Abstract
Purpose
The aim of our study was to evaluate the influence of different ejaculatory abstinence time frames (several days versus 1 h) on semen parameters, blastocysts ploidy rate, and clinical results in assisted reproduction cycles on sibling oocytes.
Methods
This is a prospective study including 22 preimplantation genetic testing for aneuploidy (PGT-A) cycles performed between November 2015 and December 2018. Male partners with oligoastenoteratozoospermia produced two semen samples on the day of oocyte retrieval: the first one after several days of abstinence and the second, 1 h after the first one. Oocytes from each patient were divided into two groups: those in group 1 were injected with spermatozoa from the first ejaculate (N = 121) and oocytes in group 2 with spermatozoa from the second one (N = 144). Outcomes of aniline blue test, fertilization, blastocyst formation, ploidy rates, and clinical results were compared between the two groups.
Results
Semen volume resulted lower in the second sperm retrieval. Sperm concentration, motility, and morphology were similar in the two groups. A total of 106 blasotcysts were biospied. Higher blastocyst euploidy rates resulted in group 2 (43.6%) than in group 1 (27.5%). A higher percentage of mature chromatine was observed in group 2.
Conclusion
Using spermatozoa from samples with a shorter abstinence could be a simple method to select higher quality spermatozoa, reducing aneuploidy rate in blastocysts. Prospective randomized controlled trials should be performed to confirm the potential advantage of using semen samples with short abstinence period to improve the outcome of assisted reproduction cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known how female factor has often been considered as the major cause of failure in assisted reproduction treatment. In the beginning, the beneficial effect of preimplantation genetic testing for aneuploidy (PGT-A) was thought to be greatest in women with advanced maternal age [1, 2]; in a second step, PGT-A has been offered also to women with a history of recurrent miscarriages or repeated implantation failure, since in the embryos of these women was observed a high rate of aneuploidies [3, 4]. Finally, it was applied even in women with a partner with low sperm quality [5]. Many factors influence semen parameters observed in ejaculates used in assisted reproduction procedures. One of these factors is the length of the abstinence period. The World Health Organization recommends an abstinence from a minimum of 2 days up to a maximum of 7 days for semen analysis [6]. Several studies recommend a longer abstinence frame because sperm volume, sperm concentration, and total sperm count increase with abstinence from 4 to 10 days [7,8,9], with a more pronounced effect in the first 24 h [10,11,12]. Studies on different abstinence periods report a lower outcome in fertility treatments conducted with spermatozoa from samples obtained after a short abstinence (abstinence varied from 3 to 1 days, up to a maximum of 21 days) [13,14,15], although other studies affirm that an improvement in sperm count, motility, and morphology is observed in ejaculates produced after only 24-h abstinence [16].
Nearly all the studies agree that semen volume decreases with frequent ejaculatory events, even if total sperm count, motility, morphology, and vitality may not be affected by abstinence length (varying from 3–4 days to 1 day) [17]; on the contrary, short ejaculation periods in normozoospermic men can cause nearly a 60% decrease in total motile sperm count in second ejaculate compared with the first [18].
Male factor infertility represents one-half of all infertility causes worldwide [19, 20] and it is reported in the literature that about 30% of common phenotypes of oligo- or azoospermia can be ascribed to genetic origin [19, 20].
Since it is thought that more than 2000 genes are involved in spermatogenesis defects [21, 22], it is possible that these are not often known.
Compromised spermatozoa quality may affect fertilization and cleavage rates as well as embryo grade [23, 24] and is often correlated with higher aneuploidy rate [25], miscarriages [26], and negative pregnancy outcomes. These negative outcomes could be explained with altered protamination associated with male infertility [27, 28]. In order to acquire good sperm quality, in terms of chromatin maturity, a proper regulation of spermatogenesis is necessary, obtained with histone replacement process by protamine. Defective spermatogenesis could be correlated with an imbalance in protamine transcription/translation regulators, affecting the expression of spermatogenesis-associated genes [29]. Aniline blue test is one of the staining methods used to detect protamination and chromatin integrity, assessing spermatozoa maturity. Histones are nuclear proteins present in somatic cells with the capability to activate and silence gene expression, modifying lysine and serine residues and acting as potent epigenetic regulators [30,31,32]. It is demonstrated that spermatozoa are unique in the regulation of their epigenome and, despite the different contribution of the female and male gametes to the embryos complement of cellular organelles, have a key role in embryogenesis and in the establishment of totipotency in embryo cells [21].
