Abstract
Background
Pulmonary vein isolation (PVI) modulates the intrinsic cardiac autonomic nervous system and reduces atrial fibrillation (AF) recurrence.
Methods
In this retrospective analysis, we investigated the impact of PVI on ECG interlead P-wave, R-wave, and T-wave heterogeneity (PWH, RWH, TWH) in 45 patients in sinus rhythm undergoing clinically indicated PVI for AF. We measured PWH as a marker of atrial electrical dispersion and AF susceptibility and RWH and TWH as markers of ventricular arrhythmia risk along with standard ECG measures.
Results
PVI acutely (16 ± 8.9 h) reduced PWH by 20.7% (from 31 ± 1.9 to 25 ± 1.6 µV, p < 0.001) and TWH by 27% (from 111 ± 7.8 to 81 ± 6.5 µV, p < 0.001). RWH was unchanged after PVI (p = 0.068). In a subgroup of 20 patients with longer follow-up (mean = 47 ± 3.7 days after PVI), PWH remained low (25 ± 1.7 µV, p = 0.01), but TWH partially returned to the pre-ablation level (to 93 ± 10.2, p = 0.16). In three individuals with early recurrence of atrial arrhythmia in the first 3 months after ablation, PWH increased acutely by 8.5%, while in patients without early recurrence, PWH decreased acutely by 22.3% (p = 0.048). PWH was superior to other contemporary P-wave metrics including P-wave axis, dispersion, and duration in predicting early AF recurrence.
Conclusion
The rapid time course of decreased PWH and TWH after PVI suggests a beneficial influence likely mediated via ablation of the intrinsic cardiac nervous system. Acute responses of PWH and TWH to PVI suggest a favorable dual effect on atrial and ventricular electrical stability and could be used to track individual patients’ electrical heterogeneity profile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atrial fibrillation (AF) affects nearly 40 million individuals worldwide [1]. Typically, AF begins as an arrhythmia triggered by pulmonary vein ectopic beats and evolves over time to a more substrate-driven arrhythmia, justifying the statement that “AF begets AF,” as paroxysmal AF (PAF) often progresses to persistent AF [1, 2]. To interrupt this pathway, catheter ablation of AF has increasingly gained importance in recent years, contributing to reduced AF burden and improved quality of life and left ventricular ejection fraction in selected patients [2,3,4,5,6]. Pulmonary vein isolation (PVI) attempts to eliminate AF by isolating triggering foci from the pulmonary vein–atrial region of the left atrium and also likely through autonomic modulation by partially destroying intrinsic cardiac autonomic ganglionated plexi [2, 6, 7]. AF recurrence after ablation is commonly secondary to pulmonary vein reconnection [2].
Assessment of electrophysiological properties of the heart could disclose pathogenic pathways of increased risk for AF recurrence. Electrical heterogeneity or electrical dispersion can be defined as spatial and/or temporal non-uniformity in depolarization and repolarization of the heart and can easily be monitored with 12-lead ECGs, long-term ECG patches, or wired ambulatory ECG recorders [8, 9]. T-wave heterogeneity (TWH) has been shown to predict near-term mortality in women due to acute coronary syndrome as well as sudden cardiac death in a 5600-subject population survey, with a nearly 2-fold greater predictive power compared to QTc interval [10, 11]. PWH reflects electrical dispersion of the atria, which participates in the underlying mechanism of AF [1, 2] and therefore might allow assessment of risk of AF recurrence after ablation. This is the first study of PWH measurement to determine electrophysiological properties before and after AF ablation [1, 2, 12].
The goals of the present retrospective analysis were to monitor atrial and ventricular electrical heterogeneity profiles before and after PVI for AF using second central moment analysis [8,9,10,11] and to compare PWH to other contemporary P-wave indices to evaluate risk for AF recurrence.
2 Methods
2.1 Patients
Medical records of adult patients with paroxysmal or persistent AF referred to the Electrophysiology Section at Beth Israel Deaconess Medical Center (BIDMC) for PVI were analyzed. In this exploratory study, eligible patients (N = 45) were individuals whose ECGs were obtained ≤ 2.5 h pre-ablation and ≤ 24 h post-ablation, selected from among nearly 2000 individuals who presented to the electrophysiology study laboratory for PVI in sinus rhythm between July 2016 and December 2021. Individuals with previous PVI or left atrial ablation were excluded. Demographic and clinical information, type of AF, cardiac risk factors, comorbidities and medications were accessed. Recurrent arrhythmia after ablation was defined as documentation of AF, atrial flutter, or atrial tachycardia lasting ≥ 30 s.
