Abstract
Background
Patients with ischemic heart disease may have implantable cardioverter defibrillators (ICDs) implanted for primary or secondary prevention of sudden cardiac death. Although ICD shocks can be life saving, in some patients, they have been associated with increased mortality and/or morbidity. Several studies have suggested that catheter ablation may be superior to non-ablative strategies at preventing ICD shocks delivered for ventricular arrhythmias; however, this is still controversial.
Methods
We performed a meta-analysis of randomized controlled trials (RCTs) comparing catheter ablation with non-ablative strategies in treatment of ventricular tachycardia (VT) in patients with ischemic heart disease and an ICD. The primary endpoints of interest were recurrent episodes of VT and death. We used a binary random effects method to calculate the cumulative odds ratios (OR) for recurrent VT and deaths.
Results
Of a total of 643 potential citations, our search yielded three citations that met our inclusion and exclusion criteria. In the three trials, a total of 262 patients were randomized to ablation (n = 129) or non-ablative interventions (beta-blockers ± use of antiarrhythmics) (n = 133) group. The cumulative OR for recurrent VT was 0.471 (95 % confidence interval (CI) = 0.176–1.257) for catheter ablation compared with non-ablative strategies, and for death, it was 0.766 (95 % CI = 0.351–1.674). Excluding one study for being appreciably smaller than the other two, the OR for recurrent VT was 0.298 (95 % CI = 0.164–0.543).
Conclusions
In this meta-analysis, the rate of recurrent VT was lower with VT catheter ablation compared with non-ablative strategies. There was not a significant difference in rate of death among patients receiving catheter ablation versus non-ablative strategies for management of VT. Given the lack of adequately powered RCTs comparing ablation versus medical management of VT in patients with ischemic heart disease and an ICD, larger studies with longer follow-up are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multicenter, randomized studies have demonstrated the value of implantable cardioverter defibrillators (ICDs) in reducing mortality in patients with heart failure and low ventricular ejection fraction as well as patients with spontaneous and inducible ventricular arrhythmias [1–3]. While the ICD treats ventricular arrhythmias such as ventricular tachycardia (VT), it does not prevent them from occurring. Thus, clinicians still have to address the morbidity that patients may experience due to recurrent VT and ICD shocks. In the Antiarrhythmics versus Implantable Defibrillators (AVID) trial data, Schron et al. first showed that even though the majority of ICD shocks are appropriate, patients with shocks have reduced quality of life and increased anxiety compared with patients with ICDs who do not receive any shocks [4]. Since then, numerous studies have been published documenting the possible detrimental psychological effects of recurrent ICD shocks [5–7]. Furthermore, data from MADIT-II and SCD-HeFT have shown that patients who experience an appropriate shock for VT experience a significantly higher rate of mortality compared with patients who do not receive device therapy, signifying the importance of preventing recurrent VT [8, 9].
In current clinical practice, patients with ICDs who experience, recurrent episodes of VT and shocks are usually managed with antiarrhythmic medications to prevent future episodes. Radiofrequency catheter ablation (RFA) was first shown to have promising results in treatment of VT in patients without structural heart disease in the early 1990s [10]. Morady et al. were the first to apply these principles to patients with ischemic heart disease and found that RFA could be successfully applied to patients with structural heart disease and recurrent monomorphic VT, refractory to antiarrhythmic therapy [11]. Since then, several prospective cohort studies have shown varying degrees of acute success of VT ablation in patients with coronary heart disease (49 [12], 75 [13], and 81 % [14]); however, recurrence rates of VT at 6 months post-ablation may be up to 50 % [12–14].
Given the paucity of existing data on the efficacy of catheter ablation compared with pharmacological therapy for VT in patients with ischemic heart disease, we performed a systematic review and meta-analysis to examine the effect of ablation on recurrent episodes of VT as well as death.
2 Methods
To be included in our analysis, a study had to meet all of the following criteria (1) patients with ischemic heart disease, (2) patients with a primary or secondary prevention ICD (implanted before or after the ablation in patients who underwent this procedure), (3) one of the comparators is catheter ablation for VT, (4) study had at least two comparators, and (5) a prospective study design. The main outcome of interest in our study was recurrent VT. Other outcomes were ICD shocks for VT and death.
We performed a systematic search of the existing literature using PubMed and clinicaltrials.gov. We limited our search on PubMed to the following MeSH terms: “catheter ablation AND ventricular tachycardia AND (ischemic heart disease OR ischemic cardiomyopathy OR coronary artery disease)”. We searched clinicaltrials.gov with the terms “catheter ablation” and “ventricular tachycardia”. In addition, the references of full-length manuscripts that resulted from the initial search were reviewed manually to find other studies not captured in our initial search.
