Abstract
The sinoatrial node, or sinus node, of humans is the principal pacemaker of the heart. Over the last century, studies have unraveled the complex molecular architecture of the sinus node and the expression of unique ion channels within its specialized myocytes. Aim of this review is to describe the embriology, the anatomy, the histology and the electrophisiology of the sinus node.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The sinoatrial node, or sinus node, of humans is the principal pacemaker of the heart. It has unique cyclical electrical activity, which initiates each beat of sinus rhythm. Over the last century, studies have unraveled the complex molecular architecture of the sinus node and the expression of unique ion channels within its specialized myocytes. The resulting interplay of anatomy and physiology is critical for the generation of each sinus node action potential, ultimately assigning the sinus node its pacemaker function. Sinus node dysfunction due to ion channel dysfunction and/or structural remodeling can occur in clinical scenarios such as heart failure, atrial fibrillation, aging, and intense athletic training, resulting in a variety of pathological syndromes.
2 Historical perspectives
The sinus node was first described in 1907 by Sir Arthur Keith when his seminal paper “Sino-auricular junction in human heart” was published [1]. His studies, undertaken in order to elucidate mechanisms of great vein closure during atrial systole while working with medical student Martin Flack, consistently revealed an interesting structure, “a small condensed area of tissue, just where the cava sinks into the right auricle.” This superior right atrial structure was present in all the subsequent hearts they studied. The two investigators proposed the region to be of functional importance, containing myocardial tissue critical to initiating a cardiac stimulus. This finding was confirmed by subsequent investigators [2]. Since the original findings by Keith and Flack, the structure has been known as the sinoatrial node, the primary pacemaker of the heart.
3 Anatomy of the sinus node
3.1 Sinus node embyrogenesis
As the heart tube forms early in embryogenesis, mesodermal cells multiply rapidly and differentiate into cardiomyocytes capable of contraction and fast conduction. Cardiac conduction system myocytes, including those comprising the sinus node, develop to form a continuity of specialized cells that direct electrical impulses through the myocardium, allowing coordinated atrial and ventricular contraction. The sinus node develops from an area within the sinus venous of the early heart tube and is the most superior structure in the cardiac conduction system.
Embryonic development of the specialized cardiac conduction system is regulated by key gene transcription factors, directing cells down a lineage differing from surrounding contractile myocardium, with expression of specialized ion channels conferring unique electrical properties [3]. The transcription factor Tbx3, is a T-box transcription factor found exclusively in the cardiac conduction system. Tbx3 may be important in the spatial expression of genes encoding particular membrane ion channels, since Tbx3 knockout mice have a diminished field of ion channel expression. These include HCN, which encodes a hyperpolarization-activated cAMP-gated channel, and Cx45 encoding a low conduction gap junction connexin. Interestingly, Tbx3 distribution may define the extent of the sinus node, with expression found surrounding the superior vena cava/right atrial junction, along the crista terminalis, and inferiorly toward the inferior vena cava and AV node [4]. Other sinus node transcription factors include Shox2 and Tbx18, both expressed in the developing sinus venosus. Shox2 indirectly promotes Tbx3, mediated by ISl-1 activation, while Tbx18 directs sinus venosus cells to develop into sinus node core cells. There is an interaction between transcription factors found in pacemaker tissue and those found in contractile myocardium such that pacemaker cells do not develop in contractile myocardium and vice versa.
3.2 Anatomical location and extent
Early depictions of the sinus node demonstrated it to be a relatively limited structure localized to the crest of the right atrial appendage, at the lateral superior vena cava (SVC)/right atrium (RA) junction [5]. More recent studies however in rabbit, canine, and later in human hearts, revealed the node to be a more diffuse, extensive, and elaborate structure, extending from the SVC/RA junction down the inferolateral aspect of the crista terminalis toward the inferior vena cana (IVC)/RA orifice [6, 7].
The human sinus node is a “crescent” or “tadpole” shaped structure. It has a wide body and is typically 10 to 20 mm in length. The body is situated cranially near the SVC orifice with a thinner tapering tail portion extending inferiorly below the body toward the inferior crista terminalis and Eustachian ridge [8]. The tail may vary from 8 to 21.5 mm in length. The body is primarily a subepicardial structure lying 0.1–1.0 mm subepicardially within the fatty tissue of the sulcus terminalis, with a long axis parallel to it. The body is closely opposed to the crista terminalis. Sinus node tissue gradually penetrates intramyocardially toward the subendocardium as the node extends inferiorly. The tail portion is thus closer to the subendocardium [6]. The sinus node is usually contained in an area that can be defined by the intersection of the sulcus terminalis, the lateral border of the SVC, and the superior border of the right atrium. A variant of sinus node anatomy is found in 10 % of human hearts, with the node situated anteriorly and extending across the crest of the right atrial appendage in a horseshoe shaped arrangement, within the inter-atrial groove [9] (Fig. 1).