The aim of the present study was to evaluate the influence of the duration of abstinence on semen parameters, blastocyst ploidy, and clinical results in assisted reproduction cycles.
Materials and methods
Study population
From November 2015 to December 2018, twenty-two couples were enrolled in this prospective study. Fourteen of them came from previous failed assisted reproduction technique (ART) cycles, and eight were facing their first treatment.
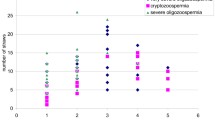
All male partners were oligoastenoteratozoospermic (OAT), 14 of them were affected by severe OAT, with sperm concentration lower than 5 million sperm/ml and progressive motility lower than 20%. Couples with genetic factor were excluded from the study. Male mean age was 39.9 ± 7.0 years old; hormonal values were recorded for all male patients: follicle-stimulating hormone (FSH) mean value was 8.9 ± 6.6 mU/ml and luteinizing hormone (LH) was 4.4 ± 2.3 mU/ml. Male body mass index (BMI) was 24.6 ± 2.8; therefore, no highly overweight patients were included in the study (Table 1).
Female partners mean age was 36.2 ± 4.3 years old, with a BMI of 22.7 ± 2. Indications for PGT-A were male infertility (N = 13) and advanced maternal age (N = 9).
Sperm processing
On the day of oocyte retrieval, a first semen sample was requested following an abstinence of 2–7 days according to WHO indications; semen collection was made in a sterile jar and semen was analyzed according to WHO 2010 at 5° percentile for sperm concentration, motility, and morphology using a Makler’s counting chamber. Morphology was assessed smearing 10 μl of the semen samples after washing and fixing the smear using MGG Quick Stain (Bio-optica, Milan, Italy). After coloration anomalies in sperm heads, necks and tails were evaluated following WHO 2010 at 5° percentile criteria. In a normal spermatozoon, the head should be smooth with regular margins and with an oval shape; the neck should be thin and regular and with the same size of the head; the tail should be thinner than the neck and uniform and with a length of about 45 μm. The total volume, evaluated using a Falcon graded pipette, was then divided into two Falcon tubes (15 ml); 5 ml of HEPES-buffered washing medium (Sage Series) was added in each tube, before 10-min centrifugation at 1880 rpm. Supernatant was removed and pellet resumed. An aliquot of the pellet was used for aniline blue (AB) staining (10 ml) and the remaining part was used for intracytoplasmatic sperm injection (ICSI). A second ejaculate was collected 1 h after the first one and treated in the same way.
Ovarian stimulation and laboratory procedures
Ovarian stimulation protocols were carried out by mean of recombinant follicle-stimulating hormone (FSH. Gonal F, Merck Serono, Geneva, Switzerland) and gonadotrophin-releasing hormone (GnRH) agonist or antagonist according to ovarian reserve and anti-mullerian hormone (AMH) values, as described elsewhere [33].
Intracytoplasmatic sperm injection was performed 38 h after human corionic gonadotropin (hCG, Gonasi, 10000 IU IBSA, Lodi, Italy) administration. In the case of severe oliagoastenoteratozoospermia, semen samples were smeared on a dish under mineral oil in order to select the best spermatozoa for ICSI with × 63 magnification. Sibling oocytes from each individual patient were equally divided into two groups: oocytes included in group 1 (N = 121) were injected with ejaculate collected after 2–5 days of abstinence. Group 2 included oocytes injected with semen produced after 1 h of abstinence (N = 144).
Embryo culture was performed in standard or time-lapse incubator at 37 °C, 6% CO2, 5% O2 in sequential media (Quinn’s Advantage Fertilization medium and Quinn’s Advantage Blastocyst medium; SAGE, USA); on day 3 of culture, assisted hatching was performed on all developing embryos. Trophoectoderm biopsy was performed as soon as the blastocysts were fully expanded on day 5, 6, or 7 of culture, collecting 4–6 cells. DNA was amplified with whole genome amplification (WGA) and analysis was made with next-generation sequencing (NGS) [34]. Biopsied blastocysts were vitrified within 1 h from biopsy, using vitrification media (Kitazato Vitrification Kit; BioPharma, Shizouka, Japan). Single frozen embryo transfers were carried out on natural or mildly stimulated cycles; euploid blastocysts were thawed with thawing media (Kitazato Thawing Kit; BioPharma, Shizouka, Japan) and transferred within 2 h.
