Abstract
Purpose
Radiofrequency catheter ablation (RFCA) of supraventricular tachyarrhythmias carries a small but non-negligible radiation risk. Studies in children already showed the feasibility of using three-dimensional mapping systems as the primary guide for catheter visualization and positioning in these RFCAs. We aim to demonstrate the feasibility and safety of such an approach in young and middle-aged patients.
Methods
Fifty patients (age 34 ± 12) with supraventricular tachyarrhythmias underwent electrophysiological study; of these, 47 patients proceeded to RFCA guided by the EnSite NavXTM system (23 with atrioventricular nodal reentry tachycardia, 16 with an accessory pathway, six with typical atrial flutter, and two with right atrial tachycardia).
Results
In 38/50 cases (76%), electroanatomical mapping avoided fluoroscopy entirely, including four cases requiring access to the left heart chambers by a retrograde approach. In the remaining 12/50 cases (24%), fluoroscopy use was limited to 122 ± 80 s, with a correspondingly low radiation exposure (dose area product 1.3 ± 1.1 mGy × m2). All procedures were acutely successful, with a procedural time of 113 ± 37 minutes, and without incurring in any major complication. Over a mean follow-up of 12 ± 3 months, we observed one recurrence of pre-excitation and one relapse of atrial flutter.
Conclusions
Our study shows that non-fluoroscopic RFCA of supraventricular tachyarrhythmias using the EnSite NavXTM system is feasible, safe, and effective in a population of relatively young adults. Our experience of a non-fluoroscopic approach in these procedures deserves consideration, particularly in the young or in other patients at higher radiation risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Radiofrequency catheter ablation (RFCA) is currently a first-line therapy for a plethora of supraventricular, atrioventricular and ventricular arrhythmias [1]. Electrophysiology (EP) procedures, including catheter ablation, are traditionally performed under fluoroscopic guidance [1] and are often complex and prolonged enough to involve a non-negligible radiation exposure [2, 3]. The relation between radiation dose from medical imaging and the attributable lifetime risk of cancer [4] and genetic anomaly [2] stresses the pivotal importance of minimization of X-ray exposure in cardiac EP practice [5].
In recent years, non-fluoroscopic three-dimensional (3D) mapping systems have been developed [6] to guide ablation during laborious electrophysiological procedures, such as electrical isolation of the pulmonary veins during ablation of atrial fibrillation and mapping of atypical atrial flutter or of ventricular tachycardia. Their use has certainly allowed to better understand and ablate complex arrhythmias, but they have been proven to confer the additional benefit of significantly reducing radiation exposure [7–9].
Non-fluoroscopic 3D navigation systems have not been used as frequently for less complex ablation procedures. In these cases, their indication does not lie in the complexity of the procedure, but only in the reduction of radiation use. In particular, studies have been published (Table 1) investigating their use for the complete or near-complete abolishment of radiation exposure during right-sided ablation of supraventricular arrhythmias in pediatric patients [10–15]. Less extensive data are available for adults [16, 17].
Our study aims to assess the feasibility, efficacy, and safety of a non-fluoroscopic approach to visualize and manipulate intra-cardiac catheters during right- and left-sided ablation procedures for supraventricular tachyarrhythmias in adults aged <50.
2 Methods
2.1 Study population
Among patients admitted to our institutions from July 2009 to July 2010 with an indication to undergo RFCA of paroxysmal SVT or atrial flutter, we enrolled 50 consecutive patients who were aged 14 to 50 and who were willing to provide informed consent to an experimental procedure (or whose parents were willing to do so, if underage). Of these, 33 patients had ECG documentation of their clinical tachycardia, ten had pre-excitation, while seven underwent an EP study on the basis of symptoms suggesting paroxysmal SVT.
