Abstract
In January 2016, Melissa Cook, a California gestational surrogate experiencing a multiple-birth pregnancy following the in vitro fertilization (IVF) transfer of three embryos comprised of donor eggs and sperm provided by the intended father, went to the media when the intended father requested that she undergo a fetal reduction because twins were less expensive to raise than triplets. Much of the legal interest in this case to date has centered on the enforceability of surrogacy contracts. However, the Cook case also raises troubling issues about fertility treatment practices involving gestational surrogates, twin preference, and third-party reproduction medical decision-making. This paper focuses on multiple-embryo transfers in the context of US surrogacy arrangements. Offering an original analysis of data obtained from the US national-assisted reproduction registry, it examines single- and multiple-embryo transfer trends over a 12-year period (2003 to 2014). Findings reveal that recommended guidelines were followed in fewer than 42% of the cases in 2014. The paper argues that ensuring equitable medical treatment for all recipients of IVF requires the adoption of treatment guidelines tailored to, and offering protections for, specific patient groups, and that, once in place, guidelines must be robustly implemented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In January 2016, Melissa Cook, a California gestational surrogate 23 weeks pregnant with triplets as a result of an in vitro fertilization (IVF) transfer of three embryos comprised of donor eggs and sperm provided by the intended father (C.M.), went to the media to protest the father’s request that she undergo selective fetal reduction. C.M. responded stating that he preferred to parent twins [2]. While much of the interest in this case has centered on the enforceability of surrogacy contracts [3–5], it also exposes some of the thorniest issues plaguing fertility medicine: the vulnerability of surrogates, soft governance embryo transfer guidelines, multiple-birth pregnancies, twin preference [6, 7], and US state surrogacy law which spans the spectrum from the most liberal in the world to a complete lack of legislation [8].
This paper considers these issues through the lens of multiple-embryo transfers and third-party reproduction decision-making. It seeks to fill an important gap in existing health policy literature, as few studies have examined the incidence of and implications for surrogates giving birth to more than one baby at a time [9–12]. It might be assumed that this problem has been addressed by embryo transfer guidelines issued jointly by the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM). However, the efficacy of these guidelines has been questioned repeatedly [13, 14] and guideline adherence studies have not examined the specific situation of the gestational surrogate patient [15, 16].
The paper begins by examining the impacts of nearly 20 years of ASRM-SART embryo transfer guidelines with some discussion of developments in other jurisdictions to provide context. This guideline review sets the scene for the analysis of embryo transfer data in the second section of the paper. Data about gestational surrogates obtained from the US Centers for Disease Control and Prevention (CDC) National Assisted Reproductive Technology Surveillance System (NASS) are used to examine multiple births and embryo transfers over a 12-year period (2003 to 2014). Findings and discussion raise a number of concerns about guideline adherence and gestational surrogate patients. The paper questions whether state surrogacy legislation and federal law could provide additional oversight [6, 17].
ASRM-SART embryo transfer guidelines
Context for guideline introduction and review: McCaughey, “Octomom,” and Cook
Assisted reproduction is costly, medically risky, and does not guarantee pregnancy. In 1981, noted embryologist John D. Biggers observed that pregnancy rates could be improved by the transfer of more than one embryo per IVF cycle [18]. By the mid-1980s, the need to achieve public confidence in assisted reproductive technology (ART) and the drive to demonstrate high clinic pregnancy success rates were such that the transfer of three to six embryos was not uncommon [19]. At the same time, other branches of medicine, notably pediatrics and gynecology, began to express concern about implications for infants and pregnant women of the growing number of IVF multiple births [20, 21].
A series of high-profile multiple births placed the practices of fertility medicine in the media spotlight. The birth of seven babies in 1997 to Bobbi and Kenny McCaughey, conceived as the result of fertility drugs, signaled that serious ethical and medical issues lurked behind the miracle of ART [22–24]. However, the “Octomom” case proved to be more significant [25].
In 2009, Nadia Suleman gave birth to eight babies as a result of the transfer of 12 embryos. International media coverage exposed troubling fertility practices, including the routine transfer of multiple embryos. The incident reopened debates about the need for national legislation, raised questions about soft governance mechanisms, and exposed tensions regarding reproductive autonomy and best interests of ART-conceived children [14, 26–29].
Mounting medical evidence has shown that multiple-birth infants are 17 times more likely to be pre-term, to require caesarean delivery, and to need expensive care at birth and throughout their lives [30–32]. Multiple-birth pregnancies often produce pregnancy and delivery complications [33–35], require longer post-delivery hospital stays [36], and may contribute to family adjustment problems [37].
In an effort to reduce the number of IVF multiple births, differing policy and legislative approaches have been adopted worldwide. Where ART is regulated and publicly financed, such as Quebec, Belgium, and Sweden, single-embryo transfer is mandated with the transfer of two or more embryos permitted in special circumstances only [38–45]. In the UK where fertility treatment can be covered under the National Health Service, the Human Fertilisation and Embryology Authority established a 15% multiple-birth target which appears to have been attained [46] notwithstanding a successful legal challenge mounted by IVF clinic directors objecting to embryo transfer restriction measures being attached to the clinic’s operating license [47].