Aniline blue assay
Sperm chromatin maturity was assessed using AB staining (Sigma®, Germany) [35]. A thin smear of pellet was prepared on a glass slide and allowed to dry. Smears were fixed for 15 min in 37% formaldehyde in phosphate-buffered saline solution (pH 7.2) and stained with 5% aqueous AB prepared in 4% acetic acid (pH 3.5) for 2 min. Slides were rinsed with water and observed at × 100 magnification under oil. For AB staining test, spermatozoa were divided into three categories: intense (dark blue coloring of the entire head), intermediate (dark staining in the post-acrosomal region), and pale (very pale staining over the entire head). A minimum of 100 spermatozoa from each sample was evaluated and the percentage of aniline blue positive heads (intense and intermediate colored) was calculated in order to determine the percentage of spermatozoa with immature chromatin.
Statistical analysis was performed using Student’s t test and chi-square test at a level of p ≤ 0.05; values are expressed as mean± SD.
Ethical approval
The study has been conducted in accordance with the principles expressed in the Declaration of Helsinki. After the approval of the internal ethical commission of European Hospital, an informed consent was obtained from all individual participants included in the study.
Results
Sperm concentration, motility, and morphology did not show any significant difference between the first and second ejaculates; sperm volume in the second ejaculate was significantly lower (p = 0.0002) (Student’s t test). Mean values of concentration in initial and consecutive ejaculates were 2.2 million/ml and 2.5 million/ml respectively. Total motility was 10.4% and 11.2% in group 1 and group 2 respectively; in the first and second ejaculates, progressive motility spermatozoa (grade A) were 0.6% and 0.5%, slow motility spermatozoa (grade B) were 4.9% and 5.7%, and non progressive spermatozoa (grade C) were 4.9% and 5.0% respectively. Median morphology did not differ in consecutive samples (1.4%) (Table 2).
After sperm washing and centrifugation, semen parameters for initial and consecutive samples respectively were as follows: concentration 2.0 million/ml and 2.4 million/ml. Total motility was 30.0% and 29.4% with 2.1% and 3.4% grade A spermatozoa, 18.7% and 17.3% grade B, and 9.1% and 8.8% grade C.
In group 1, median percentage of intense colored spermatozoa was 58.3 ± 1.3%, intermediate colored was 12.1 ± 1.7%, and pale colored was 29.6 ± 2.3%. In 1-h abstinence group, results of aniline blue staining were as follows: 35.6 ± 1.6% spermatozoa were darkly colored, 10.4 ± 2.1% presented an intermediate coloring, and 54.0 ± 1.8% showed a pale coloration. All values obtained after aniline blue test resulted to be highly statistically significant with a p < 0.05 using Student’s t test (Table 3).
Retrieved oocytes were 350, and 265 of them were mature for injection; in groups 1 and 2, 121 and 144 oocytes respectively were injected. Fertilization rates were 75.2% (N = 91) and 76.4% (N = 110) in the first and second ejaculate groups (Table 4).
No significant differences were observed in blastocyst formation timing in the two groups. After analysis, aneuploid embryos in the first and subsequent ejaculates were 54.9% (N = 28) and 41.8% (N = 23); mosaic diploid/aneuploid blastocysts were 7 (13.7%) and 8 (14.6%) in the two groups respectively. Euploid blastocyst rate resulted to be statistically higher in group 2: in this group euploid embryos were 24 (43.6%), while in the group with 2–5 days of abstinence, only 27.5% (N = 14) of the biopsied embryos resulted to be euploid (p = 0.043) (chi-square test) (Table 4).
A total of 21 frozen embryo transfers have been performed: 7 in group 1 and 14 in group 2; clinical pregnancy rates were 28.6% (N = 2) and 64.3% (N = 9) respectively (p = 0.080); implantation rates were 28.6% (N = 2) and 64.3% (N = 9) respectively (p = 0.080). All pregnancies ended in a healthy live birth.
Discussion
Many authors discussed the influence of the length of pre-ejaculation abstinence on semen parameters; the male populations considered in these studies include, however, normospermic and astenozoospermic men, or patients whose semen presented an altered DNA fragmentation. In these studies, abstinence period varied from 8 to 1 days [11, 17, 36], up to 2 h of pre-ejaculation [37].
Only few studies concentrated their attention on subfertile or OAT men, considering a second ejaculation obtained after 30–40 min [38] or after 60 min [39, 40] following the first one.
