Abstract
Purpose
Exposure to ionizing radiation during electrophysiologic procedures in children is believed to increase the risk of future malignancy. Electroanatomical navigation can reduce exposure, but the cohort of children who derive the greatest benefit from this approach is incompletely defined. We sought to determine factors associated with fluoroscopy exposure with conventional catheter ablation versus electroanatomical navigation.
Methods
A retrospective review of all ablation procedures over a 5-year period during the transition to electroanatomical navigation performed by a single electrophysiologist was performed. Fluoroscopy time >20 min was considered “prolonged.” Statistical analysis was performed to determine differences among groups.
Results
Two hundred thirty-four subjects underwent catheter ablation during the study period (conventional, n = 127; navigation, n = 107). Mean fluoroscopy decreased from 11.1 to 3.5 min with electroanatomical navigation (p < 0.0001). Overall 53/107 subjects (50 %) undergoing catheter ablation using electroanatomical navigation required no fluoroscopy, of which atrioventricular nodal reentry tachycardia (AVNRT) (n = 23) and right-sided accessory pathways (n = 22) were most common (p = 0.001). Prolonged fluoroscopic exposure was observed for 22/127 (17 %) subjects undergoing conventional fluoroscopy versus 3/107 (3 %) subjects with electroanatomical navigation (p = 0.001) and was not observed after increased experience. Flouroscopy time decreased significantly after the first 20 procedures (p = 0.04). There was no difference in success, complication, or recurrence rate between groups.
Conclusions
Electroanatomical navigation significantly reduced fluoroscopic exposure without compromising safety, efficacy, or recurrence. Subjects with AVNRT and right-sided accessory pathways derived the greatest benefit as did those requiring prolonged fluoroscopy by the conventional approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Catheter ablation is an effective therapy for multiple arrhythmia substrates in children. However, this approach traditionally relies on the use of fluoroscopy and can therefore be associated with a significant exposure to ionizing radiation [1]. Radiation doses in the range used for typical electrophysiology procedures are believed to increase the risk for future malignancy based on data derived from environmental exposures as well as long-term follow-up of patients exposed to medical radiation [2–4]. Children are inherently more susceptible to the risk of radiation-induced carcinogenesis, and therefore, these concerns are even more relevant to the pediatric electrophysiologist [5].
Electroanatomical navigation technology is uniquely available within the field of electrophysiology. With the use of navigation systems for radiofrequency ablation, the duration of fluoroscopy exposure and the subsequent radiation dose can be greatly reduced [6]. Several pediatric-specific studies have observed both similar acute success as well as significant reduction in fluoroscopy time with the use of these navigation systems compared to the conventional fluoroscopic approach [7–9].
Despite the encouraging similarity in acute safety and efficacy between these two approaches, the potential advantages of electroanatomical navigation remain incompletely defined in children, and there remains a paucity of data for factors that predict fluoroscopic exposure. We therefore sought to evaluate such factors in our own large pediatric cohort during the transition period to the use of electroanatomical navigation for catheter ablation of pediatric arrhythmia.
2 Methods
A retrospective review of all pediatric subjects undergoing catheter ablation over a 5-year period (January 2006 through December 2010) at the UCLA Medical Center was performed after obtaining approval from the local institutional review board. During the study period, electrophysiology procedures initially were performed with the exclusive use of fluoroscopy, but were later supplemented with an electroanatomical navigation system beginning in August of 2008. All procedures were performed by one electrophysiologist (KMS). Subjects undergoing procedures using conventional fluoroscopy were designated as the “conventional group,” and those in whom an electroanatomical navigation system was used during the procedure the “navigation group.”
All pediatric subjects (age <18 years) who underwent catheter ablation and all arrhythmia subtypes in the defined period were included in the analysis. Subjects with tachycardiomyopathy were not excluded, but subjects with a history of significant congenital heart disease with or without prior cardiac surgery were excluded from the analysis. The primary focus was to assess the factors influencing fluoroscopy exposure before and after the introduction of the electroanatomical navigation system in a general pediatric population. For this purpose, catheter ablation procedures that required significantly increased fluoroscopic exposure, defined as greater than 20 min of fluoroscopy (corresponding to the 90th percentile in the present cohort), were examined separately and are referred to as the “prolonged fluoroscopy group.” Conversely, procedures not requiring any degree of fluoroscopic exposure were evaluated as a distinct category to assess for associated factors.