In the USA, professional guidance rather than legislation governs the embryo transfer practices with research showing that the role played by US insurance mandate coverage in effecting IVF multiple-birth reductions is nuanced and jurisdiction specific. Findings indicate that cuts to numbers of embryos transferred per cycle can be counterbalanced by increased fertility treatment uptake, especially among older women; factors which contribute to increases in multiple-infant pregnancies and births [48–51].
Overall, studies support the position that restrictions on multiple-embryo transfers when coupled with fertility treatment insurance coverage produce substantial declines in multiple-birth deliveries. Even so, the circumstances under which multiple-embryo transfer should be recommended [52] and the ethical appropriateness of limiting reproductive choice remain topics of intense discussion [27, 53].
USA: 20 years of embryo transfer guidelines
In the USA, individual states legislate the practice of medicine. Professional bodies such as the American College of Obstetrics and Gynecology (ACOG) play prominent roles in the standard setting. In the field of reproductive medicine, organizations such as SART and ASRM develop practice guidelines, establish embryological and clinical standards, and foster best practices, though some US states legislate embryology practices, gamete infection screening and informed consent, license premises performing egg retrieval, and many oversee Institutional Review Board requirements governing research [54]. To better protect consumers of fertility medicine, the federal government enacted the Fertility Clinic Success Rate and Certification Act of 1992. This statute mandates IVF data collection, annual CDC publication of ART practices, and fertility clinic certification, but does not establish ART practice protocols nor regulate compliance with safety standards [17, 55, 56].
By the late-1990s, a clearer picture of fertility medical practices began to emerge, notably the increase in high-order births [13]. The average number of embryos transferred per IVF cycle ranged from 3.6 to 4.2 [57]; a figure all the more striking given that improved IVF techniques had led researchers to observe that pregnancy rates could be maintained with fewer embryos being transferred [58–61].
The rise in high-order multiple births [62] as well as bioethical and clinical concerns raised by McCaughey and similar cases [63] coincided with the joint ASRM-SART approval and publication in 1998 of voluntary guidelines recommending the maximum number of embryos, based on the quality and type of the embryo and age of the ova donor or IVF patient, that should be transferred to ensure the optimal chance of a successful pregnancy [64]. Revisions made in 1999 [65] decreased the number of embryos to be transferred for patients younger than 35 to a range of 2 to 3, though for other age categories 4 to 5 embryos were recommended (Table 1).
By 2001, multiple-IVF births had not declined markedly and guideline adherence came under renewed scrutiny [13, 57]. Guideline revisions made in 2004 reduced the number of embryos recommended for transfer in all patient-age categories [66]. Changes brought about by innovation in fertility medicine—notably, the use of blastocyst embryos which offers higher conception rates with fewer embryos transferred [67]—featured in the 2006 and 2008 guideline updates [68, 69].
As noted, the “Octomom” incident placed US fertility practices under the microscope [14, 27–29, 70]. Yet, updated 2009 ASRM-SART guidelines made no alteration to the number of embryos recommended for transfer [71]. Instead, revisions focused attention on counseling for patients undergoing multiple-embryo transfer, including provision of selective reduction information. Clinics were advised to document reasons for embryo transfers above recommended levels. The 2009 guidelines relaxed the requirement for a SART-initiated audit of embryo transfer practices when the threshold of 2 standard deviations above the recommended number of embryos was attained though as Jones and Schnorr [13] noted that such audits rarely, if ever, occurred. As the prosecution of Dr. Kamrava, the physician who administered fertility treatments to Nadia Suleman, demonstrates state legislation wields a tougher and more punitive stick compared with ASRM-SART voluntary guidelines [25].
The most recent guideline changes made in 2013 address clinic-level operational procedures and place greater emphasis on clinic self-monitoring [72]. The number of embryos recommended to be transferred per cycle remain unaltered from 2009 [73] even though the ASRM-SART Practice Committee had released in 2012 a position paper supportive of elective single-embryo transfer (eSET) [74].
Impact of ASRM-SART guidelines
Studies conclude that the observed decline in multiple births and the moderate decrease in the number of embryos transferred per cycle coincide with publication of the ASRM-SART embryo transfer guidelines, but no causal relationship can be demonstrated [57, 73, 75]. Change in embryology practices, notably the growth in blastocyst embryo transfers, has also contributed to the decline in the number of embryos transferred and improved IVF pregnancy rates [67].
The drop in high-order births is a welcome sign though the slow adoption of eSET and the sharp increase in twin births remain troubling [76–80]. For a subset of IVF patients—gestational surrogates—multiple birth rates remain stubbornly higher [10–12].