All authors evidenced a decrease in semen volume of the second ejaculates; the same relation between abstinence duration and semen volume was detected in our study.
Most of the studies detected an increase in total sperm count and in progressive spermatozoa rate, in ejaculates with a shorter abstinence period. Our study, on the contrary, has not evidenced the same results: concentration and progressive motility remained unvaried in the two subsequent ejaculates, although in the group with shorter abstinence frame, there was an implementation in the percentage of hyperactivated spermatozoa. It must be underlined, however, that the population of our study is essentially different from that considered in previous studies: those considering OAT men included patients with minor oligoastenoteratozoospermia whereas all men enrolled in our study were affected by severe OAT, with a history of repeated ART cycles for male infertility factor.
The greatest part of the authors studying the effect of abstinence on the ejaculate characteristics evaluated sperm DNA fragmentation and viability [17, 36, 41] and did not detect any difference related to abstinence length. Our study design did not allow us to evaluate these parameters because of the very low sperm concentration. Only sperm DNA integrity, evaluated through the percentage of mature chromatin, was investigated, using AB staining; since the percentage of mature chromatin may affect embryo development [42,43,44], we considered it an appropriate evaluation parameter.
We detected an increase in the percentage of mature chromatin in ejaculates obtained after a very short abstinence time frame (1 h); previous studies [45] evidenced a decrease of this parameter in shorter abstinence semen, although it must be underlined that subsequent ejaculates were collected after an abstinence not shorter than 18–24 h. Gill and collaborators [46] failed to report any correlation between chromatin maturity and ICSI outcomes (fertilization and embryo development up to 72 h), as confirmed in our study. However, the significant increase in euploid blastocysts obtained in the group with shorter abstinence frame highlighted by our study could be explained with a higher percentage of sperm chromatin with normal protamination in 1 h of abstinence ejaculates. Although the case number in the present study is limited, it must be underlined that the use of sibling oocytes strengthens our observations.
Nearly all differences in semen parameters between ejaculates from longer or shorter abstinence could be explained with different durations of the epididymal transit. The reason for the reduction observed in semen volume could be ascribed to shorter recovery time given to the prostatic and seminal glands after the first semen collection.
About 50% of spermatozoa in the cauda epididymis are available for ejaculation [47] and decrease in sperm concentration observed in many studies could be due to the shorter time given the spermatozoa to reach the cauda and the vas deferens [48]. Differences observed in kinematic parameters in different studies could be attributed to difficulties associated with classical evaluation of sperm motility [49]. During the epididymal transit, a series of elaborate interactions between spermatozoa and epididymal secretions occurs [50], influencing flagellar beating and sperm motility [51]. These interactions between sperms and cauda epididymal secretions involve different lipids and protein on the sperm cell surface, which is modified to ensure its fertilization capability. The existence of a “motility-inhibiting factor” has been hypothesized, whose concentration decrease with frequent ejaculation [52]. It seems to be clear that the length of abstinence could influence sperm kinetics, since it modifies storage and transfer time in the epididymis. Although our study did not evidence a significant increase in progressive spermatozoa, a higher percentage of hyperactivated sperms was observed.
This hyperactivation could reflect an intrinsic increase of capacitated spermatozoa. This hypothesis seems to be confirmed by aniline blue staining test in group 2.
A short period of abstinence could result in a reduced concentration of dead cells and a younger population of spermatozoa, with a reduced exposure to the toxic effects of reactive oxygen species (ROS), generated by granulocytes during storage in the epididymis [53]. A decreased time frame between each ejaculation speeds up the transit of sperms through the epididymis and consequently they are less exposed to the harmful effects of ROS. A reduced exposure to oxidative stress determined by ROS leads to a consequent improvement of sperm chromatin integrity [54].
Sperm chromatin structure could also be important for the maintenance of the right epigenetic patterns during spermatogenesis [55].
Epigenetic events occurring during gametogenesis include DNA methylation erasure, acquisition, and maintenance [56].
Sperm DNA methylation, which could be altered in OAT men, is essential for proper fertilization and early cellular divisions of the embryos; some studies also highlighted the importance of a “male factor” in clinical pregnancy rates and miscarriages [57,55,59]. It is known that failure of ART treatment in couples with male partner infertility could be ascribed to epigenetic disorders detected in blastocysts produced in these cycles [60].