An informed consent was obtained, and all antiarrhythmic drugs were discontinued at least five half-lives prior to the electrophysiology procedure. The protocol for electroanatomical navigation in this study consisted of placement of pairs of reference patches in three orthogonal planes on the body surface in order to simultaneously display the location of multiple intracardiac electrodes through the use of sequential low-amplitude current pulses using the Ensite NavX system (St. Jude Medical, Austin, TX) [10]. Diagnostic catheters were routinely advanced into position without supplemental fluoroscopy as described by others [9, 11]. The decapolar coronary sinus (CSL) catheter (St. Jude Medical, Inc. St. Paul, MN) was introduced via a sheath in the right internal jugular vein for all subjects in the study cohort, after which a stable electrode from this catheter was used as a fudicial reference point for the remainder of the procedure. The location of the coronary sinus (CS) catheter was tagged with a shadow to annotate its position at the beginning of the procedure in the event that the catheter was displaced during subsequent manipulation of other catheters.
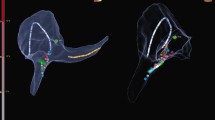
Standard electrophysiology testing was performed to document the specific arrhythmia mechanism, followed by a strategy of radiofrequency catheter ablation for all tachycardia substrates with the exception of those anticipated to be very close to the normal AV conduction tissue, in whom cryotherapy was utilized. For subjects with AVNRT, a geometric shell of the right atrium (RA) was created prior to catheter ablation. Specifically, the position of the His bundle was tagged, and the CS os was clearly defined. The tricuspid valve annulus (TVA) was delineated by annotating at least four separate points on the annulus displaying equal amplitudes of atrial and ventricular electrograms, after which the navigation system was used to color code the atrium and ventricle for the ease of catheter navigation (Fig. 1). For AVNRT, catheter ablation was performed by creating a linear set of lesions from the TVA to the lower edge of the CS os, previously reported to be associated with a very low incidence of AV block as well as low long-term recurrence [12]. This approach was used exclusively during the study period as we had previously experienced excellent acute and long-term outcomes with this particular ablation strategy (Fig. 1).
3D reconstruction of the right atrium for catheter ablation of AVNRT. The TVA is defined at the onset of the procedure and a decapolar catheter is left in place to characterize the location of the CS os. A series of lesions is placed, beginning at the ventricular aspect of the TVA and moved progressively toward the inferior portion of the CS os. This linear ablation strategy is well-suited to use with the electroanatomical navigation system
For AV accessory pathways (AP), RA geometry was constructed in a similar fashion as for subjects with AVNRT when the pathway was right-sided. After delineation of the TVA, mapping was undertaken in sinus rhythm in the setting of ventricular preexcitation or during ventricular pacing for concealed APs. Activation mapping was not performed with the navigation system, rather the catheter with the earliest ventricular electrogram in sinus rhythm (for manifest pathways) or earliest atrial activation during ventricular pacing (for concealed pathways) was used as a fudicial point as the roving catheter was manipulated to find the earliest possible signal relative to this reference electrogram. Other evidence used for the location of putative successful sites included V-delta interval >25 ms, continuous AV activity, and pathway potentials [13, 14]. Left-sided APs were targeted via a transseptal approach with limited supplemental use of fluoroscopy followed by reliance on the electroanatomical navigation system. The navigation system was rarely used for anatomic delineation of the mitral valve annulus for left-sided APs, as the position of the CS catheter was felt to provide a reasonable approximation of the posterior left atrioventricular annulus. Mapping and ablation were otherwise performed similar to right-sided APs.
Results are presented as mean ± 1 SD unless except for non-normal distributions, in which case median (with ranges) are included. P values of less than or equal to 0.05 established statistical significance. Binary outcome variables were analyzed with the use of the Chi-square analysis. Difference in means for data with continuous variables was obtained using either the Student's t test or the Wilcoxon signed-rank test as appropriate. Tachycardia recurrence was analyzed using the log-rank test for survival characteristics. Statistical analysis was performed with SPSS software (SPSS Statistics, Chicago, IL)
3 Results
A total of 234 subjects underwent catheter ablation and met the inclusion criteria during the study period. Of these, 127 (54 %) underwent catheter ablation using conventional fluoroscopy and 107 (46 %) underwent ablation supplemented with the electroanatomical navigation system. There was no difference in weight, height, or body surface area between groups although subjects in the conventional group were younger (13.3 versus 14.1 years, p = 0.02). The tachycardia substrates were comparable between the two groups, without significant difference in ablation target. There was no significant difference in the number of subjects who had a probe-patent foramen ovale for mapping and ablation versus those requiring a transseptal puncture or the number of subjects who had undergone a previous ablation procedure. See Table 1 for a description of subject demographics and baseline characteristics. Cryoablation was used for four subjects in the conventional group (two with right septal APs and two with AVNRT) and for eight subjects in the navigation group (four with right septal APs, two with right free wall APs, and two with AVNRT).