2.2 Electrophysiological procedure
Each patient underwent the procedure while fasting and without sedation. Seven skin patches were applied to guide the non-fluoroscopic EnSite NavX™ (St. Jude Medical, St Paul, MN, USA) navigation system, version 8.0. Three pairs of nominally orthogonal skin patches in x-, y-, and z-axes were positioned as usual; an additional patch was positioned on the abdomen to serve as a reference during the initial phase of catheterization. Vascular access was obtained through the right femoral vein and, if necessary, through the right internal jugular vein and/or right femoral artery. Whenever fluoroscopy was deemed necessary for orientation and confirmation of catheter location, it was performed using a Toshiba Radiographic/Fluoroscopic Unit (CAS 10A, Toshiba Medical System Corporation, Japan), using the minimum fluoroscopy dose compatible with adequate imaging.
2.3 Geometry reconstruction/chamber anatomy acquisition
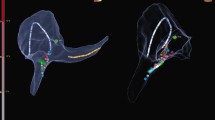
We used the EnSite NavX™ system to navigate the femoral vein, using a skin patch as a positional reference. We were able to visualize the fixed-curve quadripolar diagnostic catheter, connected to the cable, as it passed through the femoral sheath and into the venous system (Fig. 1). Once the right atrium was reached, the catheter was advanced and pulled back to mark first the inferior vena cava, then the superior one. Then, a sketchy right atrial geometry was created in the attempt to reconstruct the interatrial septum and to localize the ostium of the coronary sinus (CS), where a decapolar diagnostic catheter was then positioned through a femoral or a jugular approach. Then the CS catheter served as a positional reference for the remainder of the procedure.
Non-fluoroscopic three-view reconstruction of the venous system (olive green) up to the right atrium (gray). Collateral branches (red) are clearly visualized. Catheter advancement is continuously monitored using two simultaneous views; if the catheter tip diverges from the femoral–caval axis, a collateral branch is identified and it is possible to retract the tip to the branching point and advance it back into the correct trajectory. AP antero-posterior, RAO right anterior oblique, LAO left anterior oblique
Using the previously reconstructed venous geometry, we advanced the other diagnostic catheter as well as the ablation catheter up to the right atrium. After performing impedance calibration and compensation for respiratory movements, one of the catheters was used as roving catheter and swept through the cardiac chambers, defining endocardial boundaries and obtaining a more accurate geometry of the right atrium, including its appendage, the His bundle region, the CS ostium, and the tricuspid valve. If necessary, a separate geometry was acquired in a similar fashion for the right ventricle and its outflow tract. From this point on, the EP study and subsequent RFCA were carried out using standard protocols and procedures.
In case the EP study demonstrated a left-sided accessory pathway, right femoral artery access was achieved and the ablation/mapping catheter, connected to the cable, was advanced using the mapping system to navigate the arterial root and acquire 3D anatomy.
2.4 Procedure and fluoroscopy times
Procedure time was defined as the interval from the initial placement of the femoral venous sheath to its ultimate removal. Geometry time was defined as the interval from the insertion of the first catheter until the beginning of the EP study. We also kept track of the number of ablation pulses and the total ablation time (defined as the cumulative time ablation energy was turned on). Fluoroscopy time was defined as the cumulative duration of fluoroscopy during the entire procedure. Radiation dose was defined as the calculated dose to the patient as recorded by the dose area product (DAP) meter.
2.5 Procedural success and complications
The definition of procedural success depended on the mechanism of the patient’s tachycardia. Ablation of atrioventricular nodal reentrant tachycardia (AVNRT) was considered successful if no SVT could be induced for >30 min after the last ablation pulse, neither under basal conditions nor with intravenous isoprenaline. Ablation of tachycardias mediated by accessory pathways was deemed successful if no SVT could be induced for >30 min after the last ablation pulse, neither under basal conditions nor with intravenous isoprenaline, accompanied by documentation of transient atrioventricular block with adenosine. Ablation of accessory pathways with manifest pre-excitation but no recorded SVT was considered successful if pre-excitation disappeared and atrioventricular and ventriculo-atrial block could be induced by intravenous adenosine. Ablation of typical atrial flutter was deemed successful if bidirectional isthmus conduction block could be achieved. Finally, ablation of atrial tachycardia was considered successful if no tachycardia could be induced for >30 min after the last ablation pulse, neither under basal conditions nor with intravenous isoprenaline.