Many recent studies highlighted the involvement of various genes (USP8, TEX11, DMRT1, NR5A1) with abnormal epigenomic modifications [61,59,63], involved in implantation failure in couples with OAT or azoospermic males, even after the transfer of euploid blastocysts.
Conclusions
The present study was conducted to evaluate the effects of the duration oabstinence on semen parameters. Sibling oocytes were used as model to investigate the effect of abstincence on ART biological outcomes. Although the low number of case enrolled, the use of sibling oocytes allowed to confirm our outcomes reducing the bias due to embryo’s origin. According to our results and to previous publications, there is an agreement upon the fact that requesting a subsequent ejaculate immediately after the first one has been produced could represent a valid strategy to optimize treatment outcomes. Many authors suggested that ejaculates produced after a short abstinence could enhance the results of assisted reproductive treatments thanks to improved semen parameters, making it even possible to substitute ICSI with classical IVF [40].
These findings also raise the question whether there is an actual benefit for sexual abstinence before infertility treatment, in order to achieve superior sperm quality.
In the present study, the request of a second ejaculate allowed us comparing semen parameters and evaluating PGT-A cycle outcomes in terms of ploidy rate and clinical results, in OAT patients. We obtained an increase of the euploidy rate using semen produced after 1 h of abstinence. This observation needs to be confirmed by implementing cycles number. In case our results will be confirmed, our future routine clinical practice could envisage a single semen collection post 1-h abstinence in severe OAT patients.
References
Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91.
Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–91.
Munne S, Sandalinas M, Magli C, Gianaroli L, Cohen J, Warburton D. Increased rate of aneuploid embryos in young women with previous aneuploid conceptions. Prenat Diagn. 2004;24:638–43.
Wilding M, Forman R, Hogewind G, Di Matteo L, Zullo F, Cappiello F, et al. Preimplantation genetic diagnosis for the treatment of failed in vitro fertilization-embryo transfer and habitual abortion. Fertil Steril. 2004;81:1302–7.
Silber S, Escudero T, Lenahan K, Abdelhadi I, Kilani Z, Munne S. Chromosomal abnormalities in embryos derived from testicular sperm extraction. Fertil Steril. 2003;79:30–8.
World Health Organization. Department of Reproductive Health and Research. WHO laboratory manual for the examination and processing of human semen. 5th ed. Switzerland: WHO Press; 2010. p. 10–1.
Mortimer D, Templeton AA, Lenton EA, Coleman RA. Influence of abstinence and ejaculation-to-analysis delay on semen analysis parameters of suspected infertile men. Arch Androl. 1982;8:251–6.
Jørgensen N, Andersen AG, Eustache F, Irvine DS, Suominen J, Petersen JH, et al. Regional differences in semen quality in Europe. Hum Reprod. 2001;16:1012–9.
Jørgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, et al. Human semen quality in the new millennium: a prospective cross-sectional population based study of 4867 men. BMJ Open. 2012;2:e000990.
Makkar G, Ng EH, Yeung WS, Ho PC. A comparative study of raw and prepared semen samples from two consecutive days. J Reprod Med. 2001;46:565–72.
Levitas E, Lunenfeld E, Weiss N, Friger M, Har-Vardi I, Koifman A, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83:1680–6.
Francavilla F, Barbonetti A, Necozione S, Santucci R, Cordeschi G, Macerola B, et al. Within-subject variation of seminal parameters in men with infertile marriages. Int J Androl. 2007;30:174–81.
Frank J, Confino E, Friberg J, Dudkiewicz AB, Gleicher N. Effect of ejaculation frequency on sperm quality. Arch Androl. 1986;16:203–7.
Levin RM, Latimore J, Wein AJ, Van Arsdalen KN. Correlation of sperm count with frequency of ejaculation. Fertil Steril. 1986;45:732–4.
Tonguc E, Var T, Onalan G, Altinbas S, Tokmak A, Karakas N, et al. Comparison of the effectiveness of single versus double intrauterine insemination with three different timing regimens. Fertil Steril. 2010;94:1267–70.
Lehavi O, Botchan A, Paz G, Yogev L, Kleiman SE, Yavetz H, et al. Twenty-four hours abstinence and the quality of sperm parameters. Andrologia. 2014;46:692–7.
Mayorga-Torres BJ, Camargo M, Agarwal A, du Plessis SS, Cadavid ÁP, Cardona Maya WD. Influence of ejaculation frequency on seminal parameters. Reprod Biol Endocrinol. 2015;13:47.