3.3 Sinus node histology
Histologically, the sinus node is seen as a dense aggregation of specialized cells within a highly fibrous connective tissue matrix [9]. Sinus node myocytes are small interlacing cells, less darkly stained due to their poorly developed microfilament cytoskeleton, with an appearance distinct from neighboring atrial myocytes [10] (Fig. 2). The smallest primitive pacemaker cells are concentrated in the center of the node, with larger transitional pacemaker cells located peripherally. In the tail of the sinus node marked fragmentation is seen, with islands of nodal tissue scattered in a mix of subendocardial fibrofatty tissue and atrial myocytes.
Longitudinal sections through the cavoatrial junction, showing the variations in structure of the sinus node (within yellow dots) in four cases. The stain (Masson’s trichrome) colors the fibrous tissue green and the myocardium red. a The normal sinus node from an infant is relatively large and has abundant specialized myocytes. b In contrast, the sinus node is absent and the area replaced with fibrous tissue, from an infant with congenital heart block. c The sinus node is relatively small in this section from a 16 years old. d A normal sinus node from an adult showing increased fibrous tissue and nodal extensions. The superior extension (arrow) penetrates into the musculature of the superior vena cava
The node has a indistinct and irregular margin with the surrounding atrial myocardium. This area has been termed the “paranodal” region or “transitional zone,” comprised of loosely packed nodal and atrial myocytes, and “transitional” cells [11]. Transitional cells have histological and electrophysiological characteristics intermediate between sinus node cells and atrial myocytes. Radiations of transitional cells extend inward to the node interior and outward into the atrial myocardium, penetrating the crista terminalis in all directions, with some radiations extending into the muscular sleeve of the SVC [6] (Fig. 2). In the outer regions of the transitional zone, a gradual shift in the ratio of nodal to atrial cells is seen with a shift from nodal to atrial cell morphology. Connective tissue and fat is present around the sinus node, except where exit pathways are functionally located.
3.4 Sinus node electroanatomic coupling
The sinus node is a relatively small amount of tissue and must be electrically insulated from the large amount of hyperpolarized atrial muscle surrounding it in order to be a dominant and robust pacemaking source. It needs a failsafe way of conducting its pacemaker activity into the surrounding right atrium without being suppressed or becoming a re-entry source. This is achieved by the presence of the transitional zone, with mosaic features at the node periphery, creating complex functional and possibly anatomical block zones and exit pathways. These mosaic features may help to gradually match nodal activity with that of the right atrium and promote a gradient favoring an antegrade direction of conduction [12]. The gradation of cellular electrophysiological properties from the sinus node to right atrial myocardium is achieved by cell coupling, electrotonic interaction, and differing intrinsic properties. Mapping of electrograms in and around the node has disclosed a multicentric initiation of activation with irregular propagation to the atrial myocardium. Thus, the functional purpose of the transitional zone may be to facilitate the exit of the action potential from the node into the atrial muscle. Transitional cells are more polarized than nodal cells, with stable resting potentials close to −80 mV and faster upstroke velocities. Their pacemaking capabilities are muted by the electrotonic influence of surrounding atrial cells. More remotely connected interior nodal cells are shielded from atrial myocytes, allowing them to maintain pacemaker dominance [13]. Cells in other parts of the atrium can act as backup or subsidiary pacemakers, particularly in the inferior portions of the node near the coronary sinus. These latent pacemaker cells can respond to autonomic input and dominate heart rate control under abnormal conditions.
Cells within the sinus node are relatively poorly coupled by gap junctions, and substantial interstitial tissue is interspersed among fascicles of nodal cells [14]. This results in relatively poor intercellular communication, which slows impulse propagation from the central pacemaking region to the node periphery. The nodal wavefront travels along the radiations into atrial myocardium with nonpreferential conduction and the potential for multiple breakthroughs. On the other hand, optical mapping has demonstrated preferential narrow exit pathways at superior, middle, and inferior levels of the node, where impulses are propagated into surrounding atrial muscle along the crista terminalis [15]. Nodal conduction preferentially exits the node in the direction of the atrial septum. Narrow exit sites have physiological advantages, particularly electrical insulation from the surrounding hyperpolarizing influence of atrial muscle. There are also disadvantages however, since a small number of narrow exit pathways carrying the propagating nodal wavefront encounters a much larger mass of atrial myocardium. This source-sink mismatch reduces the safety factor for conduction. Studies to identify sinus node exit sites suggest that although they appear to be discrete, they are in fact a functional rather than an anatomical phenomenon [6]. Despite the presence of some “Purkinje-like” cells in the atrium, there is to date no evidence for sinoatrial nodal preferential conduction through an atrial conduction system as seen in the ventricle.