All patients underwent a post-procedural echocardiogram to exclude pericardial effusion or other acute complications. We also recorded any other complication that might have occurred during the procedure or during the same hospital stay.
2.6 Follow-up
A follow-up outpatient visit was scheduled for each patient at 1, 3, and 6 months to take an updated history, perform physical examination, and obtain a 12-lead ECG.
3 Results
We enrolled 50 patients (22 males, 28 females) with a mean age of 34 ± 12 years. Of these, 23 patients (46%) had AVNRT, of which two were atypical; 16 patients (32%) had an accessory pathway, of which 11 caused manifest pre-excitation while five were concealed; six (12%) had typical atrial flutter, four (8%) had right atrial tachycardia; in one case (2%), no arrhythmia could be induced during the EP study. Of the 16 accessory pathways, six were found to be located in the right postero-septal region, four in the right lateral region, one in the mid-septal region, one in the left postero-septal region, one in the left posterior region, and three in the left lateral region.
All patients had structurally normal hearts except three: one patient with atrial tachycardia in myotonic dystrophy type 1, one patient with typical atrial flutter in dilated hypertrophic cardiomyopathy, and one patient with a right-sided AP in post-myocarditis dilated cardiomyopathy. Three patients had previously undergone an attempt at RFCA, one for AVNRT, one for left lateral accessory pathway, and the other for atrial flutter.
3.1 Procedural features
Of the 50 enrolled patients, 47 (94%) underwent an ablation following the EP study. An ablation was not performed in three patients: one in whom no arrhythmia could be induced during the EP study, one in whom a junctional tachycardia could be induced, and one with myotonic dystrophy type 1 who was found to have inducible multifocal atrial tachycardia. Table 2 summarizes demographics and procedural data of the 50 enrolled patients.
The mean procedure time was 112.5 ± 36.5 min (range, 60–210 min). The mean time needed to place all catheters inside the heart, including the time for accurate geometry reconstruction of the relevant heart chambers, was 16.4 ± 7.6 min (range, 8–50 min). The mean geometry time takes into account the fact that in one patient (2%), the CS catheter became dislocated and a second geometry acquisition was required.
3.2 Procedural success
RFCA was acutely successful in all 47 patients who underwent it. The mean number of ablation pulses was 10.1 ± 7.1 (range, 3–32), the total ablation time was a mean of 8.4 ± 5.6 min (range, 2.8–23.7 min) with a mean RF pulse duration of 62.6 ± 33.1 s.
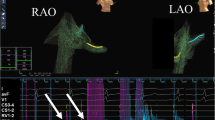
In the 23 patients with AVNRT, conduction over the slow pathway was completely eliminated in 20 cases and modified in the remaining three (Fig. 2). In the five patients with a left-sided accessory pathway, ablation pulses were delivered in two cases from both the coronary sinus and the mitral valve annulus by a retrograde approach (Fig. 3), in two cases directly at the mitral valve annulus by a retrograde approach, and in one case from the left atrium through a patent foramen ovale.
Ablation of a nodal slow pathway without fluoroscopy performed in a 35-year-old woman. The area of the triangle of Koch is marked in red. The mapping system provides 3D reconstruction of the conduction system, marking the areas where the His potential can be recorded and where the compact atrioventricular node (mechanical junctional beats). In this case, ablation pulses (white) were safely delivered in the postero-septal region. LAO left anterior oblique, LL left lateral
Panel (a) shows a three-view reconstruction of both right- and left-sided chambers in a 16-year-old with a left posterior AP, obtained without the help of fluoroscopy. To reconstruct the aortic arch, the mapping catheter was gently advanced till it reached the aortic valve, then it was pulled back and bent to give it a J-like loop shape. The looped catheter was then pushed forward while being visualized on the mapping system monitor in both RAO and LAO views. The LAO projection shows ablation pulses (white) from inside the coronary sinus. Panel (b) shows two modified views to better illustrate ablation pulses (white) at the mitral annulus, which were necessary to ablate the patient’s AP. AP antero-posterior, LAO left anterior oblique
The post-procedural echocardiogram was normal in all patients. Only one procedural complication was observed: transient second-degree atrioventricular block type 1 in a patient with AVNRT, which recovered completely 5 h after the procedure.