Olderid NB, Gordeladze JO, Kirkhus B, Purvis K. Human sperm characteristics during frequent ejaculation. J Reprod Fertil. 1984;71:135–40.
Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. 2015;13:37.
Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23:1–160.
Carrell DT. Epigenetics of the male gamete. Fertil Steril. 2012;97(2):267–74.
Yan W, McCarrey JR. Sex chromosome inactivation in the male. Epigenetics. 2009;4(7):452–6.
Loutradi KE, Tarlatzis BC, Goulis DG, Zepiridis L, Pagou T, Chatziioannou E, et al. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J Assist Reprod Genet. 2006;23:69–74.
Chapuis A, Gala A, Ferrières-Hoa A, Mullet T, Bringer-Deutsch S, Vintejoux E, et al. Sperm quality and paternal age: effect on blastocyst formation and pregnancy rates. Basic Clin Androl. 2017;27:2.
Ramasamy R, Chiba K, Butler P, Dolores J. Lamb Male biological clock: a critical analysis of advanced paternal age. Fertil Steril. Author manuscript; available in PMC 2016 Jul 21. Published in final edited form as: Fertil Steril. 2015;103(6):1402–6.
Puscheck EE, Jeyendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol. 2007;19:222–8.
Depa-Martynow M, Kempisty B, Jagodziński PP, Pawelczyk L, Jedrzejczak P. Impact of protamine transcripts and their proteins on the quality and fertilization ability of sperm and the development of preimplantation embryos. Reprod Biol. 2012;12(1):57–72.
Rogenhofer N, Dansranjavin T, Schorsch M, Spiess A, Wang H, von Schönfeldt V, et al. The sperm protamine mRNA ratio as a clinical parameter to estimate the fertilizing potential of men taking part in an ART programme. Hum Reprod. 2013;28(4):969–78.
Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update. 2007;13:313–27.
Rodenhiser D, Mann M. Epigenetics and human disease: translating basic biology into clinical applications. CMAJ. 2006;174(3):341–8.
Denomme MM, White CR, Gillio-Meina C, MacDonald WA, Deroo BJ, Kidder GM, et al. Compromised fertility disrupts Peg1 but not Snrpn and Peg3 imprinted methylation acquisition in mouse oocytes. Front Genet. 2012;3:129 Published online 2012 Jul 11.
Francis S, Yelumalai S, Jones C, Coward K. Aberrant protamine content in sperm and consequential implications for infertility treatment. Hum Fertil (Camb). 2014;17(2):80–9.
Greco E, Bono S, Ruberti A, Lobascio AM, Greco P, Biricik A, et al. Comparative genomic hybridization selection of blastocysts for repente implantation failure tretament: a pilot study. Biomed Res Int. 2014;2014:457913.
Minasi MG, Fiorentino F, Ruberti A, Biricik A, Cursio E, Cotroneo E, et al. Genetic diseases and aneuploidies can be detected with a single blastocyst biopsy: a successfull clinical approach. Hum Reprod. 2017;32(8):1770–7.
Alkhayal A, San Gabriel M, Zeidan K, Alrabeeah K, Noel D, McGraw R, et al. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet. 2013;30(11):1519–24.
Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5:935–50.
Alipour H, Van Der Horst G, Christiansen OB, Dardmeh F, Jørgensen N, Nielsen HI, et al. Improved sperm kinematics in semen samples collected after 2 h versus 4-7 days of ejaculation abstinence. Hum Reprod. 2017;32(7):1364–72.
Bahadur G, Almossawi O, IIlahibuccus A, Al-Habib A, Okolo S. Factors leading to pregnancies in stimulated intrauterine insemination cycles and the use of consecutive ejaculations within a small clinic environment. J Obstet Gynaecol India. 2016;66(Suppl 1):513–20.
Sugiyam R, Nakagawa K, Nishi Y, Sugiyama R, Shirai A, Inoue M. Improvement of sperm motility by short-interval sequential ejaculation in oligoasthenozoospermic patients. Arch Med Sci. 2008;4:438–42.
Bar-Hava I, Perri T, Ashkenazi J, Shelef M, Ben-Rafael Z, Orvieto R. The rationale for requesting a second consecutive sperm ejaculate for assisted reproductive technology. Gynecol Endocrinol. 2000;14(6):433–6.