Molecular markers have mapped the extent of the paranodal area by defining the tissue density of gap junctions [12]. Gap junctions are non-specific ion channels connecting neighbouring myocytes, resulting in electrical coupling between cardiac myocytes and the propagation of the cardiac action potential. Gap junctions are comprised of connexins (Cx). Cx43 is abundantly expressed in working myocardium, where it forms relatively large conductance gap junctions. It is responsible for electrical coupling between myocytes, and the high conduction velocity of the action potential [16]. Although it is widely expressed in working atrial myocardium, Cx43 is not expressed in the center of the sinus node. Cx45 is a connexin expressed in the sinus node centre, where it forms small low conductance gap junctions [16]. Central sinus node cells are thus poorly electrically coupled, and action potential conduction velocity is slow. The role of Cx45 may be in part to electrically insulate the central node from surrounding atrial muscle, which could otherwise enter the node and suppress its pacemaker activity. Toward the periphery of the sinus node there is coexpression of Cx43 and Cx45. Here, electrical coupling improves between sinus node and atrial myocytes. Along with interdigitation between the two cell types, this connexin coexpression may facilitate the propagation of the action potential from the sinus node into atrial muscle [12, 16].
Gap junction dysfunction significantly impacts normal sinus node pacemaker physiology and function. Fibroblasts within the node may also influence pacemaker function and conduction [17]. Through gap junctions, inexcitable fibroblasts can couple with sinus node cells and result in relative depolarization. This impairs conduction and reduces the rate of pacemaking. Conditions that cause loss of sinus node myocytes accompanied by an increase in fibroblasts, collagen, and elastin can damage functional exit sites, leading to sinus node exit block, bradycardia, syncope, and sudden cardiac death [18, 19].
3.5 Sinus impulse generation site
The first site of sinus node activation typically originates superiorly. This “leading pacemaker” site is highly variable, however, and is not limited to a single anatomical area. In response to physiological stimuli, it can shift position in the node to become more inferior or even multifocal. This phenomenon is known as “pacemaker shift” [20]. A hierarchy of pacemaker cell firing rates underlies this response. Cells that depolarize at a higher frequency generating faster heart rates are located superiorly, with slower firing cells situated inferiorly. The hierarchy mediates heart rate changes via cranio-caudal shifts in the leading pacemaker site. Sympathetic stimulation shifts the leading pacemaker site superiorly resulting in an increase in heart rate. Parasympathetic activity results in an inferior shift and heart rate slowing. Since tissue with pacemaking capability can be widely spread in the right atrium, it is possible to have a low leading pacemaker site outside the anatomical sinus node. For example, the paranodal area can extend inferiorly as far as the IVC os.
Pacemaker shifts are responsible for the commonly observed variations in P wave morphology and polarity. A dynamic cranio-caudal shift in pacemaker site occurs with a change in heart rate. Sinus node mapping has revealed the changes in P wave morphology that accompany pacemaker shift, which can be identified on a 12-lead ECG. A leading pacemaker site near the SVC has an inferiorly directed vector of atrial activation with a positive P wave in lead aVF. If the leading pacemaker is an inferior site near the IVC, there is a superior vector of atrial activation, resulting in a negative P wave in lead aVF. Negative P waves are associated with a longer R-R interval and slower heart rate. Positive P waves are associated with a shorter R-R interval, reflecting the change in the leading pacemaker site from an inferior to a superior location. During sleep, there can be a spontaneous changes in the polarity of the P wave vector accompanied by changes in the heart rate, due to cranio-caudal shift [7, 21]. Around 1 % of individuals have a negative P wave in the inferior ECG leads, indicating a low leading pacemaker site. The pacemaker shift phenomenon demonstrates that the sinus node is a heterogeneous structure both anatomically and physiologically. “Latent” pacemakers refer to “backup” pacemaker cells concentrated peripherally in the node. Impulse generation from these sites will also produce a change in P wave morphology. As these sites are more polarized than node center, they are not the leading pacemakers under normal conditions.
3.6 Arterial supply
The sinus node is supplied by the relatively large sinoatrial nodal artery [8]. In around 60–70 % of hearts, this artery arises from the right coronary artery as the first atrial artery, ascending in the interatrial groove [22]. Typically, the artery travels in an anterior direction to the node, but can also approach from a posterior direction, or an arterial circle formed around the SVC atrial junction. In 20–30 % of hearts, the artery arises from the left coronary system, typically the circumflex artery, as a branch of the left anterior atrial or left lateral atrial artery. Left-sided arteries traverse the anterior left atrium and then the right atrium superiorly, to reach the sinus node (Fig. 3). Less common variants may include blood supply from both the right and left coronary systems, or from the lateral atrial artery ascending in the lateral wall of the right atrial appendage. In most hearts, a prominent nodal artery passes centrally through the nodal body, giving rise to small side branches as it penetrates. Less frequently, the nodal artery branches before it reaches the node, with several of these branches penetrating the nodal body. Rarely, the node is supplied by an arterial circle, where arteries enter the node at both ends.