3.3 Radiation exposure
An EP study and RFCA were performed completely without fluoroscopy in 38/50 patients (76%). Notably, this included the three procedures requiring radiofrequency pulses at the mitral valve annulus. In the remaining 12/50 cases (24%) that did require some fluoroscopy, the mean fluoroscopy time was just 121.6 ± 79.7 s (range, 12–300 s) and the mean radiation dose, calculated as DAP, was 1.3 ± 1.1 mGy × m2 (range, 0.06–3.51 mGy × m2). In these 12 patients, fluoroscopy was needed in a total of 15 occurrences: to check guide wires in three cases (20%), to cannulate the CS in six cases (40%), to check catheter stability during RF delivery in five cases (33%), and to confirm catheter location in one case (7%).
3.4 Follow-up
The mean follow-up time was 12 ± 3 months, during which no procedure-related complication occurred. Of the 47 patients who had undergone a RFCA, one showed early recovery of conduction in a right-sided postero-septal accessory pathway, despite acute procedural success, and one patient had a recurrence of clinical atrial flutter. Two patients who had undergone slow-pathway ablation for AVNRT complained of palpitations without documentation of arrhythmias on ECG Holter monitoring.
4 Discussion
Our study demonstrates the feasibility, efficacy, and safety of using the non-fluoroscopic EnSite NavX™ mapping system as the sole or prevailing imaging modality to guide ablation of a wide range of supraventricular tachyarrhythmias in young adults. To the best of our knowledge, this is also the first report of its use for both left- and right-sided ablation in a non-pediatric population.
Supraventricular tachyarrhythmias such as AVRT and AVNRT are often diagnosed in young patients and considered for catheter ablation, a relatively safe, highly successful procedure, which current guidelines suggest as a first-line therapeutic option in most such cases [19]. Traditional ablation techniques, however, are based on fluoroscopic localization of catheters and are therefore inevitably associated with low-dose X-ray exposure.
Statistical limitations make it difficult to evaluate the long-term cancer risks associated with low doses of radiation, but the BEIR VII of the US National Academies concludes that current evidence supports a “linear-no-threshold” model, in which a simple linear relation exists between cancer risk and radiation dose [20]. According to this model, there is no threshold dose below which radiation carries no risks. Moreover, recent evidence suggests that even low doses may have appreciable noxious effects, as observed on chromosomal DNA damage in circulating lymphocytes of children undergoing cardiac catheterization, both acutely and in the long term [21–24]. It also bears particular importance to note that radiation risks are not distributed homogeneously among the population, as women and younger individuals are at relatively higher risk, due to both a greater vulnerability to radiation effects and a longer life expectancy [20, 25, 26].
Medical imaging accounts for most of man-made background radiation (about 79% in the USA) [20]. An extensive study in the USA showed that, over 2 years, a large population of non-elderly adults received from cumulative medical imaging a mean effective dose of 2.4 mSv, of which 75.4% depended on nuclear medicine and CT [23, 24]. More specifically, the field of cardiology is no stranger to radiation use: in a study on 50 consecutive patients admitted to a tertiary-center cardiology ward, a median cumulative dose of 60.6 mSv was observed, 48% of which depended on invasive cardiological investigations [23]. Indeed, most procedures requiring catheterization entail radiation doses at the very least equal to that of a chest CT (about 7–15 mSv) [26] and both stochastic effects (such as carcinogenesis) and deterministic effects (such as skin injury) should be considered among the possible risks. For these reasons, efforts to minimize radiation use in invasive cardiology are likely to benefit both operators and patients (especially relatively young ones, as in the present study) and represent a pivotal step in the implementation of the “ALARA” policy (radiation doses “as low as reasonably achievable”) [5].