2.2 Procedures
2.2.1 Ablation procedure
Patients were taken to the electrophysiology lab in a fasting state and placed under general anesthesia. Femoral venous access was obtained, a decapolar catheter was placed in the coronary sinus, and transseptal access was obtained under intracardiac echocardiography guidance. Multipolar mapping catheters (Pentaray® NAV ECO, Biosense Webster, Irvine CA, USA, or the Advisor™ HD Grid, Abbott, Chicago IL, USA) were used to create anatomic shells of the atria and baseline activation and voltage maps during coronary sinus pacing. Radiofrequency ablation was performed with open-irrigated contact force-sensing ablation catheters (Thermocool Smarttouch® SF, Biosense Webster, Irvine CA, USA, or Tacticath™ SE, Abbott, Chicago IL, USA) with adjacent point-by-point lesions delivered in a wide antral approach for end block isolation of the left and right pulmonary veins. Further lesions were delivered as necessary to achieve PVI.
2.2.2 Electrocardiographic analysis
12-lead electrocardiograms (ECGs) were recorded (GE Healthcare, Milwaukee WI, USA) at 500 Hz with an amplitude resolution of 4.88 µV immediately before ablation, within 24 h after the procedure, and at 4 to 13 weeks post-ablation in select patients. Heart rate, PR interval, P-wave axis, duration, terminal force in V1, and dispersion (calculated as the maximum minus the minimum P-wave duration among the 12 leads), QRS axis and duration, QT-interval dispersion (QTd), uncorrected and corrected QT interval (Bazett´s formula, QTc), and T-wave peak to T-wave end (TpTe) in lead V5 were analyzed by a single blinded investigator (G.L.F.).
2.2.3 Heterogeneity analysis
P-, R- and T-wave heterogeneity (PWH, RWH and TWH) were measured as previously described using dedicated leads (I, II, V4, V5, V6) by second central moment analysis [8, 12] from digitized standard 12-lead ECG recordings before and after the ablation. A single, blinded investigator (G.L.F.) calculated PWH, RWH, and TWH as the maximum splay in microvolts about the mean waveform throughout the P wave, R wave, and T wave, respectively, as previously described [8, 12] (Fig 1).
Signal processing and computing of the second central moment calculation of P-wave heterogeneity (PWH), R-wave heterogeneity (RWH), and T-wave heterogeneity (TWH) in a representative patient with atrial fibrillation before ablation and at 9 hours after the procedure. Greater splay (heterogeneity) can be observed before ablation
2.3 Statistical analysis
Quantitative variables were expressed as means ± standard error of mean (SEM), and qualitative variables were expressed as percentage values. The normality of the data distribution was assessed using the Kolmogorov-Smirnoff test. Student´s t-test was employed to compare continuous variables, and a p-value < 0.05 was considered significant. Fisher´s exact test or Pearson´s Chi-square analysis were used to analyze qualitative data and frequencies of occurrence. Estimation statistics were used to compare the acute effects of PVI on heterogeneity values [13]. Additionally, we used Student´s paired T-test to compare ECG and heterogeneity variables among a subgroup of the total cohort with longer follow-up (4 to 13 weeks after ablation) to pre- and earlier post-ablation variables. Hierarchical multiple linear regression was used to explore the predictive variables associated with PWH and TWH. Independent predictors of PWH and TWH among ECG parameters and demographic and clinical data by previous univariate analysis with a p-value < 0.1 were retained for the final multiple linear regression model. All statistical procedures were performed using IBM SPSS software package for Windows, version 17 (IBM, Armonk, NY, USA) and Microsoft® Excel® for Microsoft 365 MSO (Version 2211).
3 Results
3.1 Patient characteristics
The study included 45 individuals with paroxysmal (86.7%) or persistent AF (13.3%) referred for ablation who presented in sinus rhythm at the time of the elective PVI (Table 1). Women composed 60% of the cohort, and mean age at time of ablation was 66 ± 1.1 years. ECG records of 20 patients were available at 4 to 17 weeks after ablation.