Precarious position of gestational surrogates
The practice of a woman conceiving and carrying a baby for persons unable to have their own biological children has been characterized as morally troubling [81, 82]. Ethicists, policy-makers, and legal scholars have raised concerns about the vulnerability of surrogates and the potential of the practice to commercialize and commoditize reproduction [83–86]. It is not surprising that the legalization of surrogacy has been a controversial topic worldwide. Many nations including France, Germany, and Italy prohibit the practice, while others such as Canada and UK permit only altruistic surrogacy. In America, each state sets its own legal framework governing paid, altruistic, gestational, and traditional (genetic) surrogacy practices including enforceability of the contract or arrangement. For example, surrogacy in any form is illegal in New York [87], Indiana, Washington, Michigan, and District of Columbia. In the remaining states, surrogacy may be allowed even when carried out on a commercial basis (for example, California); permitted when conducted in an altruistic manner (e.g., Louisiana, Virginia and Washington State); or the law may be silent regarding it (e.g., Georgia and Hawaii) [6, 8, 88–90].
Professional associations such as SART, ASRM, and ACOG tacitly support surrogacy as a suitable option for persons unable to conceive and bear their own biological children [91–93]. Gestational surrogacy (where the surrogate is not related genetically to the child she bears for intended parents) is preferred as the practice is viewed as being less ethically troublesome, more legally acceptable, and considered to pose fewer psychological difficulties for the surrogate mother compared with traditional (genetic) surrogacy [94].
Gestational surrogacy in the US comprises a small but rapidly growing IVF patient group—2.5% of 2013 IVF treatment cycles involved gestational surrogates representing a fourfold increase since 1999 [11]. However, the practice is under-researched. The Söderström-Anttila et al. systematic review notes an absence of studies examining outcomes for surrogates and children born to them. It identified serious methodological weaknesses in the research completed to date, including reliance on small samples. None of the studies considered by the review examined embryo transfer guideline compliance [95].
Where’s Waldo?: Finding the gestational surrogate in the ASRM-SART embryo transfer guidelines
ASRM-SART embryo transfer guidelines do not specifically identify gestational surrogates as a patient group requiring particular treatment. The ASRM booklet, Multiple Pregnancy and Birth [96], informs intended parents about reducing the number of embryos transferred in order to curb multiple gestations. It advises that most IVF programs will limit the number of embryos transferred to two when the ova donor is aged 21 to 34. The booklet does not mention that the practice of transferring two embryos runs counter to ASRM-SART guidelines which recommend that one embryo be transferred when the ova donor is younger than 35 [72]: the predominate situation for gestational surrogates [11, 12].
The ASRM Ethics Committee Opinion: Consideration of a Gestational Carrier does not unambiguously recommend a single-embryo transfer for gestational surrogates. Nor does it advise that the age of the ova donor be used to determine the number of embryos to transfer. Instead, emphasis is placed on the need for counseling when multiple embryos are transferred [91]. The 2015 updated ASRM-SART Practice Committee report entitled, Recommendations for practices utilizing gestational carriers: a committee opinion, states: “Special consideration should be given to transferring a single embryo in an effort to limit the risks of multiple pregnancy for the [gestational] carrier. After appropriate counselling and agreement by all parties, additional embryos may be transferred based on the age of the genetic parent, in an effort to improve the probability of pregnancy” [97]. In contrast, the European Society for Reproduction and Embryology (ESHRE) guidelines start from the assumption that one embryo should be transferred to surrogate patients with a maximum of two embryos being considered under special conditions only [98, 99].
Returning to the Melissa Cook case, did the transfer of three donor embryos “violate accepted standards of medical practice,” as was alleged in a California court filing [100]? Is the Cook incident an isolated exception, a case of medical malfeasance, or a reflection of usual fertility treatment practice? Some clues may be found in the American ART registry data. The next section attempts to unpick these data, as a means of assessing adherence to ASRM-SART embryo transfer guidelines when the patient is a gestational surrogate.
Methodology
Data sources
Author-designed custom tabulations for the US and California were obtained from the CDC National ART Surveillance System (NASS) for 2003 through 2014 [101] showing the prevalence of one, two and three+, fresh and frozen, intended mother (IM) and donor ova used in embryos transferred per gestational surrogate and other IVF patient treatment cycle. The period spans the years during which fertility treatment and policy changes took place, including development and revision of embryo transfer guidelines, greater reliance on donor gametes (ova and sperm), and increased number of gestational surrogate cycles [11].
Methods
Descriptive statistics showing embryo transfer cycles, multiple-birth incidence, and use of embryos comprised of donor and IM own ova were prepared. Relative risk analysis using tabular data was performed using MedCalc for the period 2007 to 2014 [102, 103]. The age of the surrogate and ova donor was provided by CDC for these years only [101]. Calculation of the percentage of gestational surrogate cycles not in conformity with the ASRM-SART embryo transfer guidelines is modeled in proportion of the ova (IM and third party) donated by women under age 35.
Results
ASRM-SART guideline compliance
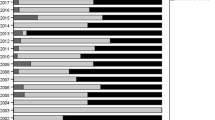
Over the 12-year study period (2003–2014), all IVF patients experienced a decline in multiple-birth deliveries. Gestational surrogates consistently demonstrated higher multiple-birth levels (41–25%) compared to other IVF patients (34–21%). California IVF patients, surrogate and non-surrogate, experienced a lower level of multiple-birth deliveries compared with other IVF patients (Fig. 1).