3.7 Nerve supply—autonomic innervation
The sinus node is the most densely innervated component of the cardiac conduction system [23]. It is richly innervated by the autonomic nervous system, which plays a crucial role in the regulation of sinus rate, by modulating ionic currents. The dense distribution of sympathetic and parasympathetic nerves and ganglia results in very sensitive autonomic responsiveness [24]. Parasympathetic fibers innervate the node via the tenth cranial or vagus nerve. The epicardium and surrounding tissue overlying the node contains clusters of ganglion cells containing vagal postganglionated nerve fibers [23]. The sinus node receives sympathetic innervation via fibers of the T1-4 spinal nerves. The result is nodal regulation of heart rate by paired yet opposed autonomic influences. Vagal parasympathetic discharge predominates at rest with acetylcholine and nitric oxide release resulting in slowing of the sinus rate. Sympathetic discharge predominates with exercise, with adrenergic stimulation leading to an increase in the sinus rate. The autonomic balance between sympathetic and parasympathetic input determines a given heart rate within the hierarchy of nodal pacemaker cells. Without any autonomic input, as demonstrated in denervated hearts, the heart rate is typically around 100 bpm.
Sympathetic heart rate effects are substantially less with high levels of vagal tone than with low vagal background activity. With increasing background sympathetic activation, vagal parasympathetic activation overrides sympathetic activation, and becomes progressively stronger. This demonstrates the predominance of parasympathetic control of the heart rate, and this complex interaction between the two autonomic influences is termed “accentuated antagonism” [25]. With increasing vagal influence, the primary pacemaker side migrates inferiorly in the node. Increasing adrenergic influence shifts the dominant site of activation to the superior aspect of the node. Parasympathetic and sympathetic limbs of the autonomic nervous system are regulated and interact at multiple levels within the central nervous system and at peripheral nerve terminals.
3.7.1 Parasympathetic activation
Parasympathetic regulation of resting sinus rate is mediated by the acetylcholine-activated, G protein-coupled, inwardly rectifying potassium channel K+ current IKAch, which is carried through Kir3.1 and Kir 3.4 channels. This atrial current is of larger amplitude in the sinus node than surrounding atrial cells. Following neuronal release of acetylcholine (ACh), it binds to the muscarinic M2 receptor. This triggers activation of a transducing G protein in the cell membrane with the exchange of GTP for GDP on its alpha subunit. The G protein beta/gamma subunit is then released and binds to the K+ channel, which opens, with an increase in K+ permeability. This outward K+ current, IKAch, hyperpolarizes the cell membrane and reduces the rate of diastolic depolarization and frequency of cellular discharge, resulting in slowing of the sinus rate. M2 receptor activation by Ach also closes the If (“funny current”) channels and T-type and L-type calcium channels, by activation of the inhibitory Gi protein. This decreases the frequency of action potentials and slows the heart rate. If may be even more sensitive to acetylcholine than the IKAch current. At autonomic nerve terminals, activation of prejunctional M2 receptors can inhibit norepinephrine release. This may be one mechanism by which vagal stimulation overrides sympathetic stimulation of the heart.
3.7.2 Sympathetic activation
Sympathetic activation occurs when the neurotransmitter norepinephrine, and the circulating hormone epinephrine, bind to and stimulate β-1 and β-2 adrenoceptors in the sinus node, both of which are important chronotropic mediators of adrenergic stimuli. Agonist binding activates a cAMP second messenger system. In the sinus node, cAMP augments the opening of If channels and T-type Ca2+ channels, resulting in an increase in the slope of spontaneous depolarization. The frequency of sinus node action potentials increases, with a concomitant increase in heart rate.
4 Electrophysiology of the sinus node
4.1 Action potential generation
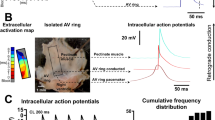
The action potential of the sinus node is very different from the action potential of contractile myocardium. Sinus node cells exhibit spontaneous diastolic depolarization, resulting in a positive “pacemaker potential,” which triggers an action potential when a threshold potential is reached [26, 27]. The smallest primitive pacemaker cells in the sinus node interior generate the dominant pacemaker potential. These cells have the least polarized maximum diastolic potentials in the range of −50 to −60 mV, the most rapid rates of diastolic depolarization, and the slowest upstroke velocities [28, 29] (Fig. 4). Latent pacemakers are concentrated more peripherally in the node. These cells are more polarized than the central sinus node, with less rapid diastolic depolarization, more abrupt action potential transition from diastole to upstroke, and more rapid upstrokes.
