Abstract
Background
Landmark trials have shown superiority of ablative therapy over medical therapy in certain subpopulation with atrial fibrillation (AF). Previous studies have demonstrated an association between weight loss and reduced rates of recurrence of AF after ablation. The objective of this study is to determine if weight loss reduces the recurrence of AF after ablation.
Methods
An extensive literature search and systematic review of studies of weight loss on recurrence of AF after ablative therapy was performed. Risk ratio (RR) and 95% confidence intervals were measured for weight loss group versus control group in each study, and comparative analysis as well as subgroup analysis was made.
Results
Eight studies with a total of 1,425 patients were included. Overall, studies of patients who lost weight demonstrated lower recurrence of AF (RR 0.35; 95% CI 0.18–0.67). However, subgroup analysis of studies which included patients who lost ≥10% weight loss from baseline showed lower recurrence of AF (RR 0.18; 95% 0.03–0.89), whereas studies which included patients with <10% weight loss did not (RR 1.00; 95% 0.51–1.96). Studies of patients who had less than 12-month history of AF (RR 0.24; 95% CI 0.11–0.57) and those who lost weight prior to ablation (RR 0.40; 95% CI 0.20–0.79) also had lower recurrence of AF.
Conclusion
Weight loss is associated with lower long-term recurrence of AF after ablative therapy. Studies of patients with ≥10% weight loss, less than 12-month history of AF, and weight loss prior to ablation experience lower recurrence of AF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Atrial fibrillation (AF) is an increasingly common cardiac condition estimated to affect greater than 1% of the adult population in the USA and Europe [1, 2]. Previous studies have shown that 596 of 100,000 males and 373 of 100,000 females are affected, indicating that more than 33 million people around the globe have AF [3] with more than 5 million people in the USA alone [4].
Management of AF largely involves two modalities: rate control strategy or rhythm control strategy, and anticoagulation. With advancements in techniques and operator experience, the role of rhythm control strategy by catheter ablation is being brought to the fore. In the subset of patients with heart failure with reduced ejection fraction, the AATAC trial showed that catheter ablation was superior to rhythm control with amiodarone in terms of AF-free survival, unplanned hospitalizations, and overall mortality [5]. CASTLE-AF trial demonstrated that AF ablation resulted in reduced death or hospitalizations for heart failure, improvement in LVEF, and longer maintenance of sinus rhythm compared to those who received only medical therapy [6]. Moreover, EAST-AFNET 4 trial revealed that early rhythm control, including ablation in 8% of patients initially that rose to 19.4% at 2 years, was superior to rate control in patients with early AF and at high risk for cardiovascular complications [7].
As catheter ablation for AF garners more attention, efforts are being made on other fronts to investigate others measures that can be done to optimize ablation outcomes. One of the strategies involves reduction of weight, a reversible risk factor, especially in obese and morbidly obese individuals. Recently, Aldass et al. performed a meta-analysis on the effect of modest weight reduction (≥10% of body weight) in AF patients and showed lower risk of recurrent AF, reduction in AF burden, and improvement in AF symptoms with weight reduction. However, the study did not specifically examine the impact of weight reduction on ablation outcomes [8]. The purpose of our current study is to investigate the impact of weight loss on the outcome of AF ablation as demonstrated by recurrence of AF. In addition, our study aims to explore the effect of the magnitude of relative weight loss, duration of AF, and chronological order of weight loss relative to ablation on the recurrence of AF.
2 Methods
2.1 Search strategy and inclusion criteria
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [9]. Two independent authors (DYP, SKA) searched relevant literatures in database including PubMed, Embase, and Cochrane Library since inception to August 20, 2021. The following keyword was used for disease: “atrial fibrillation.” To define exposure, the following keywords were used: “weight loss,” “weight reduction,” “loss of weight,” “decrease in weight,” “weight decrease,” “weight changes,” “changes in weight,” and “ablation.” The title and abstracts written in English language were reviewed to recognize eligible studies on the impact of weight loss on the outcome of AF ablation. Additional studies were also manually searched through the references cited in reviews. Cohort as well as case-control studies were included as original articles. For studies with duplicative population, the study with more informative data was selected. The following studies were excluded: descriptive studies, editorials, review articles, and studies that did not provide risk ratios or effect sizes.