3.1 Fluoroscopy exposure
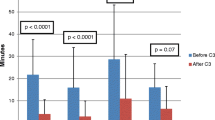
Fluoroscopy duration decreased significantly after transition from the conventional group to the navigation group, from a mean of 11.1 ± 10.1 to 3.5 ± 6.2 min overall (p < 0.001). A significant decrease in fluoroscopy exposure was observed for all tachycardia substrates with the exception of focal atrial tachycardia and idiopathic VT, the latter in whom meaningful statistical comparison could not be calculated due to an insufficient number of subjects. For those with AVNRT, the mean fluoroscopy duration decreased from 10.9 (median 7.1, range 0.4–41.0) to 1.8 (median 0.0, range 0.0–15.0) min (p = 0.0001). For right free wall APs, the mean fluoroscopy decreased from 8.8 (median 7.1, range 0.7–29.8) to 2.8 (median 0.0, range 0.0–39.0) min (p = 0.032), and for right septal APs, from 14.1 (median 10.4, range 2.0–52.5) to 2.8 (median 0.0, range 0.0–16.3) min (p = 0.050). For left-sided APs, mean fluoroscopy decreased from 10.3 (median 7.5, range 1.8–42.0) to 5.3 (median 2.6, range 0.0–20.3) min (p = 0.0065). For focal AT, fluoroscopy decreased from 27.8 (median 27.8, range 27.6–28.0) to 8.2 (median 5.0, range 0.0–22.6) min (p = 0.18). The change in fluoroscopy time by tachycardia substrate is displayed in Fig. 2.
Comparison of fluoroscopy exposure among the various tachycardia substrates before and after the introduction of the navigation system (Wilcoxon signed-rank test). Significant reductions in fluoroscopy were observed for all tachycardia substrates with the exception of focal AT and idiopathic VT. Box-and-whiskers plots are displayed (lower edge, 25th percentile; upper edge, 75th percentile; horizontal line, population median). VT is not shown, as insufficient subject numbers of subjects with this substrate were available to perform meaningful statistical analysis (see text for details)
A comparison of the first 20 subjects in the navigation group revealed a significant reduction in fluoroscopy time reflecting increased operator experience. The mean fluoroscopy duration with the first 20 procedures in the navigation group was 6.1 ± 9.9 min which decreased to a mean of 2.9 ± 4.9 min for subsequent procedures (p = 0.036).
After the introduction of the navigation system, placement of diagnostic catheters was possible without the use of fluoroscopy in 105/107 (98.1 %) of cases. For two subjects in the navigation group, brief fluoroscopy was required for acceptable placement of the His bundle catheter.
3.2 Prolonged fluoroscopy group
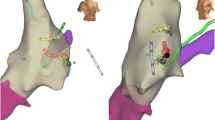
Prolonged fluoroscopy was observed in 22 subjects (17 %) in the conventional group versus three subjects (3 %) in the navigation group (p = 0.001). For these three subjects requiring prolonged fluoroscopy after transition to the navigation system, substrates included an ectopic atrial tachycardia originating from the left aspect of the atrial septum, an AP requiring ablation from within the right atrial appendage, and multiple APs (Fig. 3). These three procedures occurred during the early period of electroanatomical navigation (Fig. 4), and in each case, a review of the subject record revealed abandonment of the navigation system due to increased procedural complexity.
