Abstract
Purpose
Sleep deprivation, which is a strong stressor, can greatly affect the cardiovascular system of rescue workers. This study aimed to investigate the effect of 24-h sleep deprivation on heart rate variability (HRV) in young healthy people and the protective effect of metoprolol on arrhythmia.
Methods
Sixty young, healthy subjects (6 women and 54 men), aged 25 ± 4.5 years, were enrolled in this study. All participants received 24-h continuous ambulatory electrocardiogram monitoring. Arrhythmia, time, and frequency domain parameters were analyzed in subjects at the following three stages: normal sleep stage, sleep deprivation stage, and metoprolol treatment before sleep deprivation stage.
Results
After 24-h sleep deprivation, the high frequency (HF) of HRV was significantly decreased (p < 0.05), low frequency (LF) was remarkably increased (p < 0.05), and LF/HF was significantly increased compared with those in normal sleep (p < 0.05). Some subjects presented with mild palpitation due to premature atrial complexes and premature ventricular complexes. At the metoprolol treatment stage, compared with the sleep deprivation stage, LF and LF/HF were significantly reduced, HF of HRV was elevated (p < 0.05), and the total amount of premature atrial and ventricular complexes was decreased.
Conclusion
The underlying mechanism of arrhythmia and HRV alteration after 24-h sleep deprivation could be attributable to lower vagal activity and elevated sympathetic activity. Metoprolol improves the change in autonomic nervous system activity after 24-h sleep deprivation, which may be responsible for its protective role on arrhythmia in healthy subjects undergoing sleep deprivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the setting of earthquakes, floods, or fire disasters, rescue workers usually carry out their work without sleep. Sleep deprivation (SD), which is a strong stressor, can exert a large effect on the cardiovascular system of rescue workers. It has been reported that SD is associated with increased arrhythmogenicity and disturbed cardiac autonomic control [1]. Heart rate variability (HRV) is acknowledged as a reliable marker of cardiac autonomic control, and the frequency of premature ventricular complexes can be an indicator of arrhythmogeneity. It is known that metoprolol, a class II antiarrhythmic agent, is currently used in clinical practice for the treatment of arrhythmia and hypertension, as well as for improving cardiac autonomic function. Previous studies on the pathophysiology of people with SD receiving beta-blockers are rare. This study aimed to investigate (1) the changes in heart rate variability and occurrence of cardiac arrhythmia by 24-h continuous ambulatory electrocardiogram (ECG) (24-h Holter) in young, healthy subjects undergoing complete SD and (2) the effects of metoprolol on HRV and arrhythmia after this agent was administered prophylactically before SD.
1 Methods
1.1 Study population
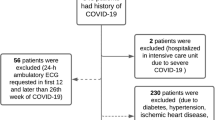
Subjects aged between 18 and 30 years of both sexes were recruited by advertisement from the army. Sixty subjects (6 women and 54 men), aged 25 ± 4.5 years, were enrolled, and all participants completed the protocols. All subjects gave written, informed consent in accordance with the PLA General Hospital before taking part in this study. All participants received financial compensation for participation. This study was carried out in the clinical investigation unit of the PLA General Hospital. Participants were free of any medical conditions (e.g., hypertension, diabetes mellitus, and hyperthyroidism) and medication known to affect cardiovascular, metabolic, gastrointestinal, or immune function (including over-the-counter medication). Participants who suffered from sleep, depression, or anxiety disorders were excluded from the study according to self-reported pre-study questionnaires and written confirmation from their general practitioner. All subjects were nonsmokers at the time of the study and did not consume alcohol.