Between 2007 and 2014, gestational surrogates nationally had a significantly greater risk of receiving two or more embryos compared to other IVF patients (RR = 1.027). California gestational surrogates were 8% more likely than other California IVF patients (RR = 1.081) and 4% more likely than gestational surrogates living elsewhere in the USA to receive two or more embryos when embryos contain donor ova (RR = 1.041). When IM ova are used, the picture becomes more nuanced. California gestational surrogates were as likely as other IVF patients to receive 2+ embryos when IM ova are used (RR = 0.992). In contrast, non-gestational surrogate Californian IVF patients using their own ova were 3% more likely to receive two or more embryos (RR = 1.035; Table 2).
The analysis reveals three important findings. First, regardless of the age of the ova donor, the average number of embryos transferred to gestational surrogates rose with the age of the surrogate (Table 3). This result suggests that the age of the gestational surrogate, rather than the age of the ova donor, may have determined the number of embryos to transfer. However, this finding is attenuated by missing age data which the CDC reports was being absent for 36.5% of reported surrogate IVF cycles and in 33% of ova donor cycles [101].
The second confirms an expected outcome: when embryos contain IM ova, more embryos per cycle are transferred. This finding is not surprising, as the median age of intended mothers is higher compared to gestational surrogates [11]. Even so, the average number of embryos transferred when the IM ova age was <35 remained high, 1.9 nationally and 2.1 for California.
The third finding reveals the degree of non-compliance to ASRM-SART guidelines. By 2014, 86% of all donor ova (IM and third party) transferred to gestational surrogates come from persons aged <35. Over the 2007–2014, the recommended number of embryos was exceeded in 89–55% all national surrogate cycles. In California, non-compliance ranged from 87 to 64% of surrogate cycles (Figs. 2 and 3).
Discussion
ASRM-SART guidelines and the gestational surrogate patient
Lack of adherence to the ASRM-SART embryo-transfer guidelines has been observed since Jones and Schnorr [13] asserted that voluntary guidelines had not solved the problem of multiple-IVF gestations. Kawwass et al. [15] reported that among non-surrogate IVF patients receiving fresh embryos, transfer of one embryo occurred in only 14.5% of cycles where the ova donors were <35. The Acharya et al.’s [16] study of blastocyst embryo-transfer cycles concluded that guideline compliance was 28%. This paper’s findings reveal a similar trend: ASRM-SART guidelines, which recommend one embryo transfer when the age of the ova donor <35, appear to have been followed in 13 to 28% of the gestational surrogate IVF cycles during the years 2007 to 2012 with compliance rising to 42% by 2014. Guideline adherence in California by 2014 was 36%.
This paper’s use of CDC information has several strengths, not the least of which being the examination of embryo transfer trends over an extensive time period. It is the first to examine compliance to ASRM-SART embryo transfer guidelines to a national gestational surrogate patient group (n = 17,359 cycles) and at the state level (California, n = 3075 cycles).
There are several limitations. Findings are attenuated by missing data for age of surrogate (36.5% cycles) and age of the ova donor (33% of cycles). It is hoped that these data gaps can be addressed in the future CDC data files. Notwithstanding this limitation, the paper demonstrates an ongoing embryo transfer guideline implementation weakness occurring when ova donors (IM and third party) are <35, a situation comprising by 2014, experienced by 86% of national and 82% of Californian gestational surrogates.
Based on these findings, the paper asks whether reluctance to implement embryo transfer guidelines has specific implications for surrogates especially given the precarious third-party reproduction position they occupy? It can be argued that clinicians and surrogates face strong pressures from prospective parents, for whom gestational surrogacy may be the only way to have a biologically related child. A lack of mandated insurance funding, the attraction of family completion that a twin birth offers, the desire of a surrogate, even when she receives payment, to assist others, and the nature of third-party reproductive arrangements privileging multi-party medical decision-making create a less-than-ideal environment within which to make embryo transfer decisions. The paper argues that the ineffectiveness of voluntary ASRM-SART embryo transfer guidelines, insufficient information about ova donor age, and clinical practices favoring two embryo transfers further complicate third-party reproduction decision-making.
A surrogate has an autonomous right to make decisions about her medical treatment. She alone can give consent on matters such as the number of embryos to transfer, prenatal testing, and selective termination. The ACOG Family Building through Gestational Surrogacy document places considerable importance on enabling surrogates to access independent legal counsel and medical advice. It reminds clinicians of the “primacy of the gestational carrier’s right to autonomous decision-making related to her body and health” [104].
Third-party reproduction produces tensions that permeate gestational surrogacy arrangements and influence decision-making. The ASRM-SART Utilizing Gestational Carrier guidance document in acknowledging these tensions underscores the legal right of surrogates to make medical decisions while emphasizing the importance of achieving agreement among all parties—intended parents, clinicians, and gestational surrogate—on decisions such as the number of embryos to transfer [105].