3.2 ECG parameters
ECGs were recorded immediately before (0.97 ± 0.08 h) and at ≤16 ± 1.1 h after the end of the procedure and the results are presented along with longer-term follow-up in 20 patients (Table 2).
3.3 P-, R- and T-wave heterogeneity
PVI acutely reduced PWH by 20.7% (p < 0.001) and TWH by 27% (p < 0.001) but did not alter RWH (Table 2, Fig. 2). Using estimation statistics [13], the paired mean differences between pre- and post-ablation for PWH, TWH and RWH were -6.46 [95% CI -9.56, -3.82], p < 0.001; -29.9 [95% CI -43.4, -17.7], p < 0.001; and -18.6 [95% CI -41.4, -1.92], p = 0.068, respectively.
The percentage decrease in P-, R- and T-wave heterogeneity was similar in PAF compared to persistent AF patients (18.5% vs. 36.2%, p = 0.14; 7.1% vs. 8.9%, p = 0.44; and 25.8% vs. 34.8%, p = 0.37, respectively).
Pre-ablation PWH and RWH values were higher in males compared to females, but TWH did not differ (36 ± 3.2 vs. 28 ± 2.0 µV, p = 0.01; 322 ± 38.6 vs. 209 ± 16.2 µV, p = 0.002; and 124 ± 9.6 vs. 102 ± 11.2 µV, p = 0.08, respectively). PVI decreased PWH and TWH similarly in males and females (23.4 vs.18.4%, p = 0.26; 15.4 vs. 36.3%, p = 0.18), respectively.
The ≥ 102 µV TWH cut point of abnormality, a marker of ventricular tachycardia/fibrillation and arrhythmic death [11], was exceeded by 55.6% of pre-ablation patients (72.2% in males and 44.4% in females) and by 26.7% of post-ablation patients (44.4% in males and 14.8% in females) (p = 0.0053). After 4 to 13 weeks, TWH ≥ 102 µV was present in 45% of the cohort (71.4% in males and 30.8% in females) (p = 0.43 compared to pre-ablation; p = 0.14 compared to hours after ablation) (Fig. 3).
Portion of patients with abnormal repolarization after ablation. Top: Patients with abnormal values of T-wave heterogeneity (≥ 102 µV) pre-ablation (N = 45), hours post-ablation (N = 45), and weeks post-ablation (N = 20). Bottom: Patients with abnormal QTc interval (Bazett’s) (≥ 450 for males and ≥ 460 for females) pre-ablation, hours post-ablation, and weeks post-ablation
In univariate regression analyses, sex, heart failure, focal ablation lines after PVI, P-wave axis and PR interval were related to PWH (Supplementary Table 1). After hierarchical multivariate regression, the full model (Model 5, Supplementary Table 2) explained 22.3% of PWH (p = 0.009 for the model), but only sex was still significantly correlated with PWH, explaining 9.6% of PWH (Model 1, Supplementary Table 2, p = 0.023 for the model).
QRS duration and age were related to TWH in univariate analysis (Supplementary Table 3). Multivariate regression showed that the full model (Model 2, Supplementary Table 4) explained 11.8% of TWH (p = 0.029 for the model), but only QRS duration remained significantly correlated with TWH, explaining 6.7% of its variance (Model 1, Supplementary Table 4, p = 0.049).
Additional exploratory evaluation of correlations between heart rate and heterogeneity adding pre- and post-ablation values revealed that heart rate exhibited a negative correlation with TWH, explaining 4.4% of its variance (standardized beta = 161.74, adjusted R² = 0.0442, p = 0.027). Heart rate had no correlation with PWH (p = 0.34) or RWH (p = 0.16).
3.4 Subgroup analyses and longer follow-up
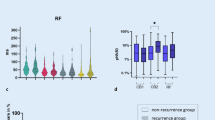
From the original 45-patient cohort, we analyzed 20 individuals (mean age = 66 ± 1.6 years, 65% female) whose ECGs were available at 4 to 13 weeks after ablation (mean 47 ± 3.7 days after ablation). In this subgroup, PWH remained reduced by 19.5% (p = 0.01) but TWH recovered to a 16% overall reduction (p = 0.16) compared to pre-ablation (Fig. 4).
