Abstract
Purpose
To compare morphokinetic parameters in embryos obtained from women with and without endometriosis.
Methods
We evaluated a total of 3471 embryos resulting from 434 oocyte retrievals performed at a single academic center. One thousand seventy-eight embryos were obtained from women affected by endometriosis and 2393 came from unaffected controls. All embryos were cultured in a time-lapse incubator chamber for up to 6 days. IVF cycle outcomes and morphokinetic parameters collected prospectively were retrospectively reviewed.
Results
Morphokinetic data suggest that embryo development is impaired in embryos obtained from women with endometriosis (EE). EE were slower to achieve the 2–8 cell stages compared to control embryos (CE) (p < 0.001); additionally, time to compaction was delayed compared to CE (p = 0.015). The timing of late developmental events, including morulation and blastulation was also delayed in the endometriosis cohort (p < 0.001). In addition to demonstrating delayed cell cycle milestones, EE were less likely than controls to progress to morula, blastocyst, and expanded blastocyst stages (p < 0.001). Furthermore, a smaller proportion of embryos in the endometriosis group fell into optimal kinetic ranges for cc2 (p = 0.003), t5 (p = 0.019), tSB (p < 0.001), and tEB (p = 0.007). There were no significant differences in clinical pregnancy or live birth rates between groups.
Conclusion
Embryos from endometriosis patients demonstrate impairments in both early and late developmental events, and progress to the morula, blastocyst, and expanded blastocyst stages at lower rates than control embryos. Despite these differences, IVF outcomes are similar for patients with and without endometriosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis, defined by the presence of ectopic endometrial implants, affects up to 50% of women with infertility [1,2,3]. In the presence of endometriosis, a couple’s monthly likelihood of conception decreases from 15–20% to 2–10% [1, 4, 5].
A number of mechanisms are thought to contribute to impaired fertility in the setting of endometriosis. First, anatomic distortion can negatively affect tubal and ovarian function. Additionally, the inflammatory environment characteristic of endometriosis negatively impacts sperm motility and tubal capacity [6]. Finally, although controversial, some data suggest that oocyte quality and embryonic development are also negatively affected. Studies of embryo development in vitro show that embryos obtained from women with endometriosis are more likely to demonstrate aberrant and arrested growth [7]. Similarly, studies of donor oocytes indicate that oocyte quality is impaired. Healthy women who receive donor oocytes from women with endometriosis have lower implantation and pregnancy rates than those who receive unaffected oocytes. Further, women with endometriosis who receive an egg from an unaffected donor have pregnancy rates equivalent to those of healthy recipients [7,8,9,10,11,12]. Despite these observations, most recent studies of IVF outcomes in women with endometriosis show pregnancy and live birth rates comparable to those of healthy women [13,14,15].
Until recently, observations about embryo quality in patients affected by endometriosis have been limited to static morphologic assessments [12, 13, 16]. The advent of time-lapse microscopy (TLM) in embryology has allowed for real-time observation of embryonic development and the identification of transient morphologic features. The quantitative assessment of cell-cycle parameters in embryos derived from endometriosis-affected oocytes may provide further insight into the impact of the disease on embryo quality and development. Cell cycle timings for early cleavage events, compaction, and blastulation have been associated with implantation potential [17,18,19,20,21,22]. Several studies, including a meta-analysis of 5 randomized trials, have demonstrated that embryo morphokinetic data derived from TLM improves clinical outcomes, increasing live birth rates, and reducing miscarriage rates [21, 23,24,25].
This study aims to compare morphokinetic parameters in embryos derived from endometriosis-affected oocytes to unaffected controls, with the goal of gaining insight into the impairments in embryonic development associated with endometriosis.
Methods
Patient cohort
The study cohort consisted of women undergoing in vitro fertilization (IVF) with a history of laparoscopy-confirmed endometriosis or an endometrioma present on imaging at the time of ovarian stimulation. The comparison group was comprised of those undergoing IVF for the indications of (1) prior salpingectomy or tubal ligation, (2) male factor infertility not requiring percutaneous epididymal sperm aspiration (PESA) or testicular sperm extraction (TESE), (3) uterine factor infertility, (4) need for donor sperm due to severe male factor infertility, same-sex couple, or lack of partner, (5) preimplantation genetic screening for unaffected carriers of an autosomal recessive disorder, (6) egg donation, or (7) elective fertility preservation. The sample was limited to patients between the ages of 18 and 39 who underwent IVF between 2014 and 2019 at a single academic center. All patients who underwent IVF during this timeframe for the indications described above were included. The year 2014 was selected as the onset of the study period, as this was the date when time-lapse microscopy was used consistently in our embryology lab.