Yet existing research tells us little about how embryo transfer decisions are made. It is revealing in the Melissa Cook case that the rationale for a transfer of three embryos as stated by Wright J in his June 6, 2016 decision was: “Knowing of Cook’s advanced age [47 years] and C.M.’s request that multiple embryos be transferred, on August 17, 2015 Dr. Steinberg implanted three six-day-old fertilized male embryos into Cook’s uterus. On August 31, 2015, her viable pregnancy with triplets was confirmed” [106]. The age of the anonymous egg donor was never disclosed suggesting that the IVF clinic may have failed to collect such information; or if in possession of it, decided to ignore it. Nor did Cook’s advanced maternal age and previous pregnancies appear to signal possible increased health risks.
Some may argue that Melissa Cook is but a statistical outlier; yet as this study has shown, California gestational surrogates have an 8% increased risk of receiving multiple-embryo transfers when donor embryos are used. Equally worrying is the evidence presented in the Cook case that ACOG and ASRM-SART third-party reproduction best practice guidance recommendations appear to have been neither referenced in court nor followed. Given this, it is worth considering some of the factors that could influence multiple-embryo transfer decisions, including: (i) assertion that a twin pregnancy does not pose a significant health risk for the mother or the children; hence, the transfer of two embryos (or three embryos in the case of Melissa Cook) reflects pragmatism not malfeasance; (ii) uneven decisional playing field; and (iii) lack of regulated limits on the number of embryos transferred per IVF cycle. We move now briefly to consider the significance of each of these factors.
Pragmatism not malfeasance: twin birth versus two singleton births
I said I always would want twin babies: C.M. [107]
Privileging twin IVF births has long been recognized as a positive ART outcome by patients and clinicians [37, 77–80]. The risks posed by a twin pregnancy compared to two singleton births are contested [35, 108, 109]. The Söderström-Anttila et al.’s systematic review of surrogate pregnancy outcomes concludes that surrogates experience similar levels of hypertensive disorders and placental complications as other IVF patients even though they are likely to be younger [95].
In the context of gestational surrogate pregnancies, clinical acceptance of multiple-embryo transfers to facilitate a twin birth outcome is worrisome given the structured nature of shared decision-making specified in many gestational surrogacy contracts and arrangements, lack of information about repeat surrogate pregnancies [95], increased risk of delivery complications [34], and greater likelihood that fetal termination decisions may be necessary when multiple embryos are transferred [75]. Directed research is needed before it will be possible to sustain the argument that pragmatism justifies multiple-embryo transfers or that a twin birth is as safe for a gestational surrogate as two singleton births.
Surrogate arrangements are complicated: making informed decisions on the uneven playing field
It is argued that the depersonalized term, “gestational carrier,” the phrase frequently used in IVF fertility circles to describe a surrogate mother [110], denies her patient status by instrumentalizing her reproductive body [111]. Further complicating the uneven playing field of third-party reproduction is the emphasis placed on the requirement for surrogates, paid and unpaid, to demonstrate altruistic motivations [105]. An additional troubling factor concerns the adequacy of information provided to surrogates. Fuchs and Berenson note that more than 10% of gestational surrogates surveyed had not been told about the risks of multiple pregnancy and over one quarter reported not being informed of the demands and risks of medical protocols and about coping with the pregnancy, attachment to the child, and risks to their own children, marriage or partnership [112].
This paper argues that the overlay of objectification, enforced altruism, costly IVF treatments, intended parents’ strong desire for children combined with an underestimation of health risks posed by multiple-birth pregnancies may be factors functioning to encourage demands for multiple-embryo transfers while exerting influence on surrogates to acquiesce to them. The desire to please intended parents by achieving pregnancy on the first IVF cycle may also play an important role in decision making as a double-embryo transfer increases the odds of achieving a successful pregnancy.
Third-party reproduction has the potential to change boundaries of consent and right to privacy. More research is needed especially on the roles played by counseling and consenting mechanisms. Beck’s work on contracting of emotion may serve as a useful starting point as her examination of surrogacy arrangements documents the loss of autonomy expected of surrogates [113].
Mandated embryo transfer limits and insurance coverage
In the aftermath of the “Octomom” incident, Daar [28] rejected proposed embryo transfer-limitation legislation [114] arguing instead for incentivizing patient decision-making through changes to medical insurance. It is a well-maintained view that any movement towards establishment of single-embryo transfer targets must be accompanied by assurance that fertility treatments will be covered, in whole or in part, by medical insurance programs [13, 27].
By 2016, 15 states had adopted insurance mandates that included fertility treatment [48, 115, 116]. The scope of the mandates varies as to the range of services permitted, patient requirements, and coverage exceptions [48–51, 117]. Applicability to surrogate IVF treatment, prenatal care, delivery, and infant medical coverage remains uncertain [90, 118]. Where state law permits surrogacy or is silent about it, medical expenses and insurance coverage often form elements of surrogacy arrangements and contracts and may be embedded in law [90, 113]. For example, the Fuchs and Berenson study reported that 94% of surveyed gestational surrogates had private medical insurance [112]. Even so, surrogates may be subject to liability which could affect medical insurance coverage [119]. More research is needed. Studies to date have not explicitly explored the relationship between insurance coverage and embryo transfer trends experienced by surrogates.