Atrial and ventricular heterogeneity in 20 patients with longer-term follow-up. At 4 to 13 weeks after ablation, P-wave heterogeneity (PWH) maintained lower values compared to pre-ablation and was similar to hours post-ablation; T-wave heterogeneity (TWH) partially returned to pre-ablation level. R-wave heterogeneity (RWH) was similar at the three different time points. Pre = pre-ablation; Hours = hours after ablation; Weeks = weeks after ablation
Heart rate remained elevated at the longer follow-up, while P-wave duration, uncorrected QT interval, QTd, and TpTe remained reduced. Although QTc interval increased from pre-ablation baseline to hours after ablation ( p < 0.001), longer follow-up showed decreased values ( p < 0.001) (Table 2, Fig. 3). QTc ≥ 450 ms for men and ≥ 460 ms for women was present in 43.2% of the cohort pre-ablation (33.3% for men; 50% for women), 66.7% at 16 h post-ablation (38.9% men; 85.2% women) and 5% (0% men; 7.7% women) at 4 to 13 weeks post-ablation (p = 0.03 for pre- vs. hours post-ablation; p = 0.002 for pre- vs. weeks post-ablation; and p < 0.001 for hours post- vs. weeks post-ablation).
To study the influence of heart rate on heterogeneity, we analyzed a subgroup of 17 patients who had < 10% variation in heart rate after ablation (mean age = 68 ± 1.9 years, 41.2% female, heart rate before and after PVI = 62 ± 2.0 vs 63 ± 2.0 bpm, p = 0.06). In this group, PWH decreased from 33 ± 2.9 to 25 ± 2.9 µV (p = 0.003) and TWH decreased from 131 ± 14.6 to 97 ± 12.7 µV (p = 0.02) after ablation.
We compared individuals who received PVI-only to those with additional ablation lines (84.4%). PWH and TWH values decreased in patients with PVI-only by 24.1% and 14.7%, but these changes were not significantly different compared to patients with additional ablation lines, namely 20.4% (p = 0.39) and 29.6% (p = 0.24).
3.5 PWH in patients with early recurrence of atrial arrhythmias
Three individuals from the original 45-patient cohort experienced early recurrence of atrial arrhythmia, defined as AF, atrial flutter, or atrial tachycardia within 3 months after ablation. Two patients had atrial flutter at 4 days and one at 77 days after ablation. In these three individuals, PVI increased PWH within hours after ablation by 8.5% while in patients without early recurrence, PVI decreased PWH by 22.7% (p = 0.048). In 18 patients with early plus later recurrence (604 ± 128 days after ablation), the difference in PWH decrease after ablation reached borderline p-value (0.06) for recurrence at any time. By comparison, neither P-wave axis, P-wave duration, nor P-wave dispersion values pre- or post-ablation, nor their absolute difference, was associated with early recurrence or recurrence at any time. P-wave terminal force in V1 at baseline was significantly greater in patients with early recurrence compared to individuals with no early recurrence. However, there were no statistical differences in their decreased values after PVI in early-recurrence or recurrence at any time compared to no recurrence (Table 3).
4 Discussion
4.1 Main findings
This is the first report of simultaneous assessment of interlead heterogeneity of P-, R-, and T-wave morphology following PVI in patients with AF. We found that PVI produced reductions in PWH and TWH in the immediate post-ablation period (Table 2). PWH remained reduced at 4 to 13 weeks following ablation, whereas TWH partially returned to pre-ablation levels. In the present cohort, PWH was superior to other contemporary P-wave metrics including P-wave axis, dispersion, duration, and terminal force in predicting early AF recurrence (Table 3).
4.2 Triggers, substrate and modulating factors and the influence of PVI on AF
Factors such as pressure or volume overload with stretch-related fibrosis, inflammation, fatty infiltration, ischemia, atrial dysfunction, genetic predisposition, ionic handling, ion-channel alterations, and neuro-humoral tone favor atrial electrical and anatomical remodeling, which may explain the progression from paroxysmal episodes to persistent forms of AF [1, 14,15,16]. AF itself and its associated risk factors and comorbid conditions favor arrhythmia perpetuation [1, 2]. Thus, early interruption of these processes is beneficial.