Samples of action potentials (APs) recorded from different regions of the sinus node (SAN) from the center (highest black point within blue region of central SAN) to the periphery of the node (lowest black point outside blue region, in SAN periphery). On the left is a schematic of the right atrium, with the SAN region shown in blue. The corresponding action potentials from each area with their maximal diastolic potential, and threshold potential, are shown on the right. Tissue nearer to the SAN center generates APs with the least negative resting potential resulting in a site of dominant pacemaking activity. Areas in the SAN periphery have a more negative resting potential. A healthy SAN cell has a maximal diastolic potential of −50 to −60 mV
The depolarizing pacemaker potential is the sum of individual ionic currents, each flowing through a unique ion channel [29]. The result is an increase in inward current and a decaying outward current. The inward ionic current is due to the flow of positively charged cations across the cell membrane with a positive shift in the membrane potential. Subsequent repolarization occurs with flow of positively charged cations out of the cell, yielding an outward ionic current and negative shift in the membrane potential [26, 27]. The operational channels in sinus node myocytes have been revealed by experiments recording ionic currents with the voltage clamp technique, by individual pharmacological blockade, and in studies using transgenic knockout animals. Contemporary molecular biological techniques have further characterized the location and function of the different ion channels in the sinus node and the role of each in pacemaker electrophysiology.
4.2 Action potential currents
Diastolic depolarization during phase 4 of the sinus node action potential is the fundamental pacemaker potential and underlies sinus node automaticity [30]. It brings the membrane potential to a triggering threshold, thereby initiating the next heart beat. The interaction of several individual membrane currents and intracellular currents, some under autonomic influence, execute this depolarization. In general terms, there are five main ionic currents which make up two key ionic systems, the membrane current or “membrane clock,” and the “calcium clock.” These two systems act synergistically in a coupled fashion to produce the sinus node action potential [31].
The membrane clock is composed of time- and voltage-dependent decay of the outward rectifier potassium current IK, and voltage-dependent activation of at least three inward currents: the If “funny” current, the ICaL L-type Ca2+ current, and the ICaT T-type Ca2+ current.
The calcium clock or INaCa current is initiated by a Ca2+ handling mechanism, which is inherently linked with the membrane clock and has an equally important contribution to automaticity [32]. It involves ryanodine receptor-mediated calcium release from the sarcoplasmic reticulum resulting in sarcolemmal Na+/Ca2+ exchange and has an important role in regulating sinus rate.
The key ion channels and their temporal currents involved in producing the sinus node pacemaker potential are depicted in Fig. 5 [33].
Ionic currents involved in producing the sinus node pacemaker potential in a sinus node myocyte. A typical action potential of spontaneously beating rabbit sinus node is shown on the top (red trace). The different phases are labeled, with phase 4 representing diastolic depolarization, the defining feature of pacemaking cells. The timing and magnitude of the components of the “membrane clock” is shown in the middle (green bracket). There is voltage-dependent decay of the outward rectifier K+ current IK, and voltage-dependent activation of inward currents: If, ICaL, and ICaT. The timing and magnitude of the components of the “Ca2+clock” are shown at the bottom (dark blue bracket). Ca2+ entry into the cell via ICaL and ICaT results in spontaneous local Ca2+release (LCRs) from the sarcoplasmic reticulum though RyR2 channels. During phase 4, this rise in total intracellular calcium activates the NaCa exchanger NCX1 which generates the net inward INCX (or INaCa) current. Toward the end of diastole, activation of L-type Ca2+ channels causes Ca2+ induced Ca2+ release from the SR via ryanodine receptors, resulting in the whole cell Ca2+ transient. Cytoplasmic Ca2+ is then removed by the SR Ca2+ pump SERCA, and by the sarcolemmal sodium-calcium exchanger. MDP maximum diastolic potential, DD diastolic depolarization, I CaT T-type voltage-dependent Ca2+ current, I CaL L-type voltage-dependent Ca2+ current, I NCX sodium-calcium exchange current, I K delayed rectifier potassium current, I f funny current, SR sarcoplasmic reticulum, SERCA sarco-endoplasmic reticulum ATPase, LCRs local Ca2+ releases
4.2.1 Potassium currents
The stable negative resting potential of working myocardium is generated by the repolarizing outward potassium currents IK1 and IK. IK1 is an outward current flowing through Kir 2.1 channels and is known as the “inward rectifier K+ current.” IK1 maintains the stable negative resting membrane potential close to the K+ equilibrium potential of −90 mV since K+ permeability dominates in the resting state. IK1 is abundant in the ventricles but scarce in the atria. IK1 is absent in the sinus node and IK is diminished, allowing the membrane potential to drift upward in early diastole [12]. This renders the sinus node cell membrane potential labile with no stable negative resting potential, allowing opposing inward currents, particularly If, but also INaCa, to depolarize nodal myocytes [34]. Early diastolic depolarization results, with pacemaker cells operating at diastolic potentials between −60 and −30 mV [35].
IK is the delayed rectifier current and has rapid and slow components, IKr and IKs, carried by the K+ channels, ERG and KvLQT1, respectively. IKr and IKs are activated during the action potential and are responsible for repolarization of the myocyte at the end of the action potential by increasing K+ permeability, thereby returning the trans-membrane potential close to the K+ equilibrium potential. In the sinus node, however, the rectifier potassium current deactivates and decays after the action potential is complete [36]. As a result, in diastole, the membrane potential drifts toward the more positive equilibrium potentials of other ions Na+, Ca2+, and Cl−, allowing these inward currents to depolarize the sinus node myocyte. IK decay contributes much of the earliest part of the pacemaker potential.