The control cohort was designed to include patients whose underlying diagnoses would be unlikely to impact embryo development or quality. Those with polycystic ovarian syndrome, recurrent pregnancy loss, unexplained infertility, or diminished ovarian reserve as defined by the Bologna criteria, were excluded, as these conditions are known or suspected to impact early embryo development. Additionally, records were excluded if there was a reported history of endometriosis but no operative report or endometrioma on imaging. An endometrioma was diagnosed on imaging when an ovarian cyst was present containing ground-glass or low-level echoes, without features of malignancy, such as Doppler flow or wall nodules. To reduce the chance that a hemorrhagic cyst was misidentified as an endometrioma, the diagnosis was made only if the cyst was present on 2 ultrasounds at least 6 weeks apart. Patients diagnosed with endometriosis on imaging were staged according to endometrioma size.
After approval by our Institutional Review Board (IRB # 19–997), patients were identified via the medical record, and cycle outcomes and embryonic morphokinetic data were reviewed retrospectively. Morphokinetic data was collected prospectively prior to this review.
Ovarian stimulation
Stimulation protocols were selected based on patient age, ovarian reserve testing, and prior response to stimulation. Oocyte retrieval was performed by transvaginal follicle aspiration under ultrasound guidance 36 h after final follicular maturation was induced by human chronic gonadotropin (hCG) and/or a gonadotropin-releasing hormone (GnRH) agonist.
Antagonist, microdose flare, standard long, agonist–antagonist, and mini-stimulation protocols were used. The agonist–antagonist protocol consisted of administration of a GnRH agonist, followed by a GnRH antagonist during ovarian stimulation. The mini-stimulation protocol included clomiphene citrate or letrozole followed by gonadotropins, with or without a GnRH antagonist. Recombinant follicle-stimulating hormone (FSH) was administered for ovarian stimulation, with or without a medication containing luteinizing hormone (LH) activity (either urinary menotropins or hCG). Initial gonadotropin dose was selected based on age, anti-Mullerian hormone (AMH) levels, antral follicle count, and stimulation history. Doses were increased as needed during stimulation, with a maximum dose of 450 IU/day.
Fertilization and embryo culture
Cumulus-oocyte complexes (COCs) were placed in HTF medium (Life Global; Guilford, CT) with 10% human serum albumin (Cooper-Surgical, Trumball, CT) with an oil overlay. The COCs were cultured at 37 °C with 6% CO2 and air for 2–3 h. In preparation for intracytoplasmic sperm injection (ICSI), COC’s were treated with hyaluronidase (Cooper-Surgical) to aid in removal of cumulus cells (Cooper-Surgical). ICSI was performed in all cycles on all metaphase II (MII) oocytes using ejaculated sperm. Sperm were obtained from ejaculated semen samples using density gradient centrifugation. Oocytes were evaluated for fertilization 16–18 h after insemination.
Fertilized zygotes were then placed into an EmbryoSlide time-lapse culture dish (VitroLife) and cultured in 25uL of growth medium overlaid with 1.4 mL washed oil (Life Global). Zygotes were cultured for a maximum of 6 days at 37°Cwith 6% CO2 and 6% O2.
Embryo grading and time-lapse microscopy
The EmbryoScope time-lapse microscopy system captured 200 × images of each embryo in 5–7 different focal planes every 15 min for the duration of culture. The embryos were evaluated by embryology staff on a daily basis by viewing time-lapse video footage and notating both embryo morphology and cell cycle events. EmbryoScope data were reviewed prospectively prior to the initiation of this retrospective study.
Cleavage-stage embryos were evaluated for blastomere symmetry, cell stage, fragmentation, and signs of increased cell:cell adherence or compaction. Embryos were also evaluated for the presence of multinucleation and abnormal division patterns such as direct uneven cleavage (division from 1 to 3 cells or 2 to 5 cells). Embryos were classified as compacting when 3 cells had merged and as morula when 90% of the embryo had undergone compaction. Blastocysts were assessed on days 5 and 6 (114–116 h and 138–140 h) for growth of the inner cell mass, appearance of the trophectoderm, and expansion.
Blastocysts were graded using the European Society of Human Reproduction (ESHRE) and Embryology-Alpha scoring system [26]. Grades were based on blastocyst maturity, growth of the inner cell mass, and organization of the trophectoderm, as published previously [27,28,29]. In frozen cycles, blastocysts were cryopreserved on day 5 if they were expanded and had an inner cell mass/trophectoderm score of 1 or 2. Embryos that did not meet this criteria were cultured until day 6 and frozen if they demonstrated good morphology and a discrete inner cell mass. In fresh cycles, blastocysts were transferred on day 5, and additional good-quality blastocysts were cryopreserved for future use. Biopsies of the trophectoderm for preimplantation genetic testing for aneuploidy (PGT-A) were performed on days 5 or 6. Embryos were cryopreserved through vitrification using the Rapid-i-carrier (Vitrolife), as described in detail elsewhere [29].