An important modulating factor in the perpetuation of AF is the autonomic nervous system [14]. Choi and colleagues demonstrated in a canine model that activation of the intrinsic cardiac nervous system precedes AF either alone (72%) or in association with (28%) sympathovagal extrinsic autonomic nervous system activation [17]. AF can be triggered by both sympathetic and parasympathetic stimuli [15, 18, 19]. Imbalance between these two components can promote uncoupling between a vagally shortened atrial effective refractory period with a sympathetic increase in calcium transients, a phenomenon called calcium transient triggering [20]. For AF onset, this seems particularly important in the pulmonary veins [21].
Adding ganglionic plexi ablation to PVI increased the success rate of AF ablation in a randomized trial [22]. However, since lesions from standard pulmonary vein ablation typically encompass areas with high ganglionic plexi concentrations, it is possible that autonomic denervation is an additive mechanism contributing to favorable PVI results [23]. Our cohort exhibited increased heart rates shortly after ablation with a sustained response weeks later. Although alternative tests such as procedural extracardiac vagal stimulation, heart rate variability, tilt-test, exercise tolerance test, and atropine test would be ideal to evaluate the effects of parasympathetic and sympathetic denervation in the follow-up, increases in minimum and mean heart rates after ablation have been related to and considered the simplest parameter changed by cardioneuroablation [24,25,26,27].
4.3 Atrial heterogeneity and ECG variables after PVI
We observed an immediate reduction in atrial electrical heterogeneity after ablation. This response was sustained 4 to 13 weeks later. An increase in atrial effective refractory period caused by decreased vagal tone, isolation of triggering activity from PV or non-PV foci, and decreased critical tissue mass available for simultaneously circulating wavelets could be responsible for reduced heterogeneity [1, 2, 15]. Inhomogeneous atrial electrical conduction is related to AF risk and has been proposed as the cause of P-wave dispersion [28, 29].
Interestingly, heart rate was not correlated with PWH, and in a subgroup with < 10% variation in heart rate after ablation, a PWH reduction was also noted. This observation suggests that decreased vagal tone evident as increased heart rate is not the sole mechanism for PWH reduction.
P-wave duration was also reduced after ablation, which could further implicate an increase in sympathetic tone [30]. Several groups reported that increased P-wave duration after ablation obtained with signal averaged P-wave analysis was associated with AF recurrence in patients with persistent or paroxysmal AF [31,32,33]. However, other studies reported that pre-ablation P-wave duration predicted recurrence [34], while others found no association between AF recurrence and P-wave duration [35].
Female patients composed 60% of our cohort, although, in unselected populations, the age-adjusted incidence, prevalence, and lifetime risk of AF are lower in women compared to men [1]. In multivariate analysis, only female sex was significantly correlated with PWH. We also demonstrated lower levels of heterogeneity before and after ablation in women compared to men. However, PWH and TWH reduction after ablation was similar for men and women. Since women tend to have smaller hearts, reduced critical tissue mass for circulating wavelets could be responsible for the observed reduced heterogeneity [1, 2, 15].
4.4 Ventricular heterogeneity and ECG variables after PVI
Within 24 h after ablation, TWH was substantially decreased, but by weeks after the procedure, it had returned partially to pre-ablation levels. Although higher sympathetic tone is linked to increased electrical dispersion, afterdepolarizations, and arrhythmias [15], other factors, such as sedation during ablation, stress relief after the end of procedure, or the acute reduction in vagal tone, sympathetic output, and optimized sympathovagal balance could be alternative explanations to our findings. In addition, bradycardia, as observed in pre-ablation patients, could have promoted repolarization dispersion, as previously demonstrated [36] and ablation, by its acute effect on heart rate secondary to parasympathetic output alterations, could explain the rapid initial decrease in TWH. These mechanisms appear to have less importance in ventricular depolarization, since QRS duration, QRS axis, and RWH were similar before and after ablation.