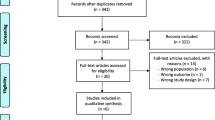
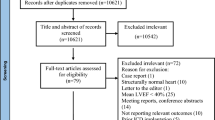
Figure 1 shows a flow chart of our review process and shows reasons for which articles were excluded. All abstracts were reviewed, and if a study appeared to have met our eligibility criteria, the full-length manuscript was then reviewed to make a final determination regarding including the study. Abstracted data included study design, baseline patient characteristics, duration of follow-up, number of deaths, and recurrent VT. Results were recorded on a form created in Distiller SR (Ottawa, CA).
Flow chart describing the review process. The initial search on PubMed identified 638 potential articles of interest, which were each thoroughly reviewed for inclusion and exclusion criteria. An additional search on clinicaltrials.gov identified 49 trials. We thoroughly examined six full manuscripts for inclusion in our study of which three met our criteria
2.1 Statistical analysis
We performed our meta-analysis using OpenMeta software (Brown University: Funded by Agency of Health Care Research and Quality Grant Number R01 HS 018574). Using the software, we performed a cumulative meta-analysis of the included studies and used binary random effects method (DerSimonian-Laird) with our confidence interval set at 95 %. We used the Cochrane statistic I 2 to calculate the heterogeneity of the results across the studies.
3 Results
3.1 Extraction of articles
Using our search strategy, we abstracted 638 articles from PubMed and 49 trials from clinicaltrials.gov (Fig. 1). The most common reason for excluding studies was the absence of a comparator (n = 151). A total of 140 abstracts were excluded because they were review articles. Additionally, 118 articles were excluded because they were case reports, and 60 articles were excluded because they included patients with non-ischemic cardiomyopathy or patients without structural heart disease. Of the 49 studies identified on clinicaltrials.gov, 41 did not have results available as most were still in process. Of the eight with results available, three were excluded because they studied effects of ablation on atrial fibrillation, two were excluded because they did not have a comparison arm, and one had a recruitment problem. The remaining two studies were studies included in our analysis.
The full citations of six studies were closely examined. We did not include Trappe et al. because some patients receiving catheter ablation did not have ICDs implanted before or after their ablation was performed [15]. We excluded Szumowski et al. because the trial was terminated early after patients randomized to receive ablation for their VT refused to have the procedure done [16]. Of the two citations that reported the results of the Ventricular Tachycardia Ablation in Coronary Heart Disease (VTACH) study [17, 18], we included the one that analyzed data according to the intention-to-treat principle [17].
3.2 Study characteristics
As seen in Table 1, we identified a total of three studies that randomized a total of 262 patients with ischemic heart disease and an ICD to either VT catheter ablation or a non-ablative strategy. All three of these were multi-center, prospective randomized controlled trial (RCT) studies. The enrollment strategies and inclusion and exclusion criteria were quite different among the three RCTs. In the study by Reddy et al., patients on class I or III antiarrhythmic medications were excluded, and the non-ablative strategy arm was an ICD along with optimal medical management of ischemic heart disease [19]. Kuck et al. enrolled patients with an ICD and allowed individual site investigators to decide on the optimal medical therapy for managing patients assigned to the non-ablation arm but recommended limiting the use of antiarrhythmic drugs [17]. In contrast to the other two trials, the non-ablative strategy arm in the study by Al-Khatib et al. included anti-arrhythmic medications as recommended by the 2006 Consensus Guidelines for Management of Patients with Ventricular Arrhythmias and Prevention of Sudden Cardiac Death in addition to optimal medical therapy for patients with ischemic heart disease [20]. In Reddy et al., about 87 % of patients had ICDs implanted before receiving their ablation, while in Kuck et al., all patients randomized to the ablation arm received their ICD after the procedure [17, 19]. In contrast, Al-Khatib et al. required all patients to have an ICD before enrolling them to be randomized to medical management or catheter ablation for ventricular tachycardia [20].
Kuck et al. allowed the use of either an electroanatomic or non-contact system for substrate mapping [17], while Reddy et al. used only electroanatomic substrate mapping [19]. Al-Khatib et al. allowed individual centers to pick their own VT mapping method and own way of identifying substrates leading to VT [20]. If VT was inducible using programmed stimulation, all three studies used non-inducibility of VT to define a successful ablation [17, 19, 20]. If VT was not inducible at the beginning of the study, Al-Khatib et al. used “modification of induced VT cycle length and non-inducibility of any VT” as its endpoint [20], while Kuck et al. used “absence of all channels inside the area of interest or ablation with linear lesions based on pace mapping along the infarct scar target sites” [17].