Scatter plot of fluoroscopy time per case arranged in chronological order during the study period. The vertical line represents transition to the electroanatomical navigation system. The horizontal line denotes fluoroscopy duration in excess of 20 min (prolonged fluoroscopy group). Prolonged fluoroscopy was observed in 22 cases prior to, and three cases after, transition to the use of the electroanatomical navigation system (p = 0.001)
Substrate analysis of prolonged fluoroscopy before and after the introduction the electroanatomical navigation system. Prolonged fluoroscopy was not observed for AVNRT, right septal accessory pathways, left-sided accessory pathways, and for ablation of ventricular arrhythmia after the transition to electroanatomical navigation. See text for a description of substrate location after the navigation system was introduced. AT focal atrial tachycardia, Mult multiple accessory pathways, left AP left-sided AP, R sept right septal AP, RFW right free wall AP, VT idiopathic VT/ectopy
3.3 Non-fluoroscopy group
Among subjects in the navigation group, 53 (50 %) were not exposed to fluoroscopy during their procedure. Specifically, fluoroscopy was not required in 23/33 subjects with AVNRT (70 %), 14/21 with right free wall APs (67 %), 8/11 with right septal APs (73 %), 5/35 with left-sided APs (14 %), 2/3 with idiopathic VT (67 %), and 1/4 with ectopic atrial tachycardia (25 %). Within this group, subjects with the diagnosis of either AVNRT or right-sided APs in the navigation group as a whole were less likely to receive any degree of fluoroscopic exposure than other subjects (p < 0.0001).
3.4 Procedural outcomes and follow-up
Total procedural time was equivalent between the two groups. Acute success was achieved in 95.5 % of subjects in the conventional group and 99.1 % of subjects in the navigation group. Of subjects in the conventional group, procedural complications included both transient AV block (n = 4) and right bundle branch block (n = 1) versus the navigation group in whom only transient AV block (n = 3) was observed. Follow-up data were available for 179/234 (76 %) subjects, including 97 subjects in the conventional group and 82 subjects in the navigation group. The median follow-up was 4.0 months (5.2 months in the conventional group and 3.1 months in the navigation group). There was no significant difference in outcome between the two groups with respect to arrhythmia recurrence at last follow-up after the catheter ablation procedure. Overall, there were 5/97 recurrences (5.2 %) in the conventional group compared to 5/82 recurrences (6.1 %) in the navigation group (log-rank, p = n.s.).
4 Discussion
This study examines the factors associated with fluoroscopic exposure during the transition period from a purely fluoroscopic approach to one of supplemental electroanatomical navigation at a single center over approximately a 5-year period. There was no difference in acute success, total procedure time, complication rate, or recurrence events among the two groups. However, mean fluoroscopy time decreased from 11.1 to 3.5 min with the use of electroanatomical navigation (p < 0.001), and the greatest benefit was observed among two groups of children: (1) those with AVNRT and/or right-sided APs, in whom the use of fluoroscopy was frequently completely eliminated (p < 0.0001), and (2) those who would otherwise be subjected to “prolonged fluoroscopy” with the conventional approach (p = 0.001).
It has now been roughly a decade since the first reports of fluoroscopy elimination using electroanatomical navigation for catheter ablation in children [7]. Although an initial report included only subjects with prior evidence of a manifest right-sided AP [7], subsequent studies demonstrated the feasibility of fluoroscopy elimination over a wide range of substrates as diverse as AVNRT and left-sided APs [15, 16]. More recently, a prospective evaluation of nonfluoroscopic navigation technology in 37 children undergoing electroanatomical navigation-guided versus 37 control subjects using conventional fluoroscopic ablation for either AVNRT or pathway-mediated tachycardia found a mean decrease in fluoroscopy time from 18.3 to 7.5 min [6]. As opposed to previous reports which were primarily feasibility studies, this report demonstrated the benefits of a nonfluoroscopic navigation system from the standpoint of the proceduralist choosing to adopt this approach, with an immediate and dramatic reduction in radiation exposure. Since then, Papagiannis and colleagues [17] described their extensive experience with catheter ablation in 192 children, focusing specifically on APs. This was the first large-scale study of electroanatomical-based ablation in children, and the group noted a dramatic improvement in fluoroscopy time for all targeted pathways, achieving an overall decrease from 39.8 ± 32.7 to 8.3 ± 8.2 min after the introduction of electroanatomical navigation.
The results of our investigation confirm those of previous reports and extend their observations. We reviewed our experience with a large cohort of pediatric subjects at a single center during the transition period to an electroanatomical navigation-based ablation approach and included all tachycardia substrates. We found a beneficial effect of the navigation system for the majority of tachycardia subgroups and observed a rapid and sustained decrease in fluoroscopy exposure after transition to this technology.