4.2.2 Sodium current
The inward Na+ current INa is abundant in working myocardium and is carried by the Nav1.5 channel encoded by the SCN5A gene. It is responsible for the fast upstroke of the action potential in working myocardium but is mostly absent in the center of the sinus node center, explaining the slower upstroke of the pacemaker action potential [37]. Transitional cells at the sinus node periphery may contain some Nav1.5 channels, albeit at low levels, providing some excitatory Na+ current in this region, and explaining the more rapid upstrokes in the node periphery than in the center [37].
4.2.3 The funny current If
The funny current If is nonspecific mixed inward cation current carried by Na+ and K+ ions [38]. This positive current is a major contributor to early diastolic depolarization [39]. If passes through membrane bound channels encoded by the gene HCN4 [34]. This gene is highly expressed in central sinus node cells, and to a lesser degree, in peripheral latent pacemakers. If is specifically activated at hyperpolarized membrane potentials and is slowly activated early in phase 4 diastole when the cell is hyperpolarized at its most negative membrane potential. There are four isoforms of the gene HCN. HCN1 and HCN4 are the predominant cardiac isoforms in the sinus node. These two genes are expressed in abundance in the node but absent in working atrial myocardium, suggesting the importance of If in pacemaking [40]. Selective blockers of If such as ivabradine have been shown to slow the sinus rate in human subjects by decreasing the slope of the pacemaker potential [34, 41].
If ion channels are voltage gated, being hyperpolarization-activated at membrane potentials below −60 mV. If channels are also cyclic-nucleotide gated. Cyclic AMP signaling modulates their electrophysiological properties rendering them responsive to adrenergic and cholinergic stimulation [42]. HCN channels and If are thus pivotal in the autonomic regulation of heart rate. Following sympathetic stimulation, β1-adrenoceptor activation of adenylate cyclase increases intracellular cAMP. This binds to and increases opening of the HCN channel with an increase in If current. The result is a rise in heart rate. Vagal stimulation reduces cAMP and the If current, resulting in slowing of the sinus rate. Block of If increases heart rate variability, indicating that If may be important in heart rate stabilization [43]. This is important since decreased heart rate variability is associated with an increased risk of arrhythmia and sudden cardiac death [44].
4.2.4 Calcium currents
Voltage-gated calcium channels are activated by the rising membrane potential late during the pacemaker potential, producing two excitatory calcium currents; the T-type current ICaT and the L-type current ICaL [45, 46]. ICaT has an activation threshold more negative than −40 mV and is therefore activated earlier in diastolic depolarization than ICaL. ICaT flows through Cav3.1 and Cav3.2, which are found in abundance in cardiac myocytes displaying automaticity, including sinus node myocytes.
ICaL is responsible for the slow action potential upstroke in the sinus node. It activates late due to its activation threshold being more positive than −40 mV. At threshold potential, phase 0 of the action potential is triggered and L-type voltage-gated Ca2+ channels open, allowing large-scale depolarization of the cell via Ca2+ influx. The current is slower and much lower amplitude than the sodium current of working myocardium, resulting in a slow action potential upstroke and slow conduction within the sinus node. ICaL is the trigger for Ca2+ release by the sarcoplasmic reticulum and is therefore the trigger for contraction in all cardiac cells. ICaL in node flows through the Ca2+ channel, Cav1.3. ICaL responds to autonomic influences.
4.2.5 The calcium clock INaCa exchange current
In the second part of diastolic depolarization, or phase 4, of the sinus node action potential, calcium is spontaneously released from the sarcoplasmic reticulum (SR) into the cytosol. These “sparks” or local Ca2+ releases leave the SR via ryanodine receptor calcium channels (RYRs). The resulting rise in intracellular calcium concentration activates the Na+Ca2+ exchanger cell membrane pump (NCX) [47]. NCX exchanges one intracellular Ca2+ ion for three extracellular Na+ ions, generating the net positive inward Na+Ca2+ exchange current INaCa (also known as INCX). Activation of INaCa in late diastole is responsible for the final exponential phase of the pacemaker potential.
In a process known as “Ca2+-induced Ca2+ release,” calcium extrusion from the sarcoplasmic reticulum via RYR increases in response to Ca2+ entry into the cell. Cellular entry of Ca2+ via ICaL and ICaT is activated by the rising membrane potential initiated by the membrane clock [31]. The result of these processes is whole-cell Ca2+ sweeping across the cell, with myofilament contraction.
The combination of the above intracellular Ca2+ cycling processes, in the late stage of depolarization of sinus node cells, is known as the calcium clock [32]. The clock is driven by high levels of phosphorylation of Ca2+-cycling proteins and is modulated by adrenergic and cholinergic stimulation. Regulation by these cAMP-driven pathways of the autonomic nervous system gives the calcium clock an important role in sinus rate determination [32].