The primary endpoint of the study by Reddy et al. was freedom from any appropriate ICD therapy, while the primary endpoint of the study by Kuck et al. was time to recurrence of sustained VT/VF [17, 19]. The study by Al-Khatib et al. was a pilot study, and so the primary endpoint was feasibility of conducting a larger multicenter randomized clinical trial that would examine mortality, but this study reported on recurrent sustained VT/VF and death [20]. Furthermore, Al-Khatib et al. had a shorter follow-up period of 6 months while Kuck et al. and Reddy et al. both had follow-up periods of close to 2 years [17, 19, 20].
Baseline characteristics of the patients included in the three studies are shown in Table 2. The majority of patients were male (90 %) and were on beta-blockers (87 %) at the time of randomization. The studies had varying rates of anti-arrhythmic use prior to randomization and during the study period. Patients in the study by Reddy et al. had no prior or current use of anti-arrhythmic in the study [19]. Kuck et al. reported that 35 % of patients were on amiodarone at the time of randomization for both the ablation and non-ablation group [17]. After 12 months of follow-up, amiodarone use was 26 % in patients who received ablation and 31 % in patients in the control group [17]. In Al-Khatib et al., 31 % of patients were previously on amiodarone and 15 % on sotalol in the ablation group, while 21 % were previously on amiodarone and 7 % on sotalol in the anti-arrhythmic group [20]. Furthermore, 5 of 11 patients (45 %) who received ablation were on an anti-arrhythmic drug during follow-up [20].
Of the two studies who reported on 30 day mortality, Reddy et al. and Kuck et al. both did not have any deaths after ablation [17, 19].
3.3 Outcomes for ablation versus non-ablative strategies
Using a random effects model, the cumulative odds ratio (OR) for recurrent VT in the follow-up period between patients who underwent VT catheter ablation compared with those who received non-ablative therapies was 0.471 (95 % CI = 0.176–1.257; Fig. 2a). Excluding the study by Al-Khatib et al. because of its relatively small sample size and shorter follow-up period compared with the other two studies, the OR for the catheter ablation arm compared with the non-ablative therapy arm for recurrent VT was 0.298 (95 % CI = 0.164–0.543; Fig. 2b). There was a non-significant (p = 0.065) trend toward heterogeneity among the three studies for recurrent VT. Excluding Al-Khatib et al., there was not significant heterogeneity for recurrent VT among the other two studies (I 2 = 0; p = 0.954).
a Forest plot of odds ratio (OR) for recurrent VT using the three cumulative studies. A greater odds ratio favored ablation over non-ablation in causing less recurrent VT. The black rectangle represents each individual study’s OR estimate with the line, left and right of the rectangle, representing the 95 % confidence interval. The center of the blue diamond represents the cumulative OR estimate while the edges of the diamond represent 95 % confidence interval. b Same as Fig. 2a but excluded Al-Khatib et al.
Using the three studies, the cumulative OR for death between patients randomly assigned to catheter ablation versus those who received non-ablative therapies was 0.766 (95 % CI = 0.351–1.674; Fig. 3a). Excluding Al-Khatib et al., the OR for deaths in the VT catheter ablation arm versus the non-ablative strategy arm was 0.745 (95 % CI = 0.285–1.949; Fig. 3b).
a Forest plot of odds ratio (OR) for deaths using the three cumulative studies. A greater odds ratio favored ablation over non-ablation in causing less deaths. The black rectangle represents each individual study’s OR estimate with the line, left and right of the rectangle, representing the 95 % confidence interval. The center of the blue diamond represents the cumulative OR estimate while the edges of the diamond represent 95 % confidence interval. b Same as Fig. 2a but excluded Al-Khatib et al.
4 Discussion
The main result of this meta-analysis is that compared with medical therapy, VT catheter ablation in patients with an ICD and ischemic heart disease appears to result in fewer VT recurrences. We found no significant difference in the rate of death whether patients are treated with catheter ablation or non-ablative strategies for VT.