Other parameters related to ventricular electrical dispersion, such as uncorrected QT interval, TpTe, and QTd, decreased hours or weeks after ablation. QTc interval (Bazett´s) increased hours after ablation and decreased weeks later. Our results are similar to those reported by Nguyen et al., who found increased QTc interval immediately after ablation, which returned to pre-ablation levels at 30–90 days [37]. Chikata et al. found that uncorrected QT interval was prolonged at 4 h after ablation and then decreased on the first day and at 1 and 3 months, but QTc interval (Bazett´s) maintained higher values until the third month after ablation [38]. Similar to our and other reports, they demonstrated that female sex was associated with a more pronounced prolongation of the uncorrected QT interval compared to men [38, 39]. Hermans et al., however, found no association between ablation and QTc interval or QTd, although there was a shorter duration of uncorrected QT interval on the first day and at three months after ablation [40]. In that study, however, individuals were younger and fewer were female. These studies also reported increased heart rates after ablation [37, 38, 40].
The fact that the directional changes in TWH and QTc interval differed in our study suggests that these measurements reflect differing electrophysiologic properties. While QTc interval includes electrical myocyte depolarization and repolarization, incorporating systolic and diastolic periods, TWH reflects temporal and/or spatial differences in repolarization of neighboring myocytes [8].
4.5 Limitations
This hypothesis-generating study was a retrospective medical records analysis that explored acute and short-term patterns of atrial and ventricular heterogeneity in patients undergoing ablation for AF. Because of the small number of individuals studied, the potential role of PWH as a tool for tracking risk for AF recurrence will require further analysis and, although patients underwent routine follow-up, underestimation of asymptomatic AF recurrence and AF burden may have occurred. Second, the absence of a control group (either in sinus rhythm or in AF but not referred for PVI) in longitudinal settings precludes further insights into thresholds for normal/abnormal levels of PWH, TWH and RWH for clinical significance. Third, although exploratory investigations of subgroups of different types of AF and of ablation procedures did not show significant differences in heterogeneity, the absence of late atrial mapping to search for PVI reconnections prevented detailed anatomical correlation between ablation lines and heterogeneity results. And last, updated echocardiographic data were not available for correlation analyses with heterogeneity values.
5 Conclusion
This is the first study to measure atrial and ventricular electrical heterogeneity concurrently before and after PVI for AF using second central moment analysis. We found that PWH was reduced within hours after ablation and remained decreased at 4 to 13 weeks after the procedure. TWH, a marker of sudden death in epidemiological studies, decreased acutely after ablation and only partially returned to pre-ablation levels weeks later. The potential role of electrical heterogeneity after ablation regarding early success rates and AF recurrence risk holds promise but requires further study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, ESC Scientific Document Group, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612.
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–160. https://doi.org/10.1093/europace/eux274.
Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag V, EARLY-AF Investigators, et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388:105–16. https://doi.org/10.1056/NEJMoa2212540.
Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, CASTLE-AF Investigators, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–27. https://doi.org/10.1056/NEJMoa1707855.
Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, CABANA Investigators, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–74. https://doi.org/10.1001/jama.2019.0693.
Kottkamp H, Tanner H, Kobza R, Schirdewahn P, Dorszewski A, Gerds-Li JH, et al. Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: Early or delayed cure? J Am Coll Cardiol. 2004;44:869–77. https://doi.org/10.1016/j.jacc.2004.04.049.
Willems S, Klemm H, Rostock T, Brandstrup B, Ventura R, Steven D, et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur Heart J. 2006;27:2871–8. https://doi.org/10.1093/eurheartj/ehl093.
Verrier RL, Nearing BD, D’Avila A. Spectrum of clinical applications of interlead ECG heterogeneity assessment: From myocardial ischemia detection to sudden cardiac death risk stratification. Ann Noninvasive Electrocardiol. 2021;26:e12894. https://doi.org/10.1111/anec.12894.
Verrier RL, Huikuri H. Tracking interlead heterogeneity of R- and T-wave morphology to disclose latent risk for sudden cardiac death. Heart Rhythm. 2017;14:1466–75. https://doi.org/10.1016/j.hrthm.2017.06.017.
Monteiro FR, Rabelo Evangelista AB, Nearing BD, Medeiros SA, Tessarolo Silva F, Pedreira GC, et al. T-wave heterogeneity in standard resting 12-lead ECGs is associated with 90-day cardiac mortality in women following emergency department admission: A nested case-control study. Ann Noninvasive Electrocardiol. 2021;26:e12826. https://doi.org/10.1111/anec.12826.