The timing of cell cycle events was expressed in hours (h) following insemination, with ICSI serving as t0. The following kinetic parameters were evaluated: timing of development to two-cell, t2, t3, t4, t5, t7, t8, t9, compaction (tSC), morulation (tM), start of blastulation (tSB), blastulation (tB), expanded blastocyst (tEB), and hatching blastocyst (tHB). The cell cycle intervals cc2 (t3-t2), s2 (t4-t3), and cc3 (t5-t3) were also assessed. Optimal kinetic ranges for specific cell cycle events were established based on work from our laboratory as well other published data [17,18,19,20, 30, 31]. Optimal timings were defined as follows: cc2 (> 5 and ≤ 11.9 h), s2 (≤ 1 h), t5 (45–57 h), cc3 (9.7–21 h), tSB (< 96.2 h), and tEB (≤ 116 h). Percentages of embryos meeting each benchmark were calculated.
Embryo transfer
Embryos were selected for transfer based on a combination of morphology grade at the cleavage or blastocyst stage as well as cell growth kinetics. Both fresh and frozen embryo transfers were included in this cohort. Decisions to proceed with fresh or frozen transfer were made based on risk for ovarian hyperstimulation syndrome, pre-trigger progesterone levels, desire for pre-implantation genetic testing, and patient preference. Fresh embryo transfer was performed on days 3 or 5, depending on the number of available zygotes, whereas frozen transfers were performed only for blastocysts. Frozen embryo transfers were performed after estrogen priming, followed by 6 days of intramuscular progesterone in oil or intravaginal micronized progesterone.
The number of embryos transferred was determined based on patient age, embryo quality, medical and infertility history, and patient preference. Embryo transfers were performed under sonographic guidance with a Wallace catheter. Implantation rates were calculated based on the number of gestational sacs visualized on ultrasound, and clinical pregnancy was defined by the presence of an embryo with cardiac activity at 6–8 weeks’ gestation.
Statistical analysis
Approximately normally distributed continuous measures were summarized using means and standard deviations and compared using two-sample t tests or ANOVA tests. Continuous measures that showed departure from normality and ordinal measures were summarized using medians and quartiles and compared using Wilcoxon rank sum tests or Kruskal–Wallis tests. Categorical factors were summarized using frequencies and percentages and were compared using Pearson’s chi-square tests or Fisher’s exact tests. Post-hoc pairwise comparisons were done using the Bonferroni adjustment. In order to account for the fact that some patients underwent multiple retrievals and generated multiple embryos, generalized estimating equation (GEE) models were fit for morphokinetic parameters, controlling for age and patient effects; exchangeable working covariance structure was assumed for embryos coming from the same patient. All analyses were done using SAS (version 9.4, The SAS Institute, Cary, NC) and p-values < 0.05 were considered statistically significant. Figures were generated using GraphPad Prism (version 8.4.0).
Results
Patient characteristics
Eight hundred nineteen patient charts were reviewed for eligibility. Four hundred thirty-four (434) ovarian stimulation cycles resulting in oocyte retrieval met inclusion criteria. Two hundred thirty-three (233) unique patients without endometriosis underwent 286 cycles, and 126 patients with endometriosis underwent 148 cycles. Among the control group, 171 (59.7%) cycles were performed for male-factor infertility not requiring surgical intervention, 52 for tubal factor infertility (18.2%), 38 cycles for uterine factor infertility (13.2%), 14 (4.9%) due to the need for donor sperm, 6 for pre-implantation genetic testing for unaffected carriers of autosomal recessive disorders (2.1%), 2 for elective fertility preservation (0.7%), 2 for a maternal cardiac condition requiring use of a surrogate (0.7%), and 1 for egg donation (0.3%). With the exception of one patient who had undergone trachelectomy, all patients with uterine factor infertility planned to use a gestational carrier or undergo uterine transplantation; therefore, we expect that neither embryo development nor implantation rate would be impaired. Those that lacked a uterus had a diagnosis of Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome (n = 30) or had undergone hysterectomy (n = 3). Two patients had Asherman syndrome and 2 had a history of placenta accreta. In the endometriosis group, 120 (95.2%) diagnoses were made by laparoscopy, and the remainder were made by the presence of an endometrioma on imaging. Fifty-five patients representing 67 cycles (43.6%) had stages 1–2 endometriosis, and 71 patients representing 81 cycles (56.3%) had stages 3–4 endometriosis. These cycles yielded 2393 embryos in the control group and 1078 in the endometriosis cohort.
Baseline characteristics are described in Table 1. Mean patient age at time of retrieval was slightly older (33.7 vs 33.0, p = 0.042), and parity was slightly lower in the endometriosis compared to the control cohort. Consistent with the known negative impact of endometriosis and adnexal surgery on ovarian reserve, mean AMH levels were lower in the endometriosis group (median 1.5 vs 2.3, p < 0.001).
Morphokinetic parameters
Morphokinetic timings suggest that the development of embryos obtained from women with endometriosis (EE) is impaired (Table 2). EE were slower than control embryos (CE) to complete nearly all developmental milestones evaluated, including the 2–8 cell stages (p < 0.001), compaction (p = 0.015), morulation (p < 0.001), start of blastulation, blastulation, and expanded blastocyst (p < 0.001).