1.2 Heart rate variability
Sixty participants received Holter monitoring using a 12-channel ambulatory ECG recorder (Pacerecorder Model MIC-12H, Beijing Jinco Medical Co., Ltd) with a sampling rate of 250 Hz (4 ms). The P waves and QRS complexes were automatically classified and manually verified as normal sinus rhythm, premature atrial or premature ventricular complexes, or noise by comparison with adjacent waves. The R–R intervals were deduced from the adjacent normal sinus beats (i.e., N–N intervals). For the entire study population, time domain measurements, including mean N–N intervals, standard deviation of N–N intervals (SDNN), and root-mean-square successive differences (RMSSD), were calculated automatically every 5 min. The power spectrum densities were estimated by Welch's averaged periodogram method, while extremely low-frequency (VLF) power (0.01–0.04 Hz), low-frequency (LF) power (0.04–0.15 Hz), and high-frequency (HF) power (0.15–0.4 Hz) were derived for each 5-min segment. HF power is considered a function of cardiac parasympathetic nervous system activity to the heart [2]. LF, although not modulated by a single arm of the autonomic nervous system [3], is considered to be normalized for total power as a representative index of sympathetic activity to the heart [4–6]. The LF-to-HF power ratio is considered an index of the balance of sympathovagal input to sinoatrial node activity and has been found to be representative of sympathetic to parasympathetic balance in both physiological and pathophysiological conditions [2, 5–8].
1.3 Mean arterial pressure
Patients rested in the supine position while blood pressure was measured each hour from the right arm by an automatic cuff recorder (Critikon, Dinamap 1846 SX, USA).
2 Protocol
No caffeine or alcohol was allowed from the 48 h preceding the laboratory studies to the completion of the study. Enrolled subjects reported to the sleep laboratory at 07:30 hours after obtaining their normal sleep at home the previous night. Each subject remained awake in the sleep lab from 17:00 hours of day 1 to 17:00 hours of day 2 at the stage of SD. All participants had a designated bedroom for the entire study. All physiological measurements were performed in the bedroom with temperature of 22 °C and illumination of 100 lx during the study. Subjects were continuously monitored by a video camera. The subjects displaying sleep onset were immediately aroused and kept awake by verbal encouragement. Caloric and fluid management was individualized according to estimated daily needs. However, snacks were permitted to be eaten in the lab. Subjects were permitted to read, watch video movies on a DVD player, play video games, do job-related work, including using the computer and Internet, and converse with the staff or visitors.
Subjects underwent the following three stages: normal sleep, SD, and metoprolol treatment before SD. At the normal sleep stage, 24-h Holter monitoring was applied to all subjects. At the SD stage, we examined subjects who received another 24-h Holter examination at the sleep 1 month after the first stage. At the third stage, subjects accepted metoprolol administration 3 days previously and underwent SD again, which was scheduled 1 month later following the second stage, and data derived from 24-h monitoring were analyzed. Metoprolol was given in the morning (08:00–09:00 hours) and at the afternoon (17:00–18:00 hours), and the dosage was 25 mg every time.
3 Statistical analysis
Continuous variables are expressed as the mean ± standard deviation. Continuous variables were compared with the Student's t test or the Wilcoxon signed-rank test, as appropriate. Categorical variables were compared by χ 2 test. A p value of <0.05 was considered statistically significant.
4 Results
The characteristics of the study population are given in Table 1. The body mass index of the participants ranged from 22 to 23.1 kg/m2. They usually needed 7 to 10 h of sleep daily.
At the normal sleep stage, some of the subjects (n = 5) complained of sleep disruption due to blood pressure measurements, but it did not generally affect the normal sleep of subjects. At the metoprolol treatment before SD stage, a few of the subjects complained of mild fatigue (n = 2), headache (n = 1), and constipation (n = 1) after the administration of metoprolol. Daily life was not significantly affected by this drug.
After SD, heart rate, mean blood pressure, LF, and LF/HF of HRV were significantly increased at the SD stage compared with those in the normal sleep stage (p < 0.05). There was also a significant decrease in HF, VLF, SDNN, and RMSSD of HRV at the SD stage compared with those in the normal sleep stage (p < 0.05). The subjects suffered from frequent premature atrial and ventricular complexes after SD. At the third stage, after prophylactic treatment of metoprolol, heart rate, mean blood pressure, LF, and LF/HF were significantly decreased compared with those in the SD stage, and HF, VLF, SDNN, and RMSSD of HRV were also elevated (p < 0.05). The frequency of premature atrial complexes and premature ventricular complexes was reduced with treatment of metoprolol compared with that in the SD stage (Table 2).