Kenttä TV, Nearing BD, Porthan K, Tikkanen JT, Viitasalo M, Nieminen MS, et al. Prediction of sudden cardiac death with automated high-throughput analysis of heterogeneity in standard resting 12-lead electrocardiograms. Heart Rhythm. 2016;13:713–20. https://doi.org/10.1016/j.hrthm.2015.11.035.
Nearing BD, Verrier RL. Tracking cardiac electrical instability by computing interlead heterogeneity of T-wave morphology. J Appl Physiol. (1985) 2003;95:2265–72. https://doi.org/10.1152/japplphysiol.00623.2003.
Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. Moving beyond P values: data analysis with estimation graphics. Nat Methods. 2019;16:565–6. https://doi.org/10.1038/s41592-019-0470-3.
Cheniti G, Vlachos K, Pambrun T, Hooks D, Frontera A, Takigawa M, et al. Atrial fibrillation mechanisms and implications for catheter ablation. Front Physiol. 2018;9:1458. https://doi.org/10.3389/fphys.2018.01458.
Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–21. https://doi.org/10.1161/CIRCRESAHA.113.302549.
Potpara TS, Stankovic GR, Beleslin BD, Polovina MM, Marinkovic JM, Ostojic MC, et al. A 12-year follow-up study of patients with newly diagnosed lone atrial fibrillation: implications of arrhythmia progression on prognosis: the Belgrade Atrial Fibrillation study. Chest. 2012;141:339–47. https://doi.org/10.1378/chest.11-0340.
Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–23. https://doi.org/10.1161/CIRCULATIONAHA.109.919829.
Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. JACC 2003;42 (7):1262–8. https://doi.org/10.1016/S0735-1097(03)00955-0.
Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–9. https://doi.org/10.1161/01.cir.0000018443.44005.d8.
Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–206. https://doi.org/10.1016/j.jacc.2005.12.023.
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. https://doi.org/10.1056/NEJM199809033391003.
Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–25. https://doi.org/10.1016/j.jacc.2013.06.053.
Lemola K, Chartier D, Yeh YH, Dubuc M, Cartier R, Armour A, et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–7. https://doi.org/10.1161/CIRCULATIONAHA.107.737023.
Pachon-M EI, Pachon-Mateos JC, Higuti C, Santillana-P TG, Lobo T, Pachon C, et al. Relation of fractionated atrial potentials with the vagal innervation evaluated by extracardiac vagal stimulation during cardioneuroablation. Circ Arrhythm Electrophysiol. 2020;13:e007900. https://doi.org/10.1161/CIRCEP.119.007900.
Pachon JC, Pachon EI, Cunha Pachon MZ, Lobo TJ, Pachon JC, Santillana TG. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace. 2011;13:1231–42. https://doi.org/10.1093/europace/eur163.
Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. https://doi.org/10.3389/fpubh.2017.00258.
Pachon-M JC, Pachon-M EI, Pachon CTC, Santillana-P TG, Lobo TJ, Pachon-M JC, et al. Long-term evaluation of the vagal denervation by cardioneuroablation using Holter and heart rate variability. Circ Arrhythm Electrophysiol. 2020;13:e008703. https://doi.org/10.1161/CIRCEP.120.008703.
Aytemir K, Ozer N, Atalar E, Sade E, Aksöyek S, Ovünç K, et al. P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:1109–12. https://doi.org/10.1111/j.1540-8159.2000.tb00910.x.
Chen LY, Ribeiro ALP, Platonov PG, Cygankiewicz I, Soliman EZ, Gorenek B, et al. P wave parameters and indices: a critical appraisal of clinical utility, challenges, and future research. A consensus document endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardiology. Circ Arrhythm Electrophysiol. 2022;15:e010435. https://doi.org/10.1161/CIRCEP.121.010435.
Cheema AN, Ahmed MW, Kadish AH, Goldberger JJ. Effects of autonomic stimulation and blockade on signal-averaged P wave duration. J Am Coll Cardiol. 1995;26(2):497–502. https://doi.org/10.1016/0735-1097(95)80028-f.
Blanche C, Tran N, Rigamonti F, Burri H, Zimmermann M. Value of P-wave signal averaging to predict atrial fibrillation recurrences after pulmonary vein isolation. Europace. 2013;15:198–204. https://doi.org/10.1093/europace/eus251.