In addition to demonstrating delayed cell cycle milestones, EE were less likely than CE to progress to morula (68.6% vs 73.8%, p < 0.001), blastocyst (59.95% vs 66.2%, p < 0.001), and expanded blastocyst stages (46.9% vs 53.5%, p < 0.001) (Supplemental Table 1). Additionally, the rate of embryo wastage, defined as the proportion of embryos discarded due to arrest or poor quality, was slightly higher in the endometriosis compared to the control group (43.9% vs 40.2%, p = 0.04; Supplemental Table 1).
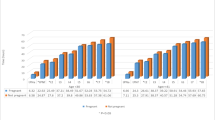
To further characterize embryos derived from women with endometriosis, we evaluated the proportion of embryos that fell into optimal kinetic ranges for cc2, s2, cc3, t5, tSB, and tEB (Fig. 1). A significantly smaller portion of EE embryos fell into optimal kinetic ranges for cc2 (43.7% vs 47.6%, p = 0.003), t5 (50.6% vs 53.7%, p = 0.019), tSB (24.9% vs 33.1%, p < 0.001), and tEB (57% vs 63.9%, p = 0.007) compared to controls, further suggesting that the early development of these embryos is impaired.
Percentage of embryos falling into optimal kinetic ranges. Optimal kinetic ranges were defined as: t3-t2 (> 5 and ≤ 11.9 h), t4-t3 (≤ 1 h), t5 (45–57 h), t5-t3 (9.7–21 h), tSB (< 96.2 h), and EB (≤ 116 h). Asterisk denotes p values that are statistically significant (< 0.05). Analysis performed using the chi-squared test. Abbreviations are as follows: tSB, timing of the start of blastulation; tEB, timing of expanded blastocyst
We also evaluated rates of multinucleation, direct uneven cleavage (division from 1 to 3 cells or 2 to 5 cells), and irregular division in the endometriosis and control groups. The rate of multinucleation was significantly higher in the EE (50.0% vs 44.5%, p = 0.003); however, there were no differences in the rates of direct uneven cleavage (8.6% vs 9.2%, p = 0.56) or irregular division (17.0% vs 16.6%, p = 0.78).
Finally, in order to account for the fact that some patients underwent multiple retrievals and generated multiple embryos, we generated GEE models, presented in Supplemental Tables 2–3. These models also controlled for age, given that the mean age in the endometriosis cohort was slightly higher than in the control cohort (33.7 vs 33.0, p = 0.042). Nearly all differences in morphokinetic parameters between the endometriosis and control groups retained statistical significance after these models were applied. Controlling for age and patient effects, EE achieved the 2–8 cell stages 1 to 1.8 h later than controls (Supplemental Table 2). Additionally, the endometriosis cohort reached the start of blastulation, blastulation, and progressed to expanded blastocyst 1.5–2 h later than controls (Supplemental Table 2).
After controlling for age and patient effects, the difference in the odds of multinucleation between the endometriosis and control groups was no longer significant (OR 1.10 CI: 0.88, 1.5, p = 0.317) (Supplemental Table 3). However, the odds of EE achieving optimal kinetic ranges for t5 and tSB remained significantly lower than that of CE (Supplemental Table 3). Furthermore, EE remained significantly less likely to reach the morula (OR 0.68 [0.52, 0.88], p = 0.004), blastocyst (OR 0.75 [0.60, 0.95], p = 0.019), and expanded blastocyst (OR 0.76 [0.60, 0.96], p = 0.022) milestones in the multivariate model.
The impact of endometriosis stage on embryo morphokinetics
Interestingly, endometriosis stage appeared to have little impact on cycle outcomes and morphokinetic parameters. Baseline characteristics between patients with stages 1–2 and 3–4 endometriosis were similar (Supplemental Table 4). The only notable difference in cycle parameters between stages 1–2 and stages 3–4 was that those with stages 3–4 disease required higher doses of FSH (median 3225; IQR [2000, 4600] vs 2550 [1800, 3450], p = 0.018) (Supplemental Table 4). Despite the increased doses of FSH needed for stimulation, there was no difference in AMH levels according to endometriosis stage (median 1.4; IQR [0.66, 3.3] for stages 3–4 vs 1.5 [1.09, 2.5] for stages 1–2, p = 0.69) (Supplemental Table 4). Furthermore, there were no differences in number of oocytes retrieved, implantation rate, pregnancy rate, or live birth rate according to endometriosis stage.
With regard to morphokinetic parameters, the only significant difference between embryos obtained from women with stages 1–2 versus stages 3–4 endometriosis, was in the timing of tSB, which was longer in the stages 3–4 cohort (mean 104.1 ± 10.7 vs 102.2 ± 9.6, p = 0.015) (Supplemental Table 5). The timing of blastulation was also delayed by approximately 2 h in the stages 3–4 group (mean 109.1 ± 10.3 vs 107.5 ± 10.0, p = 0.058); however, this difference did not reach statistical significance. Additionally, there were no differences in rates of embryo wastage or progression to morula, blastocyst, or expanded blastocyst between the stages 1–2 and 3–4 endometriosis groups (Supplemental Table 6).
