower altitude parts of the E. cassioides altitudinal range seem mainly to be attributable to climate change. The observed changes to the E. cassioides could result mainly from the combination of warming and precipitation decline leading to habitat deterioration at lower altitudes, versus habitat amelioration at higher altitudes where population density of the species increased.
Physiological studies carried out on some Erebia species could provide a direct explanation of the upward shift of E. cassioides on the Pollino Massif, consistent with the precipitation deficit observed during last decades (Fig. 1). Vrba et al. (2012) found the lower lethal temperature of the alpine Erebia tyndarus higher than that of Erebia medusa, a species inhabiting lower altitude habitats with higher mean winter temperatures. They explain this reverse altitudinal cline by the stronger buffering function of snow cover in the hibernacula of caterpillars living at higher altitudes, while at lower ones snow pattern is irregular in space and time and thus unreliable at lower altitude. The anthropogenous treeline seems to act as a physical delimitation of the actual optimal habitat for this species on the Pollino Massif, maybe due to the effective buffering function of snow cover. Furthermore, in mountain valleys often thermic inversion occurs, causing accumulation of cold air especially in springtime and thus increasing mortality of non-adapted caterpillars.
The Pollino Massif is inhabited by several Lepidoptera species at their low-latitude range boundaries such as Setina irrorella (Parenzan and Scalercio 2001), Epipsilia grisescens, Xestia ashworthii, Pareulype berberata, Entephria flavicinctata (Scalercio 2009), all recorded only over the treeline and potentially threatened by climate changes. Another Erebia species is known for this massif, E. gorge (Balletto et al. 1977), but it has not been recorded since many years and we cannot exclude that the relict population of this species has gone extinct in recent decades. Although a great diversity loss is expected in the future for this Mediterranean mountain, Settele et al. (2008) identified the Pollino Massif as a potential refuge area suitable for Erebia ottomana and E. melas, eastern representatives of the genus Erebia. If E. cassioides will go extinct as expected and its niche will become available, it is possible to hypothesise an artificial introduction on the Pollino Massif of E. ottomana and E. melas in order to preserve them from extinction for a longer time, as successfully done for Erebia epiphron in the Krkonoše Mountains in Eastern Europe (Cizek et al. 2003).
Conclusions
Both good and bad news for the future of E. cassioides in the Appennine can be drawn from this paper. The good news is that the observed range reduction of this cold-adapted butterfly was coupled to an increased abundance at high altitude in the study area that decreases its extinction risk. This pattern was largely unexpected and poses new questions for insect conservation. If it will be observed for other insect species too, extinction risks due to climate changes should be re-evaluated for mountain areas, or at least for some orophilous species in order to avoid generalisations.
The bad news is that the species range decline could only be delayed and slightly mitigated by reducing the magnitude of human pressure on natural habitats. In fact, climate change is able to drive species to high elevations and change species assemblages, even in protected areas as observed also in Greece (Zografou et al. 2014), where human induced habitat perturbations decreased in recent decades. Conservation strategies must tend to increase habitat quality to preserve relict populations of cold adapted species, but the only result could be the delay of several local extinctions. In fact, further temperature increase and precipitation decrease could deteriorate habitat quality and suitability of mountaintop prairies of the Pollino Massif and other refuge areas around the Mediterranean Basin for all the species having a natural history similar to that of E. cassioides.
References
Albre J, Gers C, Legal L (2008) Molecular phylogeny of the Erebia tyndarus (Lepidoptera, Rhopalocera, Nymphalidae, Satyrinae) species group combining CoxII and ND5 mitochondrial genes: a case study of a recent radiation. Mol Phylogenet Evol 47:196–210
Balletto E, Toso G, Barberis G, Barberis G, Rossaro B (1977) Aspetti dell’ecologia dei Lepidotteri Ropaloceri nei consorzi erbacei alto Appenninici. Animalia 4:277–343
Blondel J (1979) Biogèographie et ècologie. Masson, Paris
Brandmayr P, Mingozzi T, Scalercio S, Passalacqua D, Rotondaro F, Pizzolotto R (2002) Stipa austroitalica garigues and mountain pastureland in the Pollino National Park (Calabria, Southern Italy). In: Redecker B, Finck P, Härdtle W, Riecken U, Schröder E (eds) Pasture Landscapes and Nature Conservation. Springer, Berlin, Heidelberg, pp 53–66
Brandmayr P, Pizzolotto R, Scalercio S (2003) Overview: invertebrate diversity in Europe’s alpine regions. In: Nagy L, Grabherr G, Körner C, Thompson DBA (eds) Alpine biodiversity in Europe. Springer, Berlin, Heidelberg, pp 233–237
Brunetti M, Maugeri M, Fabio M, Nanni T (2006) Temperature and precipitation variability in Italy in the last two centuries from homogenised instrumental time series. Int J Climatol 26:345–381