It is notable that with almost all previous pediatric studies, catheter ablation of AVNRT in children was performed with the use of the cryoablation and by targeting areas associated with slow pathway potentials. A second approach advocated for modification of the slow pathway input into the AV node consists of a set of lesions placed in a linear fashion between the tricuspid valve annulus and the lower edge of the CS os [12]. Ablation performed in such a way has been associated with a favorably low incidence of AV block and improved recurrence rates, presumably due to the complete abolition of slow pathway conduction that often ensues [12, 18]. This approach is particularly appealing when used in conjunction with an electroanatomical navigation system, since successively placed lesions can be easily visualized on a geometric shell and catheter placement at adjacent sites can be readily confirmed (Fig. 1). Importantly, we found that a high percentage of AVNRT cases (70 %) in our cohort could be safely ablated without the use of any fluoroscopy using such an approach.
In this population, the safe delivery of radiofrequency energy to the posteroseptal region for modification of the AV nodal slow pathway required a clear understanding of the major anatomic landmarks of the triangle of Koch. Considered essential to this approach was the constant visualization of a properly placed catheter within the CS, as lesions were placed between the lower margin of this structure and the TVA. Simultaneous visualization of both diagnostic and mapping catheters was only available with the Ensite NavX system during the present study and was therefore the preferred navigation system for our procedures. Recently, similar visualization capability became available with a mapping system that uses sensor-based technology whereby catheter location is derived by triangulation within a magnetic field (CARTO 3, Biosense-Webster, Diamond Bar, CA). The approach to modification of the AV node described here should be equally feasible with such a system.
In the present study, both AVNRT and right-sided APs were significantly associated with complete elimination of fluoroscopic exposure. This finding is not surprising, as transseptal puncture is not a prerequisite for the ablation of these arrhythmias, and the supplemental use of fluoroscopy is not inherently a part of the procedure. Although transseptal puncture without the use of fluoroscopy has been described in children [16], we did not adopt this approach during our initial experience with the use of electroanatomical navigation. It is likely that an experience similar to our own (i.e., persistent fluoroscopy requirement for left-sided APs) would be noted by others utilizing electroanatomical navigation for the first time in catheter ablation in children, at least until greater familiarity with the nonfluoroscopic approach is achieved.
Similar to the findings of Miyake et al. [6], we also identified an important subgroup of patients (17 % in the conventional group) who required increased fluoroscopy exposure during extensive mapping and repeated attempts at ablation due to difficulty in eliminating the target substrate. This group of children was exposed to increased doses of radiation. This group (the prolonged fluoroscopy group) was almost completely eliminated with the supplemental use of the electroanatomical navigation system in the present study, and it seems likely that this particular group derives great benefit from its use. Although some of the navigation group subjects (3 %) were exposed to prolonged fluoroscopy during the initial learning curve portion of the study, this problem was no longer observed after greater familiarity with the navigation system had been achieved.
5 Limitations
This was a single center, retrospective review which resulted in a lack of data collection in certain instances. Specifically, the radiation-absorbed dose (rad) could not be determined in the present study due to its retrospective design. Despite this, a decrease in fluoroscopy duration is usually considered the primary indicator of a successful nonfluoroscopic ablation strategy being more directly comparable than rad from one study to the next (due to variations in operator technique), making fluoroscopy duration a superior benchmark. Similarly, we were unable to determine total case duration in our sample and were instead limited in our description to the actual procedure duration. Clearly, total case duration is an important consideration when weighing the benefits of a nonfluoroscopic approach for catheter ablation in children since this, in addition to use of the navigation system itself, results in increased financial costs. We therefore feel that large-scale, prospective studies examining the superiority of electroanatomical navigation during catheter ablation in children with consideration of these and other issues are still needed before universal implementation by the pediatric electrophysiologist can be wholeheartedly recommended.
6 Conclusions
Supplemental use of an electroanatomical navigation system significantly reduced fluoroscopy exposure for children with a variety of tachycardia substrates using radiofrequency energy for ablation. Children deriving the greatest benefit of this approach were those with targets most readily accessible from the systemic venous chamber and those in whom prolonged fluoroscopy was required with the conventional fluoroscopic approach. This navigation approach was particularly suited to an anatomical slow pathway modification for children with AVNRT. These effects were observed early during the experience of a single operator and were sustained throughout the study period.
References
Van Hare, G. F., Javitz, H., Carmelli, D., Saul, J. P., Tanel, R., Fischbach, P. S., et al. (2004). Prospective assessment after pediatric cardiac ablation: demographics, medical profiles, and initial outcomes. Journal of Cardiovascular Electrophysiology, 15, 759–770.
Pierce, D. A., & Preston, D. L. (2000). Radiation-related cancer risks at low doses among atomic bomb survivors. Radiation Research, 154, 178–186.