Advanced electrophysiological procedures (such as ablation of atrial fibrillation or ventricular tachycardia) are often quite prolonged in time, technically complex, and traditionally associated with abundant use of fluoroscopy. Electroanatomical mapping systems have become an important part of such procedures mainly because they help guide ablation, with reduced radiation use being a welcome added value. The latter benefit would instead represent the main advantage justifying their use in simpler ablation procedures (e.g., atrial flutter, AVRT, or AVNRT) and is of course most relevant to patients at higher risk from radiation, such as in pediatric practice.
Several reports have indeed been published on SVT ablation guided by different electroanatomical mapping systems in children [10–15] although the greatest reduction in radiation use has been documented in three studies employing the EnSite NavX™ system. A 2006 study by Papagiannis and colleagues [10] showed an overall reduction in fluoroscopy time in AVNRT and AVRT ablation (including left-sided APs), although the reduction was not statistically significant in AVNRT cases and no procedure was performed without radiation entirely. Two 2007 studies by Tuzcu [14] and Smith [13] completely eliminated fluoroscopy in 80–86% of cases and significantly reduced it in the remaining 14–20%, with the latter study also including left-sided ablations. Of note, no procedure requiring access to left heart chambers was performed without fluoroscopy. In adults, completely non-fluoroscopic ablation of supraventricular tachyarrhythmias using the EnSite NavX™ system was described in a few case reports [27, 28] and in one randomized trial [16], in which fluoroscopy was completely avoided in only 27% of patients treated with the EnSite NavX™ system and in none of those treated using the CARTO system. Furthermore, Alvarez and colleagues recently demonstrated the feasibility of a completely non-fluoroscopic approach in 98% of patients undergoing RFCA of AVNRT [17].
Our own experience strikes a middle ground between previous reports, as we were able to avoid fluoroscopy entirely in 76% of adult cases, which included a wide variety of both right and left supraventricular arrhythmias. While this is not as high a percentage as that previously reported in children, we believe the difference may partly depend on the greater anatomical complexity of catheter ablation in adult hearts and on the wider range of tachyarrhythmias in our population. On the other hand, our percentage was higher than that reported in the study by Earley et al. [16], probably also because complete elimination of fluoroscopy was not the principal aim of that work.
With regard to other procedural data, we found that our total procedural times are comparable to previous studies despite longer geometry times. We believe this highlights the importance of accurate geometry reconstruction, as it suggests that spending more time obtaining an accurate geometry reconstruction may speed up subsequent phases of the procedure. Indeed, basing ablation on an accurate 3D model of cardiac anatomy provides certain remarkable technical advantages, which both save time and make the procedure easier. Electroanatomical mapping confers a deeper insight into the patient’s individual cardiac anatomy, as geometry reconstruction will already have compelled the operator to exercise the movements necessary to reach the various intra-cardiac sites relevant to the subsequent ablation procedure. The latter is also sped up by the fact that the 3D model enables the operator to continuously observe two projections at the same time. Certain advantages are more specific to the exact type of arrhythmic substrate. For example, conventional ablation of an accessory pathway is sometimes complicated by either minimization or transient disappearance of pre-excitation after the initial radiofrequency pulse, which makes accurate localization of the AP more difficult for subsequent pulses and requires further time-consuming electrophysiological maneuvers. This problem can be avoided using a 3D mapping system, as the location of the AP can be marked on the reconstructed geometry and still used to direct radiofrequency pulses at a time when pre-excitation becomes minimal or intermittent. AVNRT ablation is also facilitated by a precise definition of the anatomy of the triangle of Koch (particularly the area just anterior to the CS ostium) and of the His bundle (which is visualized as an area rather than just a point). The 3D view of this region is particularly important to understand the anatomical continuum of the transition from right atrium to CS ostium, which in some patients may take a windsock shape. Finally, during ablation of atrial flutter, if an incomplete line has been drawn and the arrhythmia has been slowed down but not interrupted, an activation map will make localization of the gap in the line much easier.