2.2 Statistical analysis
Risk ratio (RR) and 95% confidence intervals (95% CI) were estimated by using DerSimonian and Laird-based random effects model, which calculated the integrated summary statistics obtained from the literature. To assess the heterogeneity of the studies, Higgins and Thompson’s I2 statistics were conducted. The I2 measure ranges from 0 to 100% and was categorized to low (I2 < 50%), moderate (50% ≥ I2 < 75%), and substantial heterogeneity (I2 ≥ 75%). Funnel plot showing the log of risk ratio on sample size of selected studies was performed to evaluate for publication bias, after which Begg-Mazumdar and Egger tests were applied whose P-value <0.05 indicates statistically significant publication bias. Sub-group analyses were also conducted to evaluate for outcome in specific subsets. Firstly, the risk ratio of recurrent AF in patients who lost ≥ 10% of body weight and < 10% of their body weight was examined. This cut-off was chosen as many of the selected studies aimed to have their treatment group reduce the body weight by 10% [10,11,12]. Secondly, the duration of AF as defined by how long it lasted prior to ablation (< 12 months, ≥ 12 months, and not specified) was considered. Lastly, risk ratios of AF recurrence after pre-ablative and post-ablative weight loss were compared. All statistical analyses were conducted using R version 4.0.2.
3 Results
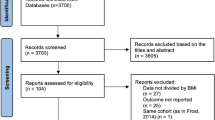
Initial search yielded 56 studies, of which 8 studies were included in our final analysis: one randomized controlled trial, three prospective cohort studies, and four retrospective cohort studies (Fig. 1). Funnel plot analysis of the included studies showed no evidence of publication bias on the effect of weight loss on the recurrence of AF after ablative therapy (Supplementary Figure 1).
Main characteristics and the demographics of the selected studies are summarized in Table 1. A total of 1,425 patients were included, of whom 659 patients (46.2%) experienced weight loss and 766 patients (53.8%) did not experience weight loss. The duration of follow-up ranged from 365 to 1095 days, with five of the eight studies having a follow-up of 365 days. The mean follow-up period was 513 days. Two studies did not mention the absolute amount or percentage of weight loss in their case groups [13, 14]. Three studies reported weight loss of ≥10% from baseline in their case groups, with a mean weight loss of 18.9% [10, 11, 15]. Three studies reported weight loss of <10% from baseline in their case groups, with a mean weight loss of 5.6% [12, 16, 17]. Inclusion and exclusion criteria and designs of each study are summarized in Supplementary Tables 1 and 2. Weight loss in each study is summarized in Table 2.
Six studies, labeled as “pre-ablation” in Table 1, took into account weight loss from the baseline to the day of ablation [10, 12,13,14,15, 17], while two studies, labeled as “post-ablation” considered weight loss from the day of ablation to the final day of follow-up [11, 16]. Recurrence was defined as atrial fibrillation lasting for at least 30 s after 3 months of blanking period. Modalities of monitoring primarily consisted of electrocardiogram in regular clinic visits and cardiac monitoring devices (7–30 days), and continuous monitoring with implantable loop recorders, but monitoring protocols varied in each study (Supplementary Table 3).
Studies of patients who lost weight demonstrated lower recurrence of AF (RR 0.35; 95% CI 0.18–0.67, p<0.01) at final follow-up in a random effects model (Fig. 2). The heterogeneity, however, was substantial with the Higgins I2 at 81%. Sensitivity analyses accounting for the magnitude of weight loss, duration of atrial fibrillation, and timing of the ablative therapy were successively conducted. The first sub-group analysis considering the magnitude of weight loss demonstrated that studies of patients who experienced ≥10% weight loss showed lower recurrence of AF (RR 0.18; 95% 0.03–0.89, p=0.04), which was not found in studies of those who underwent <10% weight loss (RR 1.00; 95% 0.51–1.96, p=0.99) (Fig. 3).
In a second sub-group analysis considering the duration of AF, studies of patients who had less than 12-month history of AF prior to ablation had lower risk of recurrence of AF (RR 0.24; 95% CI 0.11–0.57, p<0.01) with RR lower than that of all studies combined (Fig. 4). Only one study included patients with long-standing persistent AF (>12 months), so a comparative analysis was not possible, but this single study did not show any improvement in lowering AF recurrence [10].