Sarcoplasmic reticulum calcium is replenished by reuptake of Ca2+ via sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), the speed of which is enhanced by adrenergic and slowed by cholinergic receptor activation. Store-operated Ca2+ channels at the cell surface membrane also replenish sarcoplasmic reticulum calcium. These channels may set the frequency of the Ca2+ clock by regulating the amount of Ca2+ within the sarcoplasmic reticulum [48].
References
Keith, A., & Flack, M. (1907). The form and nature of the muscular connections between the primary divisions of the vertebrate heart. Journal of Anatomy and Physiology, 41, 172–189.
Lewis, T. (1910). Galvanometric curves yielded by cardiac beats generated in various areas of the auricular musculature: the pacemaker of the heart. Heart, 2, 23–46.
Hoogaars, W. M., Engel, A., Brons, J. F., Verkerk, A. O., De Lange, F. J., Wong, L. Y., Bakker, M. L., et al. (2007). Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes and Development, 21, 1098–1112.
Hoogaars, W. M., Tessari, A., Moorman, A. F., De Boer, P. A., Hagoort, J., Soufan, A. T., Campione, M., et al. (2004). The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovascular Research, 62, 489–499.
Truex, R. C., Smythe, M. Q., & Taylor, M. J. (1967). Reconstruction of the human sinoatrial node. Anatomical Record, 159, 371–378.
Sanchez-Quintana, D., Cabrera, J. A., Farre, J., Climent, V., Anderson, R. H., & Ho, S. Y. (2007). Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart, 91, 189–194.
Lee, R. J., Kalman, J. M., Fitzpatrick, A. P., Epstein, L. M., Fisher, W. G., Olgin, J. E., Lesh, M. D., et al. (1995). Radiofrequency catheter modification of the sinus node for “inappropriate” sinus tachycardia. Circulation, 92, 2919–2928.
Anderson, K. R., Ho, S. Y., & Anderson, R. H. (1979). Location and vascular supply of sinus node in human heart. British Heart Journal, 41, 28–32.
Hudson, R. E. B. (1960). The human pacemaker and its pathology. British Heart Journal, 22, 153–156.
James, T. N., Sherf, L., Fine, G., & Morales, A. R. (1966). Comparative ultrastructure of the sinus node in man and dog. Circulation, 34, 139–163.
Anderson, R. H., & Ho, S. Y. (1998). The architecture of the sinus node, the atrioventricular conduction axis, and the internal atrial myocardium. Journal of Cardiovascular Electrophysiology, 9, 1233–1248.
Chandler, N. J., Greener, I. D., Tellez, J. O., Inada, S., Musa, H., Molenaar, P., Difrancesco, D., et al. (2009). Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation, 119, 1562–1575.
Watanabe, E., Honjo, H., Anno, T., et al. (1995). Modulation of pacemaker activity of sinoatrial node cells by electrical load imposed by an atrial cell model. American Journal of Physiology, 269, H1735–H1742.
Anumonwo, J. M. B., Jalife, J., Zipes, D. P. (1995). Cellular and subcellular mechanisms of pacemaker activity initiation and synchronization in the heart. Cardiac electrophysiology: from cell to bedside. Philadelphia: Saunders.
Fedorov, V. V., Schussler, R. B., Hemphill, M., et al. (2009). Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circulation Research, 104, 915–923.
Boyett, M. R., Inada, S., Yoo, S., Li, J., Liu, J., Tellez, J., Greener, I. D., et al. (2006). Connexins in the sinoatrial and atrioventricular nodes. Advances in Cardiology, 42, 175–197.
Fahrenback, J., Mejia-Alvarez, R., & Banach, K. (2007). The relevance of non-excitable cells for cardiac pacemaker function. Journal of Physiology, 585(2), 565–578.
Alings, A. M., Abbas, R. F., & Bouman, L. N. (1993). Age-related changes in structure and relative collagen content of the human and feline sinoatrial node. A comparative study. European Heart Journal, 14, 1278–1288.
Demoulin, J. C., & Kulbertus, H. E. (1978). Histopathological correlates of sinoatrial disease. British Heart Journal, 40, 1384–1389.
Schuessler, R. B., Boineau, J. P., & Bromberg, B. I. (1996). Origin of the sinus impulse. Journal of Cardiovascular Electrophysiology, 7, 263–274.
Dilaveris, P. E., Farborn, P., Batchvarov, V., Ghuran, A., & Malik, M. (2001). Circadian behavior of P-wave duration, P-wave area and PR interval in healthy subjects. Annals of Noninvasive Electrocardiology, 6, 92–97.
Busquet, J., Fontan, F., Anderson, R. H., et al. (1984). The surgical significance of the atrial branches of the coronary arteries. International Journal of Cardiology, 6, 223–234.