As far as procedural safety is concerned, the only complication of our series was a transient type 1 atrioventricular block during AVNRT ablation. It bears emphasis that, in such case, the position of the ablation catheter was validated also by fluoroscopy due to the presence of a far-field His potential on the ablation catheter. In fact, in our experience, a careful and continuous monitoring of intracavitary electrograms during a completely non-fluoroscopic procedure is even more important than during conventional fluoroscopic approaches, in order to better comprehend the position of catheters and also to increase the safety of radiofrequency delivery.
4.1 Study limitations
The subject of our work certainly requires further studies, particularly prospective randomized trials. We recognize that our work is limited by its own nature as a feasibility study, by the small population, and possibly, also by the fact that it was conducted by operators who were very experienced with conventional ablation procedures. We also acknowledge that using the EnSite NavX™ carries with it certain costs, but these can only be properly evaluated by a cost/benefit analysis, which is beyond the scope of the present study. Finally, we regret that no data were available on radiation exposure for the operators, particularly considering that recent evidence from Venneri and colleagues demonstrates that invasive cardiologists have non-negligible lifetime attributable risk of cancer [28].
5 Conclusion
Our study illustrates that non-fluoroscopic ablation of supraventricular tachyarrhythmias guided by the EnSite NavX™ system is feasible, effective, and safe in a population of relatively young adults, and that fluoroscopy use can be avoided even in ablations requiring access to left heart chambers. We believe our experience justifies consideration of a non-fluoroscopic approach in these procedures, particularly in the young or in other patients at higher radiation risk.
References
Cappato, R., & Kuck, K. H. (2000). Catheter ablation in the year 2000. Current Opinion in Cardiology, 15, 29–40.
Perisinakis, K., Damilakis, J., Theocharopoulos, N., Manios, E., Vardas, P., & Gourtsoyiannis, N. (2001). Accurate assessment of patient effective radiation dose and associated detriment risk from radiofrequency catheter ablation procedures. Circulation, 104, 58–62.
Smith, I. R., Rivers, J. T., Hayes, J., Stafford, W., & Codd, C. (2009). Reassessment of radiation risks from electrophysiology procedures compared to coronary angiography. Heart, Lung & Circulation, 18, 191–9.
Einstein, A. J. (2009). Medical imaging: the radiation issue. Nature Reviews Cardiology, 6, 436–8.
Hirshfeld, J. W., Jr., Balter, S., Brinker, J. A., Kern, M. J., Klein, L. W., Lindsay, B. D., et al. (2005). ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. Circulation, 111, 511–32.
Packer, D. L. (2005). Three-dimensional mapping in interventional electrophysiology: techniques and technology. Journal of Cardiovascular Electrophysiology, 16, 1110–6.
Estner, H. L., Deisenhofer, I., Luik, A., Ndrepepa, G., von Bary, C., Zrenner, B., et al. (2006). Electrical isolation of pulmonary veins in patients with atrial fibrillation: reduction of fluoroscopy exposure and procedure duration by the use of a non-fluoroscopic navigation system (NavX). Europace, 8, 583–7.
Rotter, M., Takahashi, Y., Sanders, P., Haissaguerre, M., Jais, P., Hsu, L. F., et al. (2005). Reduction of fluoroscopy exposure and procedure duration during ablation of atrial fibrillation using a novel anatomical navigation system. European Heart Journal, 26, 1415–21.
Sporton, S. C., Earley, M. J., Nathan, A. W., & Schilling, R. J. (2004). Electroanatomic versus fluoroscopic mapping for catheter ablation procedures: a prospective randomized study. Journal of Cardiovascular Electrophysiology, 15, 310–5.