A third sub-group analysis was performed evaluating the timing of weight loss relative to the time of ablation (Fig. 5). Studies of patients who underwent weight loss prior to ablation showed lower recurrence of AF (RR 0.40; 95% CI 0.20–0.79, p<0.01), whereas studies of patients who underwent weight loss after ablation did not reveal a statistically significant reduction in recurrence of AF (RR 0.15, 95% 0.01–3.60, p=0.24). There were only two studies included in the post-ablative weight loss group, however, and the heterogeneity was substantially high with Higgins I2 at 91%.
4 Discussion
The results of our meta-analysis show that in studies of AF patients, ≥10% weight loss and weight loss before ablation were associated with lower recurrence of AF post-ablation during 12-month follow-up. We also showed that lower recurrence of AF was consistently seen in the subset of studies including only subjects with less than 12-month history of AF prior to ablation. To the best of our knowledge, our study is the largest systematic review and meta-analysis done to date on this topic.
The association of obesity as a risk factor for AF was first described by Wang et al. in their prospective study of the Framingham cohort in 2004 [18]. Since then, multiple studies have validated the consistent relationship between obesity and AF [19,20,21,22]. Obesity has been implicated as a substrate in the initiation and maintenance of AF. The electro-structural remodeling seen in obesity, including increasing left atrial volume, conduction system alteration, and accelerated atrial fibrosis, has been associated with spontaneous and more persistent AF [23]. In a study involving ovine models, moderate to severe fatty infiltration of the posterior left atrial wall by epicardial fat was more prominent in obese animals. The obese animals were associated with additional episodes and prolonged duration of AF [24].
Given the role of obesity in AF, the impact of weight loss on AF course has been a topic of great interest. The landmark LEGACY and REVERSE-AF trials showed that weight loss was associated with reduction in AF recurrence as well as reversal of existing AF [11, 25]. Weight loss has also been shown to slow the progression of paroxysmal to persistent and eventually permanent AF [26]. The effect of weight loss on AF recurrence has been postulated to be multifactorial. Studies have shown that obese individuals have larger left atrial dimensions compared to non-obese counterparts [18]. In AF patients experiencing weight loss, relative reduction of left atrial volume has been described, suggesting that favorable left atrial remodeling is one of the mechanisms for reduced AF recurrence in these individuals [11].
Reduced long-term efficacy after catheter ablation in patients with AF has been potentially attributed to several cardiovascular risk factors including hypertension, heart failure, hyperlipidemia, obesity, and obstructive sleep apnea with obesity being an independent risk factor [27]. The mechanism of how each of these risk factors is associated with AF recurrence is beyond the scope of this study. However, the opposing effects of obesity and weight loss on each of these cardiovascular comorbidities is well documented in the literature [28].
Our subgroup analysis demonstrated that there was a significant reduction in recurrence of AF in studies of patients with less than 12-month history of AF. Although different studies have shown a conflicting relationship between AF duration and recurrence after catheter ablation, presence of persistent forms of AF has been shown to be a predictor of reduced procedural efficacy [29, 30]. This may be partly because of the effect of sustained episodes of AF on remodeling of the left atrium and common presence of other risk factors in these individuals [27]. The results of our study also showed that pre-ablative weight loss is associated with lower recurrence of AF in comparison to weight loss post-ablation. A plausible explanation is that losing weight prior to ablative therapy can ameliorate insulin sensitivity and glycemic control, lowering epicardial fat deposition and low-grade inflammation [31, 32]. Pre-ablative weight loss may also offset obesity-mediated structural remodeling of the atrium, optimizing outcomes of ablation [33]. Studies that implemented post-ablative weight loss each had RR below 1, but their sample sizes were smaller and only 2 studies were available, demonstrating the need for more data to better assess the impact of post-ablative weight loss.
Based on our findings, patients with obesity being considered for catheter ablation for AF may benefit from undergoing structured and goal-directed (≥10% body weight) weight loss therapy especially before ablative therapy. Such goals can be achieved by dietary modifications, exercise, or surgical intervention. Our results emphasize that initiation of weight loss regimens should strongly be considered prior to catheter ablation and as early as possible after AF diagnosis, in order to maximize freedom from AF and durability of outcomes after catheter ablation.