Crick, S. J., Sheppard, M. N., Ho, S. Y., & Anderson, R. H. (1999). Localisation and quantitation of autonomic innervation in the porcine heart I: conduction system. Journal of Anatomy, 195, 341–357.
Crick, S. J., Wharton, J., Sheppard, M. N., et al. (1994). Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation, 89, 1697–1708.
Uijtdehaage, S. H., & Thayer, J. F. (2000). Accentuated antagonism in the control of human heart rate. Clinical Autonomic Research, 10(3), 107–110.
Robinson, R. B., & Difrancesco, D. (2001). Sinoatrial node and impulse initiation. In P. Spooner & M. Rosen (Eds.), Foundations of cardiac arrhythmias: basic concepts and clinical approaches. New York: Marcel Dekker.
Vassalle, M., Yu, H., Cohen, I. S., Zipes, D. P., Jalife, J. (1995). Pacemaker channels and cardiac automaticity. Cardiac electrophysiology: from cell to bedside. Philadelphia: Saunders.
Bleeker, W. K., MacKaay, A. J. C., Masson-Pevet, M., et al. (1980). Functional and morphologic organization of the rabbit sinus node. Circulation Research, 46, 11–22.
West, T. C. (1955). Ultramicroelectrode recording from the cardiac pacemaker. Journal of Pharmacology and Experimental Therapeutics, 115, 283–290.
Bozler, E. (1943). The initation of impulses in cardiac muscle. American Journal of Physiology, 138, 273–282.
Maltsev, V. A., & Lakatta, E. G. (2008). Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovascular Research, 77, 274–284.
Maltsev, V. A., & Lakatta, E. G. (2007). Normal heart rhythm is initiated and regulated by an intracellular calcium clock within pacemaker cells. Heart, Lung & Circulation, 16(5), 335–348.
Monfredi, O., Maltsev, V. A., & Lakatta, E. G. (2013). Modern concepts concerning the origin of the heartbeat. Physiology, 28, 74–92.
DiFrancesco, D., & Borer, J. (2007). The funny current: cellular basis for the control of heart rate. Drugs, 67(Suppl 2), 15–24.
Shibata, E. F., & Giles, W. R. (1985). Ionic currents that generate the spontaneous diastolic depolarization in individual cardiac pacemaker cells. Proceedings of the National Academy of Sciences of the United States of America, 82, 7796–7800.
Irisawa, H., Brown, H. F., & Giles, W. (1993). Cardiac pacemaking in the sinoatrial node. Physiological Reviews, 73, 197–227.
Dobrzynski, H., Boyett, M. R., & Anderson, R. H. (2007). New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation, 115, 1921–1932.
DiFrancesco, D. (1981). A new interpretation of the pace-maker current in calf Purkinje fibres. Journal of Physiology, 314, 359–376.
Verkerk, A., Wilders, R., van Borren, M. M., et al. (2007). Pacemaker current (If) in the human sinoatrial node. European Heart Journal, 28, 2472–2478.
Nof, E., Luria, D., Brass, D., et al. (2007). Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation, 116, 463–470.
Bucchi, A., Barbuti, A., Baruscotti, M., & DiFrancesco, D. (2007). Heart rate reduction via selective ‘funny’ channel blockers. Current Opinion in Pharmacology, 7, 208–213.
Wainger, B. J., DeGennaro, M., Santoro, B., Siegelbaum, S. A., & Tibbs, G. R. (2001). Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature, 411, 805–810.
Noble, D., Denyer, J. C., Brown, H. F., & DiFrancesco, D. (1992). Reciprocal role of the inward currents ib, Na and if in controlling and stabilizing pacemaker frequency of rabbit sino-atrial node cells. Proceedings of the Royal Society of London B: Biological Sciences, 250, 199–207.
Lombardi, F., Makikallio, T. H., Myerburg, R. J., & Huikuri, H. V. (2001). Sudden cardiac death: role of heart rate variability to identify patients at risk. Cardiovascular Research, 50, 210–217.
Kodama, I., Nikmaram, M. R., Boyett, M. R., Suzuki, R., Honjo, H., & Owen, J. M. (1997). Regional differences in the role of the Ca2+ and Na+ currents in pacemaker activity in the sinoatrial node. American Journal of Physiology, 272, H2793–H2806.
Hagiwara, N., Irisawa, H., & Kameyama, M. (1988). Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. Journal of Physiology, 395, 233–253.
Bogdano, K. Y., Vinogradova, T. M., & Lakatta, E. G. (2001). Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circulation Research, 88, 1254–1258.
Ju, Y. K., Chu, Y., Chaulet, H., Lai, D., Gervasio, O. L., Graham, R. M., Cannell, M. B., et al. (2007). Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circulation Research, 100, 1605–1614.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murphy, C., Lazzara, R. Current concepts of anatomy and electrophysiology of the sinus node. J Interv Card Electrophysiol 46, 9–18 (2016). https://doi.org/10.1007/s10840-016-0137-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-016-0137-2