Known implantation data (KID)
In order to characterize morphokinetic parameters associated with successful implantation, a subgroup analysis was performed comparing embryos that successfully implanted to those that failed to implant (Supplemental Fig. 1). The analysis was limited to embryo transfers with known implantation data (KID), where either all or none of the transferred embryos implanted. In the control group, 212 implanted embryos and 173 that failed implantation were included in the analysis; in the endometriosis cohort, 111 implanted embryos and 90 implantation failures were evaluated.
The morphokinetic characteristics of successfully implanting embryos (KID-positive) were similar in the control and endometriosis groups (Supplemental Fig. 1). Likewise, non-implanting (KID-negative) embryos from women with and without endometriosis showed no difference in morphokinetics. However, there were significant differences between implanting and non-implanting embryos. In the control cohort, mean tM (91.8 ± 10.7 vs 87.7 ± 8.2, p < 0.001), tSB (100.8 ± 7.9 vs 97.2 ± 7.3, p < 0.001), tB (105.7 ± 8.1 vs 102.3 ± 7.5, p < 0.001), tEB (113.6 ± 8.4 vs 109.8 ± 7.1, p < 0.001), and tSC (84.7 ± 9.7 vs 81.3 ± 9.0, p < 0.001) were all substantially delayed in implantation failures compared to implanted embryos. In the endometriosis cohort, t7 (58.6 ± 8.2 vs 55.2 ± 7.1, p = 0.003), tSB (102.1 ± 9.2 vs 97.5 ± 7.1, p < 0.001), and tB (106.3 ± 8.6 vs 102.5 ± 7.0, p < 0.001) occurred later in KID-negative than in KID-positive embryos. The timings of tM (91.7 ± 10.7 vs 87.8 ± 9.0), tEB (113.9 ± 8.2 vs 110.6 ± 8.1), and tSC (86.0 ± 11.7 vs 81.6 ± 8.8) were also delayed in the EE embryos that failed to implant; however, these differences did not reach statistical significance. Notably, the rate of multinucleation was significantly higher in failed compared to successful implantations in both the endometriosis (43.3% vs 33.3%) and control groups (40.5% vs 27.8%) (p = 0.019) (data not shown). Additionally, embryos that fell into optimal kinetic ranges for s2, t5, tSB, and tEB were more likely to implant than those that did not (Supplemental Fig. 2). Together, these data suggest that a delay in timepoints associated with late developmental events, including compaction, morulation, and blastulation, is associated with implantation failure, and that the morphokinetic factors associated with implantation are similar in EE and CE.
Cycle outcomes
Although morphokinetic data suggest that embryonic development in women with endometriosis is impaired, cycle outcomes were similar between the control and endometriosis groups (Table 1). Higher doses of FSH were required for stimulation in the endometriosis compared to the control group (median 2850; IQR [1912.5, 4275.0] vs 2250 [1800.0, 3375.0], p < 0.001). Additionally, peak estradiol levels were lower among women with endometriosis than controls (median 2033.0; IQR [1392.0, 2690.0 vs 2323.0 [1659.0, 2780.0], p = 0.004). The numbers of total oocytes retrieved (12.6 ± 8 vs 15.2 ± 8.2, p < 0.001) and mature oocytes (mean 8.4 ± 4.8 vs 10.5 ± 5.9, p < 0.001) were lower in the endometriosis compared to the control group. Conversely, the fertilization rate in the endometriosis cohort was slightly higher than in the control group (median 0.80; IQR [0.67, 0.94] vs 0.78 [0.63, 0.89], p = 0.036); however, this difference was not thought to be clinically significant. A total of 11.7% of cycles utilized PGT-A, 11.5% in the endometriosis group, and 11.9% in the control group (p = 1.0) (Table 1).
Two hundred and forty-eight (248) retrievals in the control group and 132 retrievals in the endometriosis group resulted in at least one embryo transfer. The majority of patients underwent day 5 (86%), fresh (71%) embryo transfers. The mean number of embryos transferred at the first embryo transfer was 1.4. Cycle outcomes are reported in Table 3 after the first embryo transfer (primary outcome) and after all embryo transfers resulting from the retrieval (cumulative outcome). The cumulative outcome represents the percentage of retrievals that resulted in at least one clinical pregnancy or live birth, whether it occurred as a result of the first or a subsequent embryo transfer. Despite the higher doses of FSH required for stimulation and smaller number of oocytes retrieved in the endometriosis group, there was no significant difference in pregnancy (63.6% vs 66.5%, p = 0.56), miscarriage (10.7% vs 10.3%, p = 0.92), or live birth rates (56.1% vs 58.7%, p = 0.62) following the first embryo transfer. There were similarly no differences in cumulative clinical pregnancy or live birth rates between groups. Fresh and frozen cycles were evaluated separately, with fresh embryo transfers stratified by day of transfer. In the cohort of women who had fresh, day 5 transfers, clinical pregnancy rates were higher in controls compared to women with endometriosis (76.7% vs 63.3%, p = 0.04); however, there was no significant difference in live birth rate between the endometriosis and control groups (62.4% vs 55.6%, p = 0.38). These results must be interpreted cautiously due to the small subgroup sample sizes.