Brunetti M, Caloiero T, Coscarelli R, Gullà G, Nanni T, Simolo C (2012) Precipitation variability and change in the Calabria region (Italy) from a high resolution daily dataset. Int J Climatol 32:57–73
Chen I-C, Hill JK, Shiu H-J, Holloway JD, Benedick S, Chey VK, Barlow HS, Thomas CD (2011) Asymmetric boundary shifts of tropical montane Lepidoptera over four decades of climate warming. Glob Ecol Biogeogr 20:34–45
Cizek O, Bakesová A, Kuras T, Benes J, Konvicka M (2003) Vacant niche in alpine habitat: the case of an introduced population of the butterfly Erebia epiphron in the Krkonoše Mountains. Acta Oecol 24:15–24
De Groot M, Rebeušek F, Grobelnik V, Govedič M, Šalamun A, Verovnik R (2009) Distribution modeling as an approach to the conservation of a threatened alpine endemic butterfly (Lepidoptera: Satyridae). Eur J Entomol 106:77–84
Franco AMA, Hill JK, Kitschke C, Collingham YC, Roy DB, Fox R, Huntley B, Thomas CD (2006) Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Glob Chang Biol 12:1545–1553
Haslett JR (1997) Mountain ecology: organism responses to environmental change, an introduction. Glob Ecol Biogeogr Lett 6:3–6
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Chang Biol 12:450–455
Higgins LG, Riley ND (1983) Farfalle d’Italia e d’Europa. Rizzoli Editore, Milano
Kleckova I, Konvicka M, Klecka J (2014) Thermoregulation and microhabitat use in mountain butterflies of the genus Erebia: importance of fine-scale habitat heterogeneity. J Therm Biol 41:50–58
Konvicka M, Maradová M, Beneš J, Fric Z, Kepka P (2003) Uphill shifts in distribution of butterflies in the Czech Republic: effects of changing climate detected on a regional scale. Glob Ecol Biogeogr 12:403–410
Lattes A, Mensi P, Cassulo L, Balletto E (1994) Genotypic variability in western European members of the Erebia tyndarus species group (Lepidoptera, Satyridae). Nota Lepidopterol 5:93–104
Olff H, Ritchie ME (1998) Effects of herbivores on grassland plant diversity. Trends Ecol Evol 13:261–265
Parenzan P, Scalercio S (2001) Presenza di Setina irrorella (Linnaeus, 1758) in Italia meridionale e di Setina roscida (Denis & Schiffermüller, 1775) in Abruzzo; considerazioni sulle specie europee del genere Setina Schrank (Lepidoptera: Arctiidae, Lithosiinae). Entomologica 35:89–101
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–41
Pizzolotto R, Sapia M, Rotondaro F, Scalercio S, Brandmayr P (2010) A georeferenced biodiversity databank for evaluating the impact of climate change in southern Italy mountains. In: Körner C, Spehn EM (eds) Data mining for global trends in mountain biodiversity. CRC Press, Taylor & Francis, Boca Raton, pp 137–147
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation. Chapman & Hall, London
Scalercio S (2009) On top of a mediterranean massif: climate change and conservation of orophilous moths at the southern boundary of their range (Lepidoptera: Macroheterocera). Eur J Entomol 106:231–239
Schmitt T, Hewitt G (2004) The genetic pattern of population threat and loss: a case study of butterflies. Mol Ecol 13:21–31
Schmitt T, Cizek O, Konvicka M (2005) Genetics of a butterfly relocation: large, small and introduced populations of the mountain endemic Erebia epiphrion silesiana. Biol Conserv 123:11–18
Settele J, Kudrna O, Harpke A, Kühn I, Van Swaay C, Verovnik R, Warren M, Wiemers M, Hanspach J, Hickler T, Kühn E, Halder I, Veling K, Vliegenthart A, Wynhoff I, Schweiger O (2008) Climatic risk atlas of European butterflies. Biorisk 1 (Special Issue). Pensoft Publishers, Sofia
Shoo LP, Williams SE, Hero J-M (2005) Potential decoupling of trends in distribution area and population size of species with climate change. Glob Chang Biol 11:1469–1476
Tolman T, Lewington R (1999) Guide des papillons d’Europe et d’Afrique du Nord. HarperCollins, Lausanne
van Swaay C, Wynhoff I, Verovnik R, Wiemers M, López Munguira M, Maes D, Sasic M, Verstrael T, Warren M, Settele J (2010) Erebia cassioides. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2. www.iucnredlist.org. Accessed 19 March 2013
Vrba P, Konvicka M, Nedved O (2012) Reverse altitudinal cline in cold hardiness among Erebia butterflies. CryoLetters 33(4):251–258
Wilson RJ, Gutiérrez D, Gutiérrez J, Martinez D, Agudo R, Monserrat VJ (2005) Changes to the elevational limits and extent of species ranges associated with climate change. Ecol Lett 8:1138–1146
Zografou K, Kati V, Grill A, Wilson RJ, Tzirkalli E, Pamperis LN, Halley JM (2014) Signals of climate change in butterfly communities in a mediterranean protected area. PLoS ONE 9(1):e87245. doi:10.1371/journal.pone.0087245
Acknowledgments
Research supported by the Ministry of University and Education of Italy (PRIN D.M. 19 marzo 2010, n. 51, prot. 200947YRB9 Impact of global change on ecosystems, animal communities and species of alpine and mediterranean areas of italy: models, scenarios and evaluation from macro-to microscale, based on ecology and philogeography of vertebrates and invertebrates).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scalercio, S., Bonacci, T., Mazzei, A. et al. Better up, worse down: bidirectional consequences of three decades of climate change on a relict population of Erebia cassioides . J Insect Conserv 18, 643–650 (2014). https://doi.org/10.1007/s10841-014-9669-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9669-x