De Jonge C, LaFromboise M, Bosmans E, Ombelet W, Cox A, Nijs M. Influence of the abstinence period on human sperm quality. Fertil Steril. 2004;82(1):57–65.
Asmarinah, Syauqy A, Umar LA, Lestari SW, Mansyur E, Hestiantoro A, et al. Sperm chromatin maturity and integrity correlated to zygote development in ICSI program. Syst Biol Reprod Med. 2016;62(5):309–16.
Hammadeh ME, al-Hasani S, Stieber M, Rosenbaum P, Küpker D, Diedrich K, et al. The effect of chromatin condensation (aniline blue staining) and morphology (strict criteria) of human spermatozoa on fertilization, cleavage and pregnancy rates in an intracytoplasmic sperm injection programme. Hum Reprod. 1996;11(11):2468–71.
Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82:378–83.
Shubhashree U, Sherine EM, Sujith RS, Dayanidhi K, Vikram JS, D’Souza F, et al. Sperm chromatin immaturity observed in short abstinence ejaculates affects DNA integrity and longevity in vitro. Plos One. 2016.
Gill K, Rosiak A, Gaczarzewicz D, Jakubik J, Kurzawa R, Kazienko A, et al. The effect of human sperm chromatin maturity on ICSI outcomes. Hum Cell. 2018;31(3):220–31.
Björndahl L, Kvist U. Human sperm chromatin stabilization: a proposed model including zinc bridges. Mol Hum Reprod. 2010;16(1):23–9.
Falcone T, Hurd WT, editors. Clinical reproductive medicine and surgery: a practical guide. New York: Springer Sciences; 2013. p. 31–42.
Rivera-Montes AM, Rivera-Gallegos A, Rodríguez-Villasana E, Juárez-Bengoa A, Díaz-Pérez Mde L, Hernández-Valencia M. Estimate of the variability in the evaluation of semen analysis. Ginecol Obstet Mex. 2013;81(11):639–44.
Turner TT. On the epididymis and its role in the development of the fertile ejaculate. J Androl. 1995;16(4):292–8 Review.
Sullivan R, Mieusset R. The human epididymis: its function in sperm maturation. Hum Reprod Update. 2016;22(5):574–87.
Tur-Kaspa I, Maor Y, Levran D, Yonish M, Mashiach S, Dor J. How often should infertile men have intercourse to achieve conception? Fertil Steril. 1994;62(2):370–5.
du Plessis SS, McAllister DA, Luu A, Savia J, Agarwal A, Lampiao F. Effects of H(2)O(2) exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010;42(3):206–10.
Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21(1):33–44.
Rousseaux S, Caron C, Govin J, Lestrat C, Faure AK, Khochbin S. Establishment of male-specific epigenetic information. Gene. 2005;345(2):139–53.
Rodenhiser D, Mann M. Epigenetics and human disease: translating basic biology into clinical applications. CMAJ. 2006;174(3):341–8.
Jenkins TG, Carrell DT. The sperm epigenome and potential implications for the developing embryo. Reproduction. 2012;143(6):727–34.
Marchetti F, Lowe X, Bishop J, Wyrobek AJ. Absence of selection against aneuploid mouse sperm at fertilization. Biol Reprod. 1999;61(4):948–54.
Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Horm Behav. 2011;59(3):306–14.
Denomme MM, McCallie BR, Parks JC, Booher K, Schoolcraft WB, Katz-Jaffe MG. Inheritance of epigenetic dysregulation from male factor infertility has a direct impact on reproductive potential. Fertil Steril. 2018;110(3):419–428.e1.
Hammoud SS, Nix DA, Hammoud AO, Gibson M, Cairns BR, Carrell DT. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod. 2011;26(9):2558–69.
Tüttelmann F, Ruckert C, Röpke A. Disorders of spermatogenesis: perspectives for novel genetic diagnostics after 20 years of unchanged routine. Med Genet. 2018;30(1):12–20.
Kosova G, Scott NM, Niederberger C, Prins GS, Ober C. Genome-wide association study identifies candidate genes for male fertility traits in humans. Am J Hum Genet. 2012;90(6):950–61.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scarselli, F., Cursio, E., Muzzì, S. et al. How 1 h of abstinence improves sperm quality and increases embryo euploidy rate after PGT-A: a study on 106 sibling biopsied blastocysts. J Assist Reprod Genet 36, 1591–1597 (2019). https://doi.org/10.1007/s10815-019-01533-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01533-7