The duration of experimental time was divided into two periods: night time and day time. Night time was defined as from 17:00 hours of day 1 to 05:00 hours of day 2 and day time was 05:00 hours of day 2 to 17:00 hours of day 2. With a prolonged SD, especially at day time compared with the night time, LF was significantly increased (p < 0.05; Fig. 1), and HF was remarkably decreased after SD (p < 0.05; Fig. 2). With the progression of SD, the participants suffered from more atrial premature beats and ventricular premature beats (Figs. 3 and 4).
5 Discussion
In this study, we identified the changes regarding HRV parameters among three different stages. We found that HF, VLF, SDNN, and RMSSD were significantly decreased, and LF and LF/HF were remarkably elevated among participants with SD compared with the same individuals during normal sleep. After the prophylactic administration of metoprolol before SD, HF, VLF, SDNN, and RMSSD were recovered, and LF and LF/HF were nearly reduced to the original values among SD participants.
Spectral analysis techniques have been used to determine changes in central nervous system activity. Power in specific frequency bands can be related to parasympathetic and sympathetic nervous system activity. Specifically, relative power in HF areas, usually from 0.15 to 0.5 Hz, has been used to infer parasympathetic nervous system activity. A range of lower frequencies from 0.05 to 0.15 Hz has typically been related to a combination of parasympathetic and sympathetic influences [9–11]. Because LF power is a combination of sympathetic and parasympathetic effects, investigators frequently infer sympathetic nervous system activity from the ratio of low (parasympathetic and sympathetic) to high (predominantly sympathetic) power so that parasympathetic power is extracted out of the ratio to some extent [10, 12, 13], leaving a better indicator of sympathetic activity. VLF, SDNN, and RMSSD are also considered as representative indices of parasympathetic nervous system activity [14]. Acute SD is associated with increased sympathetic activity and decreased parasympathetic modulation [15]. In addition, sleep disturbance may also result in sympathovagal imbalance [15, 16] and an increase in premature ventricular complexes [16]. Lower HF, VLF, SDNN, and RMSSD reflect lower parasympathetic activity, and higher LF and LF/HF indicate higher sympathetic activity. All these factors are associated with a higher risk of cardiovascular disease [14, 17]. The current study found that premature atrial complexes and premature ventricular complexes were significantly increased in participants with 24-h SD. The change in arrhythmia after 24-h SD has been found to correlate with elevated sympathetic activity [17]. Therefore, in our study, the above-mentioned findings are consistent with results of a previous research.
Metoprolol can bind to beta-adrenoceptors located in cardiac nodal tissue, the conducting system, and contracting myocytes. The heart has both β1 and β2 adrenoceptors, and the predominant receptor type in number and function is β1. Metoprolol acts by blocking the effects of catecholamines at β1-adrenergic receptors, thereby decreasing sympathetic activity on the heart. Metoprolol is useful in the treatment of supraventricular and ventricular tachycardia. In the current study, at the third stage after administration of metoprolol, an increase in HF, VLF, SDNN, and RMSSD reflected a recovery of parasympathetic activity, and a decrease in LF and LF/HF indicated the return of sympathetic activity. Therefore, beta-blockers can play an important role in the prevention of arrhythmia in healthy people with 24-h SD.
In conclusion, the mechanism of alteration of arrhythmia and HRV after 24-h SD could be related to lower vagal activity and elevated sympathetic activity. Metoprolol can improve this change in the autonomic nervous system after 24-h of SD, and it can play a preventive role of arrhythmia in healthy people with SD. The main limitations of this study are the small number of subjects and a non-placebo-based study protocol was carried out.
References
Wehrens, S. M., Hampton, S. M., & Skene, D. J. (2012). Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Scandinavian Journal of Work, Environment & Health, 38, 171–181.
Howorka, K., Pumprla, J., & Jirkovska, A. (2010). Modified orthostatic load for spectral analysis of short-term heart rate variability improves the sensitivity of autonomic dysfunction assessment. Journal of Diabetes and its Complications, 24, 48–54.