“One for sorrow, two for joy?”
Normative privileging of a surrogate’s altruistic intentions to assist childless couples and individuals even when in receipt of payment, attraction of twin births, uneven provision of information about procedures and risks, and the tensions inherent in third-party reproduction contribute to the precarious decision-making position of gestational surrogates. These factors create a perfect climate for non-compliance with voluntary ASRM-SART embryo transfer guidelines, especially as specific recommendations have not been developed for gestational surrogates.
In the defense of patient reproductive autonomy, Tremellen et al. [53] argue that fear of litigation and professional censure would influence clinicians to adopt recommendations of ASRM-SART and ESHRE to publish statements clearly outlining the clinical scenarios where double-embryo transfer is never acceptable. While identification of such situations might serve a useful clinical purpose, surely guidance compliance must be the goal. As it now stands, except in extreme cases like the Octomom incident, US IVF clinicians face no penalty for non-compliance to voluntary guidelines [25]. The Cook case, which involved the transfer of three donor-ova embryos, a practice counter to ASRM-SART recommendations though not unknown in clinical practice, is unlikely to result in negligence litigation or professional sanctions.
To be truly effective, guidelines need jurisdictional teeth. Increasing federal powers of audit, enforcement, and compliance under the Fertility Clinic Success Rate and Certification Act of 1992 might be an option [55]. However, to achieve this objective would require US federal authorities to legislate commercial interests of fertility medicine, a step they have been reluctant to take. At the state level, it is not unknown for national standards established by professional bodies to be incorporated into law, though to do so can be controversial as evidenced by the Common Core Standards Initiative [120]. Regarding fertility medicine, American state surrogacy laws could be expanded to mandate adoption of and compliance with ASRM-SART and ACOG treatment guidelines as was attempted when the Washington State House Bill 1267 on surrogacy was first introduced in 2011 [85].
Where US states legislate surrogacy, opportunity exists to provide greater protections for surrogates. For example, the 2015 New York State Child–Parent Security Act [87] proposed to mandate surrogate medical insurance extending for a period of 8 weeks post-birth. It included access to independent legal counsel and medical advice and sought to reinforce a surrogate’s ability to “safeguard her health.” Other American states, notably Virginia, Texas, and Louisiana, have mandated counseling on topics such as health risks associated with multiple-fetal pregnancies [121]. Other states such as Maine, Texas, and Utah have legislated health protection provisions for the surrogate and the fetus [122–124]. As these initiatives demonstrate, legislative means could be found to promote adherence to recommended professional standards.
For nearly 20 years, American multiple-embryo transfer practices can be characterized as one of pragmatism: “one for sorrow, two for joy?” This paper questions the ethics and clinical efficacy of this fertility objective for gestational surrogate patients especially given the tensions involved with third-party reproductive decision-making, normative privileging of altruism, twin preference, and intended parents’ intense desire for children. Research findings strongly support revision of ASRM-SART guidelines to recommend single-embryo transfers for gestational surrogates, with the goal being a healthy singleton birth. As it is clear that voluntary guidelines are not sufficient, adoption of regulatory measures is advisable. In the case of gestational surrogacy, state laws may offer the opportunity to do so. Clinical beneficence and non-malfeasance demand no less.
References
Traditional English children’s nursery rhyme, c 1780.
Bever L. I am pro-life: A surrogate mother’s stand against ‘reducing’ her triplets. Washington Post. January 7 2016. https://www.washingtonpost.com/news/morning-mix/wp/2016/01/07/i-am-not-having-an-abortion-a-surrogate-mothers-stand-against-reducing-her-triplets/
Melissa K Cook v. Cynthia Anne Harding United States District Court Central District of California, Case No. 2:16-cv-00742 ODW (AFM) June 6, 2016.
Johnson v. Calvert (1993) [No. S023721. May 20, 1993].
California, AB-1217, C.466 Surrogacy Arrangements, (2011–2012).
Gabry LI. Procreating out pregnancy: surrogacy and the need for a comprehensive regulatory scheme. C J Law Soc Probs. 2012;45:415–50.
O’Reilly, K. When parents and surrogates disagree on abortion. The Atlantic. February 18 2016. http://www.theatlantic.com/health/archive/2016/02/surrogacy-contract-melissa-cook/463323/
Storrow R. Surrogacy American style. In Surrogacy, law and human rights. In Gerber P. O’Byrne K., editors. Abingdon, UK: Routledge. 2015, 193–216.
Ragoné H. Incontestable motivations. In Franklin S. Ragoné H., editors, Reproducing reproduction: kinship, power and technological innovation. Philadelphia: University of Pennsylvania Press, 1998, pp.118-31.
Gugucheva M. Surrogacy in America. Council for Responsible Genetics: Cambridge Mass. 2010. www.councilforresponsiblegenetics.org
Perkins KM, Boulet SL, Jamieson DL, Kissin DM. Trends and outcomes of gestational surrogacy in the United States. Fertil Steril. 2016;106:435–42.