One meta-analysis that studies VT catheter ablation compared to medical management has been published. It showed that VT catheter ablation in addition to standard medical therapy reduces recurrent VT but has no effect on mortality [21]. The main limitation of that study is the authors analyzed both patients with non-ischemic and ischemic cardiomyopathy. This may be problematic because the techniques for VT catheter ablation are different in patients with ischemic cardiomyopathy who frequently have well-defined reentrant circuits, than patients with non-ischemic cardiomyopathy in whom the mechanism of VT may be more heterogeneous and may not all be reentrant. In fact, recent data from the Heart Centre of Leipzig Ventricular Tachycardia (HELP-VT) study revealed that patients with ischemic cardiac disease who received catheter ablation were significantly less likely to have recurrent VT episodes compared with patients with non-ischemic cardiac disease during 1 year of follow-up [22]. Other limitations of the previous meta-analysis include using a fixed effects model to calculate the cumulative OR which is not as robust as a random effects model, inclusion of studies with non-randomized patients, and inclusion of abstracts whose final results were never published in manuscript form [21].
The optimal management of VT in patients with ischemic heart disease and a primary or secondary prevention ICD still remains controversial. The OPTIC study first showed a significantly lower rate of recurrent shocks with a combination of amiodarone and beta blocker compared with sotalol or beta blocker alone [23]. However, because of the adverse side effects and poor tolerance of antiarrhythmic medications, patients in the study had a high discontinuation rate of amiodarone and sotalol at 1 year post-initiation (18 and 24 %, respectively) [23]. Current guidelines recommend considering catheter ablation for VT that recurs despite antiarrhythmic drug therapy or when antiarrhythmic drugs are not tolerated or desired [24]. Future prospective studies and RCTs are needed to answer whether catheter ablation is more effective than antiarrhythmic medications for the management of recurrent VT and whether there are any survival benefit associated with catheter ablation, especially given the recently published retrospective data indicating that patients treated with ablation have decreased mortality compared to patients medically managed for recurrent VT [25].
4.1 Limitations
The main limitation of the current study is the small number of studies included in the analysis, though it should be noted that we performed an exhaustive search of the published literature for available articles. There are not many prospective controlled and/or matched studies on the topic of interest likely because most centers may have difficulty convincing patients to participate in a trial in which there is a 50:50 chance of receiving “a pill” versus undergoing an ablation as seen in Szumowksi et al. [16]. However, the study by Al-Khatib et al. showed that only 8 % of patients declined participating in the trial comparing catheter ablation versus antiarrhythmic medications [20]. Furthermore, with an I 2 value of 63 the interpretation of our cumulative OR may be limited, though it should be noted that we performed a re-analysis without the smaller study and arrived at a more homogeneous study population. Al-Khatib et al.’s results may be so different compared to the other two studies due to its shorter follow-up of (6 months compared close to 2 years for the other two studies), smaller sample size of only including 27 patients, and possibility of having sicker baseline patients with higher VT recurrence rates and deaths in both arms [20].
5 Conclusion
In patients with ischemic heart disease, catheter ablation of VT results in decreased recurrent VT compared with medical therapy alone. Although we found no difference in mortality between the two strategies, the total number of patients was too small to allow a definitive result. Future prospective studies are needed with more patients and longer follow-up to better define the benefits from VT catheter ablation and determine if these benefits persist beyond 1–2 years of follow-up. Furthermore, future studies need to investigate whether VT catheter ablation or antiarrhythmic medications such as amiodarone or sotalol is better first line therapy for the treatment of recurrent VT in patients with ischemic heart disease.
References
Moss, A. J., Zareba, W., Hall, W. J., Klein, H., Wilber, D. J., Cannom, D. S., et al. (2002). Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med, 346(12), 877–83.
Buxton, A., Lee, K., DiCarlo, L., Gold, M., Greer, S., Prystowsky, E., et al. (2000). Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. N Engl J Med, 342(26), 1937–45.
The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. (1997). A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med, 337, 1576–84.
Schron, E. B., Exner, D. V., Yao, Q., Jenkins, L. S., Steinberg, J. S., Cook, J. R., et al. (2002). Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation, 105(5), 589–94.
Irvine, J., Dorian, P., Baker, B., O’Brien, B. J., Roberts, R., Gent, M., et al. (2002). Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J, 144(2), 282–89.
Kamphuis, H. C., De leeuw, J. R., Derksen, R., Hauer, R. N., & Winnubst, J. A. (2003). Implantable cardioverter defibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study. Europace, 5(4), 381–9.
Passman, R., Subacius, H., Ruo, B., et al. (2007). Implantable cardioverter defibrillators and quality of life: results from the defibrillators in nonischemic cardiomyopathy treatment evaluation study. Arch Intern Med, 167(20), 2226–32.
Moss, A. J., Greenberg, H., Case, R. B., Zareba, W., Hall, W. J., Brown, M. W., et al. (2004). Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation, 110(25), 3760–65.