Forslund, L., Björkander, I., & Ericson, M. (2002). Prognostic implications of autonomic function assessed by analyses of catecholamines and heart rate variability in stable angina pectoris. Heart, 87, 415–422.
Montano, N., Porta, A., & Cogliati, C. (2009). Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neuroscience and Biobehavioral Reviews, 33, 71–80.
Lombardi, F., & Stein, P. K. (2011). Origin of heart rate variability and turbulence: an appraisal of autonomic modulation of cardiovascular function. Frontiers in Physiology, 2, 90–95.
Takase, B., Kitamura, H., & Noritake, M. (2002). Assessment of diabetic autonomic neuropathy using twenty-four-hour spectral analysis of heart rate variability: a comparison with the findings of the Ewing battery. Japanese Heart Journal, 43, 127–135.
Taelman, J., Vandeput, S., & Gligorijević, I. (2011). Time-frequency heart rate variability characteristics of young adults during physical, mental and combined stress in laboratory environment. Conference Proceedings IEEE Engineering Medicine and Biology Society, 2011, 1973–1976.
Karmakar, C. K., Khandoker, A. H., & Voss, A. (2011). Sensitivity of temporal heart rate variability in Poincaré plot to changes in parasympathetic nervous system activity. Biomedical Engineering Online, 3, 10–17.
Lee, Y. H., Park, B. N., & Kim, S. H. (2011). The effects of heat and massage application on autonomic nervous system. Yonsei Medical Journal, 52, 982–989.
Lin, G., Xiang, Q., & Fu, X. (2012). Heart rate variability biofeedback decreases blood pressure in prehypertensive subjects by improving autonomic function and baroreflex. Journal of Alternative and Complementary Medicine, 18, 143–152.
Porta, A., Bari, V., & Badilini, F. (2011). Frequency domain assessment of the coupling strength between ventricular repolarization duration and heart period during graded head-up tilt. Journal of Electrocardiology, 44, 662–668.
Kumae, T. (2012). Assessment of training effects on autonomic modulation of the cardiovascular system in mature rats using power spectral analysis of heart rate variability. Environ Health Prev Med, 11, 84–85.
Sun, J., Li, X., Guo, J., & Han, F. (2011). Identification of obstructive sleep apnea syndrome by ambulatory electrocardiography: clinical evaluation of time-domain and frequency-domain analyses of heart rate variability in Chinese patients. Cell Biochemistry and Biophysics, 59, 165–170.
Kunikullaya, K. U., Kirthi, S. K., & Venkatesh, D. (2010). Heart rate variability changes in business process outsourcing employees working in shifts. Indian Pacing Electrophysiol J, 10, 439–446.
Hong, X., Hilton, H. J., Gates, G. J., et al. (2005). Acute sleep deprivation is associated with increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans. Journal Applied Phsiology, 98, 2024–2032.
Schubert, C., Lambertz, M., & Nelesen, R. A. (2009). Effects of stress on heart rate complexity—a comparison between short-term and chronic stress. Biological Psychology, 80, 325–332.
Van Amelsvoort, L. G., Schouten, E. G., Maan, A. C., et al. (2001). Changed in frequency of premature complexes and heart rate variability related to shift work. Occupational and Environmental Medicine, 58, 678–681.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial commentary
JICE1978R3
Protective effect of metoprolol on arrhythmia and heart rate variability in healthy people with 24-h sleep deprivation
Dr. Xiang-min Shi
Sleep deprivation can have an adverse effect on markers of arrhythmia susceptibility such heart rate variability (HRV). This study shows that the beta-adrenergic blocker metoprolol ameliorates sleep deprivation changes on HRV and may therefore be advantageous for reducing arrhythmia propensity in sleep-deprived individuals.
D.G. Benditt
Rights and permissions
About this article
Cite this article
Chen, Wr., Shi, Xm., Yang, Ts. et al. Protective effect of metoprolol on arrhythmia and heart rate variability in healthy people with 24 hours of sleep deprivation. J Interv Card Electrophysiol 36, 267–272 (2013). https://doi.org/10.1007/s10840-012-9728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-012-9728-8