Notably, cycles performed in women with endometriosis were less likely to result in a suitable embryo for transfer than those performed in controls. Among the retrievals that did not result in embryo transfer, 16 were cycles in which there was no embryo available to transfer; 11 of the failed cycles (68.7%) were in the endometriosis group (11/148, 7.4%) and 5 were in the control group (5/286, 1.7%) (OR = 4.95; CI: 1.41, 16.84, p = 0.005). In the remainder of cycles (n = 38), patients opted to defer embryo transfer.
Discussion
Time-lapse microscopy offers an innovative approach to characterizing differences among embryo cohorts. Our application of this technology to embryos from endometriosis patients provides unique insight into the subtle impairments in embryonic development when compared to control patients without endometriosis. Embryos obtained from women with endometriosis demonstrate delayed cell cycle parameters for both early and late developmental events. Additionally, EE were significantly less likely than controls to progress to the morula, blastocyst, and expanded blastocyst stages. Whereas 66.2% of control embryos became blastocysts, only 59.9% of embryos in the endometriosis group blastulated. These differences remained statistically significant after controlling for age and repeated patient events in a multivariate GEE model. Interestingly, the negative impact of endometriosis on embryonic development appears to be independent of stage and occurred regardless of whether patients had minimal-to-mild or moderate-to-severe disease.
Impairments in embryo development may result from the inflammatory milieu that characterizes endometriosis. It is well established that the peritoneal fluid of patients with endometriosis contains increased numbers of macrophages, prostaglandins, proteases, and cytokines, including IL-6, TNF-alpha, and VEGF [32,33,34]. Exposure to this pro-inflammatory environment is thought to negatively affect oocyte and embryo quality, and may contribute to the impaired embryonic development seen in this study.
Despite the notable differences in morphokinetic parameters, there were no significant differences in clinical pregnancy or live birth rates. This suggests that once a good-quality embryo is available to transfer, pregnancy rates are equivalent between women with and without endometriosis. Consistent with the findings of other retrospective studies [35, 36], a subgroup analysis indicates that frozen embryo transfer may result in improved pregnancy outcomes compared to fresh transfer in women with endometriosis; however, this finding requires confirmation in a larger, prospective dataset. An important finding from this study was that cycles performed in women with endometriosis were 4 times more likely to fail to produce an embryo for transfer. This finding is likely attributable in part to the lower AMH levels and smaller number of oocytes retrieved among patients with endometriosis; however, impaired oocyte quality, resulting in impaired embryo development, may also play a role. These results are consistent with meta-analysis data showing that women with endometriosis undergoing IVF have a higher rate of cycle cancellation and a lower mean number of oocytes retrieved than those without the disease; however, this does not lead to a decrease in clinical pregnancy or live birth rates [15].
Analysis of KID embryos showed that several kinetic markers were associated with implantation in embryos derived from women with and without endometriosis, including tM, tSB, tB, and tEB. These data are consistent with previously published literature showing that the timing of the start of blastulation has been associated with blastocyst quality, implantation, and aneuploidy [18, 29, 37]. Additionally, our group and others have previously demonstrated a relationship between implantation and time to morula, blastocyst, and expanded blastocyst [37, 38]. The observation that morphokinetic parameters were similar among successfully implanted embryos derived from women with and without endometriosis supports the finding that clinical pregnancy and live birth rates per transfer are equivalent regardless of the presence of endometriosis.
To our knowledge, only three studies have examined the impact of endometriosis on morphokinetic parameters [39,40,41]. Schenk et al. observed a shortening of s2, but no other significant changes in morphokinetic parameters [40]. Conversely, another study reported that the duration of the first cell cycle (cc1) and s2 were delayed in embryos from women with endometriosis compared to control embryos from women with tubal factor infertility [39]. A study by Freis and colleagues showed alterations in the timing of early cell division kinetics (t8-t2) and (t4-t2). [41]. All three of these studies were relatively small and underpowered, evaluating 264, 552, and 213 EE, respectively [39,40,41]. Additionally, two of the three studies included patients with unexplained infertility as controls, a group that is likely to contain cases of undiagnosed endometriosis [40, 41]. Demirel et al. investigated the effect of ovarian endometriomas on embryo morphokinetics [42]. The authors did not include unaffected controls, but rather compared oocytes retrieved from ovaries with and without an endometrioma in the same patient; they showed no difference in morphokinetic parameters between embryos derived from an oocyte adjacent to an endometrioma compared to an unaffected oocyte [42]. This is consistent with our finding that the impact of endometriosis on embryonic development is independent of disease severity. Freis et al. also showed that morphokinetic parameters did not differ according to endometriosis stage [41].