4.1 Limitations
This study is subject to several limitations. Only published data were included and individual patient-level data were unavailable. The variable protocol of included studies (retrospective versus prospective) may have impacted the quality of the meta-analysis. There were also significant heterogenicities among the selected studies. However, random effects models were used to mitigate the effects of heterogenicity on the results of pooled analyses that remained significant between study groups. This study did not compare the effect of different methods of weight loss, rapidity of weight loss, ablation techniques, and the usage of anti-arrhythmic drugs that may have had an impact on AF recurrence. How the comorbidities changed after weight loss was not reported by the studies. The meta-analysis only included a relatively small number of studies that were presently available in the literature. Randomized clinical trials are required to prospectively assess the impact of weight loss on outcomes after AF ablation more accurately.
5 Conclusion
Weight loss was associated with lower long-term recurrence of AF after ablative therapy. Studies of patients with ≥10% weight loss, less than 12-month history of AF, and weight loss prior to ablative therapy were associated with lower recurrence of AF on follow-up. More randomized controlled trials are required to better evaluate the impact of weight loss on recurrence of AF after ablative therapy.
References
Krijthe BP, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–51.
Piccini JP, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93.
Chugh SS, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–47.
Colilla S, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–7.
Di Biase L, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637–44.
Brachmann J, et al. Atrial fibrillation burden and clinical outcomes in heart failure: The CASTLE-AF Trial. JACC Clin Electrophysiol. 2021;7(5):594–603.
Kirchhof P, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–16.
Aldaas OM, et al. Meta-analysis of effect of modest (>/=10%) weight loss in management of overweight and obese patients with atrial fibrillation. Am J Cardiol. 2019;124(10):1568–74.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71.
Mohanty S, et al. Impact of weight loss on ablation outcome in obese patients with longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29(2):246–53.
Pathak RK, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64(21):2222–31.
Yew Ding W, et al. Feasibility of weight loss in obese atrial fibrillation patients attending a specialist arrhythmia clinic and its impact on ablation outcomes. J Arrhythm. 2020;36(6):984–90.
Donnellan E, et al. Impact of risk-factor modification on arrhythmia recurrence among morbidly obese patients undergoing atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2020;31(8):1979–86.
Peigh G, et al. Impact of pre-ablation weight loss on the success of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32(8):2097–104.
Donnellan E, et al. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace. 2019;21(10):1476–83.
Gessler N, et al. Supervised obesity reduction trial for AF ablation patients: results from the SORT-AF trial. Europace. 2021.
Yaeger A, et al. Impact of a nurse-led limited risk factor modification program on arrhythmia outcomes in patients with atrial fibrillation undergoing catheter ablation. J Cardiovasc Electrophysiol. 2020;31(2):423–31.
Wang TJ, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–7.
Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118(5):489–95.
Foy AJ, et al. Relation of obesity to new-onset atrial fibrillation and atrial flutter in adults. Am J Cardiol. 2018;121(9):1072–5.
Berkovitch A, et al. Body mass index and the risk of new-onset atrial fibrillation in middle-aged adults. Am Heart J. 2016;173:41–8.
Lee H, et al. Atrial fibrillation risk in metabolically healthy obesity: a nationwide population-based study. Int J Cardiol. 2017;240:221–7.
Abed HS, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10(1):90–100.
Mahajan R, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66(1):1–11.
Middeldorp ME, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on atrial fibrillation: the REVERSE-AF study. Europace. 2018;20(12):1929–35.
Abed HS, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. Jama. 2013;310(19):2050–60.
Al-Kaisey AM, Parameswaran R, Kalman JM. Atrial fibrillation structural substrates: aetiology, identification and implications. Arrhythm Electrophysiol Rev. 2020;9(3):113–20.
Powell-Wiley TM, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984–e1010.
Balk EM, et al. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. 2010;21(11):1208–16.
Sultan A, et al. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German Ablation Registry. Sci Rep. 2017;7(1):16678.
Clamp LD, et al. Enhanced insulin sensitivity in successful, long-term weight loss maintainers compared with matched controls with no weight loss history. Nutr Diabetes. 2017;7(6):e282.
Tsalamandris S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14(1):50–9.
Nalliah CJ, et al. The role of obesity in atrial fibrillation. Eur Heart J. 2015;37(20):1565–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was exempt from ethics approval as only data from previously published studies were retrieved and synthesized.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 45 kb)
Rights and permissions
About this article
Cite this article
Park, D.Y., An, S., Murthi, M. et al. Effect of weight loss on recurrence of atrial fibrillation after ablative therapy: a systematic review and meta-analysis. J Interv Card Electrophysiol 64, 763–771 (2022). https://doi.org/10.1007/s10840-022-01168-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01168-2