Intzes S, Zagoridis K, Symeonidou M, Spanoudakis E, Arya A, Dinov B, et al. P-wave duration and atrial fibrillation recurrence after catheter ablation: a systematic review and meta-analysis. Europace. 2023;25:450–9. https://doi.org/10.1093/europace/euac210.
Ogawa M, Kumagai K, Vakulenko M, Yasuda T, Siegerman C, Garfinkel A, et al. Reduction of P-wave duration and successful pulmonary vein isolation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:931–8. https://doi.org/10.1111/j.1540-8167.2007.00890.x.
Okumura Y, Watanabe I, Ohkubo K, Ashino S, Kofune M, Hashimoto K, et al. Prediction of the efficacy of pulmonary vein isolation for the treatment of atrial fibrillation by the signal-averaged P-wave duration. Pacing Clin Electrophysiol. 2007;30:304–13. https://doi.org/10.1111/j.1540-8159.2007.00670.x.
Redfearn DP, Skanes AC, Gula LJ, Griffith MJ, Marshall HJ, Stafford PJ, et al. Non-invasive assessment of atrial substrate change after wide area circumferential ablation: a comparison with segmental pulmonary vein isolation. Ann Noninvasive Electrocardiol. 2007;12:329–37. https://doi.org/10.1111/j.1542-474X.2007.00182.x.
Kim JJ, Němec J, Papp R, Strongin R, Abramson JJ, Salama G. Bradycardia alters Ca(2+) dynamics enhancing dispersion of repolarization and arrhythmia risk. Am J Physiol Heart Circ Physiol. 2013;304:H848–60. https://doi.org/10.1152/ajpheart.00787.2012.
Nguyen DD, Akoum N, Hourmozdi J, Prutkin JM, Robinson M, Tregoning DM, et al. Catheter ablation of atrial fibrillation results in significant QTc prolongation in the postoperative period. Heart Rhythm. 2021;O2(2):500–10. https://doi.org/10.1016/j.hroo.2021.08.004.
Chikata A, Kato T, Usuda K, Fujita S, Maruyama M, Otowa KI, et al. Prolongation of QT interval after pulmonary vein isolation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31:2371–9. https://doi.org/10.1111/jce.14625.
Nakagawa M, Ooie T, Ou B, Ichinose M, Takahashi N, Hara M, et al. Sex differences in autonomic modulation of ventricular repolarization in humans. J Cardiovasc Electrophysiol. 2005;16:278–84. https://doi.org/10.1046/j.1540-8167.2005.40455.x.
Hermans BJM, Zink MD, van Rosmalen F, Crijns HJGM, Vernooy K, Postema P, et al. Pulmonary vein isolation in a real-world population does not influence QTc interval. Europace. 2021;23(23 Suppl 1):i48–54. https://doi.org/10.1093/europace/euaa390.
Funding
The authors did not receive support from any organization in the public, commercial, or not-for-profit sectors. Dr. Fialho holds a CNPq (Brazilian Council for Scientific and Technologic Development) APQ Research Fellowship (Process No. 401087/2022–0).
Author information
Authors and Affiliations
Contributions
Conceptualization: Guilherme L. Fialho: Richard L. Verrier: André D´Avila
Methodology: Bruce D. Nearing
Formal Analysis and Investigation: Guilherme L. Fialho; Bruce D. Nearing; Jonathan W. Waks: Timothy R. Maher: John-Ross Clarke: Alyssa Shepherd
Writing: original draft preparation: Guilherme L. Fialho
Writing: review and editing: Guilherme L. Fialho, Richard L. Verrier: André D´Avila
Corresponding author
Ethics declarations
Ethics approval
This medical records study conformed to requirements laid down in the 1964 Declaration of Helsinki and its amendments and was approved by the institutional review board of BIDMC (Protocol 2003–000420). Patient information was retrospectively obtained and deidentified.
Informed consent
The informed consent requirement was waived.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Permission to reproduce material from other sources
No material from other sources was used.
Competing interests
None of the authors declares a financial or non-financial interest relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fialho, G.L., Nearing, B.D., Waks, J.W. et al. Reduction in atrial and ventricular electrical heterogeneity following pulmonary vein isolation in patients with atrial fibrillation. J Interv Card Electrophysiol (2023). https://doi.org/10.1007/s10840-023-01543-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10840-023-01543-7