White PM. Hidden from view: Canadian gestational surrogacy practices and outcomes, 2001–2012. Reprod Health Matters. 2016;24:205–17.
Jones HW, Schnorr JA. Multiple pregnancies: call to action. Fertil Steril. 2001;75:11–3.
Davidson CM. Octomom and multi-fetal pregnancies: why federal legislation should require insurers to cover in vitro fertilization. William Mary J Women Law. 2010;17:135–86.
Kawwass JF, Monsour M, Crawford S, Kissin DM, Session DR, Kulkarni AD, et al. Trends and outcomes for donor oocyte cycles in the United States, 2000–2010. J Am Med Assoc. 2013;310(22):2426–34.
Acharya KS, Keyhan S, Acharya CR, Yeh JS, Provost MP, Goldfarb JM, et al. Do donor oocyte cycles comply with ASRM/SART embryo transfer guidelines? An analysis of 13,393 donor cycles from the SART registry. Fertil Steril. 2016;103:603–7.
Fertility Clinic Success Rate and Certification Act of 1992 (FCSRC) Pub, L No.102-493 (October 24, 1992).
Price F. Establishing Guidelines and Regulations: The Clinical management of Fertility. In Birthright: Law an Ethics at the Beginning of Life. In R. Lee R. Morgan D. editors, London: Routledge. 1989, p.42.
Seppälä M. The world collaborative report on in vitro fertilization and embryo replacement: current state of the art in January 1984. In Seppälä M. and R.G. Edwards R.G. editors. In Vitro Fertilisation and Embryo Transfer. Annals of New York Academy of Science 1985; 442: 558–63.
Price F. Establishing guidelines and regulations: the clinical management of fertility. In Birthright: law an ethics at the beginning of life. In R. Lee R. Morgan D. editors, London: Routledge. 1989, pp. 37–55.
American College of Obstetricians and Gynecologists Educational bulletin. Special problems of multiple gestation. International Journal of Obstetrics and Gynecology 1989 (revised 1999) 64:323–33.
Lemonick MD. Septuplets: it’s a miracle. Time Magazine December 1 1997. http://content.time.com/time/magazine/article/0,9171,987455,00.html
Schreuder C. Fertility experts see a dark side to the septuplets’ birth controls needed, some ethicists say. Chicago Tribune. 1997. http://articles.chicagotribune.com/1997-11-23/news/9711230361_1_fertility-treatment-septuplets-babies.
Christie J. Party for seven! Record-breaking McCaughey septuplets turn 18 and prepare to graduate high school. Daily Mail. 2015. http://www.dailymail.co.uk/news/article-3237111/Party-seven-Record-breaking-McCaughey-septuplets-turn-18-prepare-graduate-high-school.html.
Before the Medical Board of California Department of Consumer Affairs, State of California In the Matter of the First Amended Accusation Against Michael Kamrava MD. Physician and Surgeon Certificate No. G41227. Agency Case No. 06-2009-197098. OAH Case No. 2010010877. 2011. http://documents.latimes.com/michael-kamrava-disciplinary-decision.
Robertson JA. Procreative liberty and harm to offspring in assisted reproduction. Am J Law Med. 2004;30:7–40.
Manninen BA. Parental, medical, and sociological responsibilities: “Octomom” as a case study in the ethics of fertility treatments. J Clin Res Bioeth. 2011;S1:002. doi:10.4172/2155-9627.S1-002.
Daar J. Federalizing embryo transfers: taming the wild west of reproductive medicine? C J Gend Law. 2012;23:257–325.
Cahn NR. Collins JM. Eight is enough. Northwestern University Law Review Colloquy 2009; 103: 501–13.
Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–7.
Pharoah PO. Risk of cerebral palsy in multiple pregnancies. Clin Perinatol. 2006;33:301–13.
Expert Panel on Infertility and Adoption. 2009. Raising expectations. Toronto: Ontario Government.
MacKay AP, Berg JC, King JC, Duran C, Chang J. Pregnancy-related mortality among women with multifetal pregnancies. Obstet Gynecol. 2006;107:563–8.
Sazonova A, Källen K, Thurin-Kjellberg A, Ulla-Britt Wennerholm U-B, Bergh C. Neonatal and maternal outcomes comparing women undergoing two in vitro fertilization (IVF) singleton pregnancies and women undergoing one IVF twin pregnancy. Fertil Steril. 2013;99:731–7.
Stillman RJ, Richter KJ, Jones Jr HW. Refuting a misguided campaign against the goal of single-embryo transfer and singleton birth in assisted reproduction. Hum Reprod. 2013;28:2599–607.
Koivurova S, Hartikainen AL, Gissler M, Hemminki, Klemetti R, Jarvelin MR. Health care costs resulting from IVF: prenatal and neonatal periods. Hum Reprod. 2004;19:2798–805.
Klock SC. Psychological adjustment to twins after infertility. Best practice and research. Clin Obstet Gynecol. 2004;18:645–56.