Poole, J. E., Johnson, G. W., Hellkamp, A. S., Anderson, J., Callans, D. J., Raitt, M. H., et al. (2008). Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med, 359(10), 1009–17.
Klein, L. S., Shih, H. T., Hackett, F. K., Zipes, D. P., & Miles, W. M. (1992). Radiofrequency catheter ablation of ventricular tachycardia in patients without structural heart disease. Circulation, 85(5), 1666–74.
Morady, F., Harvey, M., Kalbfleisch, S. J., el-Atassi, R., Calkins, H., & Langberg, J. J. (1993). Radiofrequency catheter ablation of ventricular tachycardia in patients with coronary artery disease. Circulation, 87(2), 363–72.
Calkins, H., Epstein, A., Packer, D., Arria, A. M., Hummel, J., Gilligan, D. M., et al. (2000). Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. J Am Coll Cardiol, 35(7), 1905–14.
Stevenson, W. G., Wilber, D. J., Natale, A., Jackman, W. M., Marchlinski, F. E., Talbert, T., et al. (2008). Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation, 118(25), 2773–82.
Tanner, H., Hindricks, G., Volkmer, M., et al. (2010). Catheter ablation of recurrent scar-related ventricular tachycardia using electroanatomical mapping and irrigated ablation technology: results of the prospective multicenter Euro-VT-study. J Cardiovasc Electrophysiol, 21(1), 47–53.
Trappe, H. J., Klein, H., Wenzlaff, P., Fieguth, H. G., Wahlers, T., Kielblock, B., et al. (1993). Role of interventional therapy n patients with coronary heart disease and life-threatening ventricular tachyarrhythmias. Eur Heart J, 14(Suppl E), 120–7.
Szumowski, L., Przybylski, A., Maciag, A., Derejko, P., Bodalski, R., Zakrzewska, J., et al. (2009). Outcomes of a single centre registry of patients with ischaemic heart disease, qualified for an RF ablation of ventricular arrhythmia after ICD intervention. Kardiol Pol, 67(2), 123–7.
Kuck, K. H., Schaumann, A., Eckardt, L., Willems, S., Ventura, R., Delacretaz, E., et al. (2010). Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicenter randomized controlled trial. Lancet, 375(9708), 31–40.
Delacretaz, E., Brenner, R., Schaumann, A., Eckardt, L., Willems, S., Pitschner, H. F., et al. (2013). Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): an on-treatment analysis. J Cardiovasc Electrophysiol, 24(5), 525–29.
Reddy, V. Y., Reynolds, M. R., Neuzil, P., Richardson, A. W., Taborsky, M., Jongnarangsin, K., et al. (2007). Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med, 357(26), 2657–65.
Al-Khatib, S. M., Daubert, J. P., Anstrom, K. J., Daoud, E. G., Gonzalez, M., Saba, S., et al. (2015). Catheter ablation for ventricular tachycardia in patients with cardioverter defibrillator (CALYPSO) pilot trial. J Cardiovasc Electrophysiol, 26(2), 151–7.
Mallidi, J., Nadkarni, G. N., Berger, R. D., Calkins, H., & Nazarian, S. (2011). Meta-analysis of catheter ablation as an adjunct to medical therapy for treatment of ventricular tachycardia in patients with structural heart disease. Heart Rhythm, 8(4), 503–10.
Dinov, B., Fiedler, L., Schonbauer, R., Bollmann, A., Rolf, S., Piorkowski, C., et al. (2014). Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation, 129(7), 728–36.
Connolly, S. J., Dorian, P., Roberts, R. S., Gent, M., Bailin, S., Fain, E. S., et al. (2006). Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA, 295(2), 165–71.
Aliot, E. M., Stevenson, W. G., Almendral-Garrote, J. M., Bogun, F., Calkins, C. H., Delacretaz, E., et al. (2009). EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Europace, 11(6), 771–817.
Bunch, T. J., Weiss, J. P., Crandall, B. G., Day, J. D., May, H. T., Bair, T. L., Osborn, J. S., Mallender, C., Fischer, A., Brunner, K. J., & Mahapatra, S. (2014). Patients treated with catheter ablation for ventricular tachycardia after an ICD shock have lower long-term rates of death and heart failure hospitalization than do patients treated with medical management only. Heart Rhythm, 11(4), 533–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, D., Hasselblad, V., Jackson, K.P. et al. Catheter ablation for ventricular tachycardia (VT) in patients with ischemic heart disease: a systematic review and a meta-analysis of randomized controlled trials. J Interv Card Electrophysiol 45, 111–117 (2016). https://doi.org/10.1007/s10840-015-0083-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-015-0083-4