Papagiannis, J., Tsoutsinos, A., Kirvassilis, G., Sofianidou, I., Koussi, T., Laskari, C., et al. (2006). Nonfluoroscopic catheter navigation for radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing and Clinical Electrophysiology, 29, 971–8.
Drago, F., Silvetti, M. S., Di Pino, A., Grutter, G., Bevilacqua, M., & Leibovich, S. (2002). Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. Journal of Cardiovascular Electrophysiology, 13, 778–82.
Papez, A. L., Al-Ahdab, M., Dick, M., & Fischbach, P. S. (2007). Impact of a computer assisted navigation system on radiation exposure during pediatric ablation procedures. Journal of Interventional Cardiac Electrophysiology, 19, 121–7.
Smith, G., & Clark, J. M. (2007). Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing and Clinical Electrophysiology, 30, 510–8.
Tuzcu, V. (2007). A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing and Clinical Electrophysiology, 30, 519–25.
Kammeraad, J., Udink ten Cate, F., Simmers, T., Emmel, M., Wittkampf, F. H., & Sreeram, N. (2004). Radiofrequency catheter ablation of atrioventricular nodal reentrant tachycardia in children aided by the LocaLisa mapping system. Europace, 6, 209–14.
Earley, M. J., Showkathali, R., Alzetani, M., Kistler, P. M., Gupta, D., Abrams, D. J., et al. (2006). Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. European Heart Journal, 27, 1223–9.
Alvarez, M., Tercedor, L., Almansa, I., Ros, N., Galdeano, R. S., Burillo, F., et al. (2009). Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm, 6, 1714–20.
Hindricks, G., Willems, S., Kautzner, J., De Chillou, C., Wiedemann, M., Schepel, S., et al. (2009). Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use: a prospective randomized multicenter study. Journal of Cardiovascular Electrophysiology, 20(7), 734–40.
Blomström-Lundqvist, C., Scheinman, M. M., Aliot, E. M., Alpert, J. S., Calkins, H., Camm, A. J., et al. (2003). ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias-executive summary. Circulation, 108, 1871–909.
Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation BoRER, Division on Earth and Life Studies, National Research Council of the National Academies (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII-phase 2. Washington, DC; National Academies Press.
Ait-Ali, L., Andreassi, M. G., Foffa, I., Spadoni, I., Vano, E., & Picano, E. (2010). Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart, 96(4), 269–74.
Andreassi, M. G., Ait-Ali, L., Botto, N., Manfredi, S., Mottola, G., & Picano, E. (2006). Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. European Heart Journal, 27, 2703–8.
Bedetti, G., Botto, N., Andreassi, M. G., Traino, C., Vano, E., & Picano, E. (2008). Cumulative patient effective dose in cardiology. The British Journal of Radiology, 81, 699–705.
Beels, L., Bacher, K., De Wolf, D., Werbrouck, J., & Thierens, H. (2009). gamma-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation, 120, 1903–9.
Brenner, D. J., & Hall, E. J. (2007). Computed tomography—an increasing source of radiation exposure. The New England Journal of Medicine, 357, 2277–84.
Fazel, R., Krumholz, H. M., Wang, Y., Ross, J. S., Chen, J., Ting, H. H., et al. (2009). Exposure to low-dose ionizing radiation from medical imaging procedures. The New England Journal of Medicine, 361, 849–57.
Pachon, M., Arias, M. A., Castellanos, E., & Puchol, A. (2009). No fluoroscopy for cavotricuspid isthmus-dependent right atrial flutter ablation. Heart Rhythm, 6, 433–4.
Venneri, L., Rossi, F., Botto, N., Andreassi, M. G., Salcone, N., Emad, A., et al. (2009). Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council's Biological Effects of Ionizing Radiation VII Report. American Heart Journal, 157, 118–24.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casella, M., Pelargonio, G., Dello Russo, A. et al. “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavX™ mapping system: personal experience and review of the literature. J Interv Card Electrophysiol 31, 109–118 (2011). https://doi.org/10.1007/s10840-011-9553-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-011-9553-5