Baruch, M., Lital, K., Tzvia, B., & Sadetzki, S. (2000). Cancer following cardiac catheterization in childhood. International Journal of Epidemiology, 29, 424–428.
Infante-Rviard, C., Mathonnet, G., & Sinnett, D. (2000). Risk of childhood leukemia associated with diagnostic irradiation and polymorphisms in DNA repair genes. Environmental Health Perspectives, 108, 495–498.
Strauss, K. J., & Kaste, S. C. (2006). ALARA in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients—a white paper executive summary. Journal of the American College of Radiology, 3, 686–688.
Miyake, C. Y., Mah, D. Y., Atallah, J., Oikle, H. P., Melgar, M. L., Alexander, M. E., et al. (2011). Nonfluoroscopic imaging systems reduce radiation exposure in children undergoing ablation of supraventricular tachycardia. Heart Rhythm, 8, 519–525.
Drago, F., Silvetti, M. S., Pino, A. D., Grutter, G., Bevilacqua, M., & Leibovich, S. (2002). Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. Journal of Cardiovascular Electrophysiology, 13, 778–782.
Alvarez, M., Tercedor, L., Almansa, I., Ros, N., Galdeano, R. S., Burillo, F., et al. (2009). Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm, 6, 1714–1720.
Smith, G., & Clark, J. M. (2007). Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing and Clinical Electrophysiology, 30, 510–8.
Sra, J., Krum, D., Schweitzer, J., & Hauck, J. (2003). Three-dimensional right atrial geometry construction and catheter tracking using cutaneous patches. Journal of Cardiovascular Electrophysiology, 14, 897.
Tuzco, V. (2007). A nonfluoroscopic approach for electrophysiogy and catheter ablation procedures using a three-dimensional navigations system. Pacing and Clinical Electrophysiology, 30, 519–525.
Antz, M., McClelland, J., Gonzalez, M., Beckman, K., Otoma, K., Tondo, C., et al. (1996). Ablation along a line between the tricuspid annulus and the coronary sinus and within the coronary sinus ostium results in a low recurrence of atrioventricular nodal reentrant tachycardia despite residual slow pathway conduction. Circulation, 94, 1–683 [abstract].
Calkins, H., Kim, Y. N., Schmaltz, S., Sousa, J., El-Atassi, R., & Leon, A. (1992). Electrogram criteria for identification of appropriate target sites for radiofrequency catheter ablation of accessory atrioventricular connections. Circulation, 85, 565–573.
Haisaguerre, M., Fisher, B., Warin, J. F., Dartigues, J. F., Lemetayer, P., & Egloff, P. (1992). Electrogram patterns predictive of successful radiofrequency catheter ablation of accessory pathways. Pacing and Clinical Electrophysiology, 15, 2138–2145.
Papagiannis, J., Tsoutsinos, A., Kirvassilis, G., Sofianidou, I., Koussi, T., Laskari, C., et al. (2006). Nonfluoroscopic catheter navigation for radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing and Clinical Electrophysiology, 29, 971–978.
Clark, J., Bockoven, J. R., Lane, J., Patel, C. R., & Smith, G. S. (2008). Use of three-dimensional catheter guidance and transesophageal echocardiography to eliminate fluoroscopy in catheter ablation of left-sided accessory pathways. Pacing and Clinical Electrophysiology, 31, 283–289.
Papagiannis, J., Avramidis, D., Alexopoulos, C., & Kirvassilis, G. (2011). Radiofrequency ablation of accessory pathways in children and congenital heart disease patients: impact of a nonfluoroscopic navigation system. Pacing and Clinical Electrophysiology, 34(10), 1288–1396.
Hayashi, M., Kobayashi, Y., Miyauchi, Y., Ino, T., Atarashi, H., & Takano, T. (2001). A randomized comparison of the straight linear approach with electrogram mapping focal approach in selective slow pathway ablation. Journal of Cardiovascular Electrophysiology, 24, 1187–1197.
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Additional information
This study provides additional evidence on the importance of 3D mapping in clinical practice. With the introduction of 3D mapping systems, the investigators were able to reduce the fluoroscopy exposure during ablation procedures in pediatric patients.
Rights and permissions
About this article
Cite this article
Wan, G., Shannon, K.M. & Moore, J.P. Factors associated with fluoroscopy exposure during pediatric catheter ablation utilizing electroanatomical mapping. J Interv Card Electrophysiol 35, 235–242 (2012). https://doi.org/10.1007/s10840-012-9701-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-012-9701-6