The present study is the largest of its kind, evaluating a total of 3471 embryos, including 1078 obtained from women affected by endometriosis. It is also the first study to show that the presence of endometriosis alters embryo morphokinetics as well as the timing of late developmental events, including morulation, blastulation, and time to expanded blastocyst. The strengths of this study are its large sample size and the fact that the majority of included endometriosis cases (95%) were confirmed by laparoscopy. The primary limitation is the retrospective nature of the study. Additionally, because patients in the control group did not always have a laparoscopy, there is a possibility of undiagnosed endometriosis in this group. Finally, mean patient age was slightly older in the endometriosis compared to the control group (33.7 vs 33.0, p = 0.042); however, a multivariate GEE model was used to control for this factor. Additional factors that may confound our results include the heterogenous nature of the control group, the inclusion of multiple ovarian stimulation protocols, and a combination of day-3 and day-5 transfers, as well as fresh and frozen transfers.
In conclusion, time-lapse microscopy provides insight into the stages of embryonic development that are negatively impacted by the presence of endometriosis. Embryos derived from oocytes affected by endometriosis demonstrate impairments in both early and late developmental events, and progress to the morula, blastocyst, and expanded blastocyst stages at lower rates than control embryos. Despite these differences in morphokinetic parameters, IVF outcomes are similar for patients with and without endometriosis. Further investigation in prospective trials is needed to confirm these findings.
References
M. B. Evans and A. H. Decherney, “Fertility and endometriosis,” Clin. Obstet. Gynecol., 2017.
N. C. Llarena, T. Falcone, and R. L. Flyckt, “Fertility preservation in women with endometriosis,” Clin. Med. Insights Reprod. Heal., 2019.
M. A. Luna Russo, J. N. Chalif, and T. Falcone, “Clinical management of endometriosis,” Minerva Ginecologica. 2020.
E. G. Hughes, D. M. Fedorkow, and J. A. Collins, “A quantitative overview of controlled trials in endometriosis-associated infertility,” Fertil. Steril., 1993.
“Endometriosis and infertility: A committee opinion,” Fertility and Sterility. 2012.
E. Oral, D. L. Olive, and A. Arici, “The peritoneal environment in endometriosis,” Human Reproduction Update. 1996.
A. Pellicer, N. Oliveira, A. Ruiz, J. Remohi, and C. Simon, “Exploring the mechanism(s) of endometriosis-related infertility: an analysis of embryo development and implantation in assisted reproduction,” in Human Reproduction, 1995.
J. A. Garcia-Velasco and A. Arici, “Is the endometrium or oocyte/embryo affected in endometriosis?,” in Human Reproduction, 1999.
C. Simón et al., “Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation,” Hum. Reprod., 1994.
L. Sung, T. Mukherjee, T. Takeshige, M. Bustillo, and A. B. Copperman, “Endometriosis is not detrimental to embryo implantation in oocyte recipients,” J. Assist. Reprod. Genet., 1997.
I. Díaz, J. Navarro, L. Blasco, C. Simón, A. Pellicer, and J. Remohí, “Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study,” Fertil. Steril., 2000.
N. Garrido, J. Navarro, J. García-Velasco, J. Remohí, A. Pellicer, and C. Simón, “The endometrium versus embryonic quality in endometriosis-related infertility,” Hum. Reprod. Update, 2002.
F. Filippi et al., “Ovarian endometriomas and oocyte quality: insights from in vitro fertilization cycles,” Fertil. Steril., 2014.
A. M. Sanchez et al., “Does endometriosis influence the embryo quality and/or development? Insights from a large retrospective matched cohort study,” Diagnostics, 2020.
M. Hamdan, G. Dunselman, T. C. Li, and Y. Cheong, “The impact of endometrioma on IVF/ICSI outcomes: a systematic review and meta-analysis,” Hum. Reprod. Update, 2015.
N. Garrido, J. Navarro, J. Remohí, C. Simón, and A. Pellicer, “Follicular hormonal environment and embryo quality in women with endometriosis,” Human Reproduction Update. 2000.
N. Basile et al., “The use of morphokinetics as a predictor of implantation: a multicentric study to define and validate an algorithmfor embryo selection,” Hum. Reprod., 2015.
A. Campbell, S. Fishel, N. Bowman, S. Duffy, M. Sedler, and C. F. L. Hickman, “Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics,” Reprod. Biomed. Online, 2013.
S. Chamayou et al., “The use of morphokinetic parameters to select all embryos with full capacity to implant,” J. Assist. Reprod. Genet., 2013.
M. Meseguer, J. Herrero, A. Tejera, K. M. Hilligsøe, N. B. Ramsing, and J. Remoh, “The use of morphokinetics as a predictor of embryo implantation,” Hum. Reprod., 2011.
I. Rubio et al., “Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope,” Fertil. Steril., 2014.
I. Rubio et al., “Limited implantation success of direct-cleaved human zygotes: a time-lapse study,” Fertil. Steril., 2012.
C. Siristatidis et al., “Morphokinetic parameters of early embryo development via time lapse monitoring and their effect on embryo selection and ICSI outcomes: a prospective cohort study,” J. Assist. Reprod. Genet., 2015.
C. Pribenszky, A. M. Nilselid, and M. Montag, “Time-lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: a meta-analysis,” Reproductive BioMedicine Online. 2017.
P. Kovacs, S. Matyas, V. Forgacs, A. Sajgo, L. Molnar, and C. Pribenszky, “Non-invasive embryo evaluation and selection using time-lapse monitoring: results of a randomized controlled study,” Eur. J. Obstet. Gynecol. Reprod. Biol., 2019.
B. Balaban et al., “Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting,” Reprod. Biomed. Online, 2011.
N. Desai, J. M. Goldberg, C. Austin, and T. Falcone, “Are cleavage anomalies, multinucleation, or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy?,” Fertil. Steril., 2018.
N. Desai, M. Yao, E. G. Richards, and J. M. Goldberg, “Randomized study of G-TL and global media for blastocyst culture in the EmbryoScope: morphokinetics, pregnancy, and live births after single-embryo transfer,” Fertil. Steril., 2020.
N. Desai et al., “Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles,” Fertil. Steril., 2016.
N. Desai, S. Ploskonka, L. R. Goodman, C. Austin, J. Goldberg, and T. Falcone, “Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles,” Reprod. Biol. Endocrinol., 2014.
J. Herrero and M. Meseguer, “Selection of high potential embryos using time-lapse imaging: the era of morphokinetics,” in Fertility and Sterility, 2013.
J. L. Herington, K. L. Bruner-Tran, J. A. Lucas, and K. G. Osteen, “Immune interactions in endometriosis,” Expert Rev. Clin. Immunol., 2011.
J. Zhou et al., “Peritoneal fluid cytokines reveal new insights of endometriosis subphenotypes,” Int. J. Mol. Sci., 2020.
Y. Y. Fan, H. Y. Chen, W. Chen, Y. N. Liu, Y. Fu, and L. N. Wang, “Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis,” Gynecol. Endocrinol., 2018.
A. M. F. Mohamed, S. Chouliaras, C. J. P. Jones, and L. G. Nardo, “Live birth rate in fresh and frozen embryo transfer cycles in women with endometriosis,” Eur. J. Obstet. Gynecol. Reprod. Biol., 2011.
Ji. Wu, X. Yang, J. Huang, Y. Kuang, and Y. Wang, “Fertility and neonatal outcomes of freeze-all vs. fresh embryo transfer in women with advanced endometriosis,” Front. Endocrinol. (Lausanne)., 2019.
L. R. Goodman, J. Goldberg, T. Falcone, C. Austin, and N. Desai, “Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial,” Fertil. Steril., 2016.
Y. Motato, M. J. de los Santos, M. J. Escriba, B. A. Ruiz, J. Remohí, and M. Meseguer, “Morphokinetic analysis and embryonic prediction for blastocyst formation through an integrated time-lapse system,” Fertil. Steril., 2016.
F. K. Boynukalin, M. Serdarogullari, M. Gultomruk, O. Coban, N. Findikli, and M. Bahceci, “The impact of endometriosis on early embryo morphokinetics: a case-control study,” Syst. Biol. Reprod. Med., 2019.
M. Schenk, J. M. Kröpfl, M. Hörmann-Kröpfl, and G. Weiss, “Endometriosis accelerates synchronization of early embryo cell divisions but does not change morphokinetic dynamics in endometriosis patients,” PLoS One, 2019.
A. Freis, J. E. Dietrich, M. Binder, V. Holschbach, T. Strowitzki, and A. Germeyer, “Relative morphokinetics assessed by time-lapse imaging are altered in embryos from patients with endometriosis,” Reprod. Sci., 2018.
Demirel C, Bastu E, Aydogdu S, Donmez E, Benli H, Tuysuz G, Keskin G, Buyru F. The presence of endometrioma does not impair time-lapse morphokinetic parameters and quality of embryos. Reprod Sci. 2016;23(8):1053-7.
Acknowledgements
We would like to acknowledge Lin Mei for her excellent assistance with extracting patient medical records from the electronic medical record system.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Cleveland Clinic Institutional Review Board (IRB # 19–997).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Llarena, N.C., Hur, C.E., Yao, M. et al. The impact of endometriosis on embryo morphokinetics: embryos from endometriosis patients exhibit delayed cell cycle milestones and decreased blastulation rates. J Assist Reprod Genet 39, 619–628 (2022). https://doi.org/10.1007/s10815-022-02406-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02406-2