Bissonnette F, Phillips S, Gunby J, Holzer H, Mahutte N, St-Michel P, et al. Working to eliminate multiple pregnancies: a success story in Québec. Reprod Biomed Online. 2011;23:500–4.
CBC News. Quebec in vitro fertilization: a breakdown of new restrictions on treatment. 2015. http://www.cbc.ca/news/canada/montreal/quebec-ivf-treatment-new-law-1.3317682.
Ferraretti AP, Goossen V, de Mouzon J, Bhattacharya S, Castilla JA, Korsak V, et al. Assisted reproductive technology in Europe, 2008: results generated from European registers by ESHRE. Hum Reprod. 2012;27:2571–84.
Chambers GM, Hoang VP, Sullivan EA, Chapman MG, Ishihara O, Zegers-Hochschild F, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101:191–8.
De Neubourg D, Bogaerts K, Wyns C, Albert A, Camus M, et al. The history of Belgian assisted reproduction technology cycle registration and control: a case study in reducing the incidence of multiple pregnancy. Hum Reprod. 2013;28:2709–19.
Karletröm PO, Bergh C. Reducing the number of embryos transferred in Sweden: Impact on delivery and multiple birth rates. Hum Reprod. 2007;22:2202–7.
Velez MP, Connelly MP, Kadoch IJ, Phillips S, Bissonnette F. Universal coverage of IVF pays off. Hum Reprod. 2014;29:1313–9.
Kresowick JD, Stegmann BJ, Sparks AE, Ryan GL, van Voorhis BJ. Five years of mandatory single-embryo transfer (mSET) policy dramatically reduces twinning rate without lowering pregnancy rates. Fertil Steril. 2011;96:1367–9.
Harbottle S, Hughes C, Cutting R, Roberts S, Brison D, On behalf of the Association of Clinical Embryologists & The (ACE) British Fertility Society (BFS). Elective single embryo transfer: an update to UK best practice guidelines. Hum Fertil. 2015;18:165–83.
Regina (Assisted Reproduction and Gynaecology Centre and Another) v Human Fertilisation and Embryology Authority [2013] EWHC 3087 (Admin) [2013] WLR (D) 416.
Martin JR, Bromer JG, Sakkas D, Patrizio P. Insurance coverage and in vitro fertilization outcomes: a U.S. perspective. Fertil Steril. 2011;95:964–9.
Buckles KS. Infertility insurance mandates and multiple birth rates. Health Econ. 2013;22:775–89.
Boulet SL, Crawford S, Zhang Y, Sunderham S, Cohen B, Bernson D, et al. Embryo transfer practice and perinatal outcomes by insurance mandate status. Fertil Steril. 2015;104:403–9.
Crawford S. Boulet SL. Jamieson DL. Stone C. Mullen J. Kissin DM. Assisted reproductive technology use, embryo transfer practices and birth outcomes after infertility insurance mandates: New Jersey, and Connecticut. Fertility and Sterility 2016;105: 347–55 at 349.
Monteleone PAA, Mirisola RJ, Gonçalves SP, Baracat EC, Serafini PC. Outcomes of elective cryopreserved single or double embryo transfers following failure to conceive after fresh single embryo transfer. Reprod Biomed Online. 2016;33:161–7.
Tremellen K, Wilkinson D, Savulescu J. Is mandating elective single embryo transfer ethically justifiable in young women? Reprod BioMed Soc Online. 2016;1:81–7.
Adamson D. Regulation of assisted reproductive technologies in the United States. Family Law Q. 2005;39:727–44.
Preisler A. Assisted reproductive technology: the dangers of an unregulated market and the need for reform. DePaul J Health Care Law. 2013;15:213–36.
Thompson C. Making parents: the ontological choreography of reproductive technologies. Cambridge: Mass: MIT Press; 2005.
Jain T. Missmer SA. Hornstein MD. Trends in embryo-transfer practice and in outcomes of the use of assisted reproductive technology in the United States. The New England Journal of Medicine 2004; 350: 1639–45 at 1642.
Tasdemir M, Tasdemir I, Kodama H, Fukuda J, Tanaka T. Two instead of three embryo transfer in in-vitro fertilization. Hum Reprod. 1995;10:2155–8.
Roest JP, Mous HVH, van Heusden AM, Zeilmaker GH, Verhoeff A. A triplet pregnancy after in vitro fertilization is a procedure-related complication that should be prevented by replacement of two embryos only. Fertil Steril. 1997;67:290–5.
Templeton A, Morris JK. Reducing the risk of multiple births by transfer of two embryos after in vitro fertilitzation. N Engl J Med. 1998;339:573–7.
Viska S, Tiitinen A, Hyden-Granskog C, Hovatta O. Elective transfer of embryo results in an acceptable pregnancy rate and eliminates the risk of multiple births. Hum Reprod. 1999;14:2392–5.
Martin JA. Park MA. Trends in twin and triplet births: 1980–97. National Vital Statistics Reports September 14 1999; 47(24). Centers for Disease Control and Prevention.
Ryan M. The cost of longing. 2001. Georgetown University Press.
American Society for Reproductive Medicine. Practice Committee opinion: guidelines on number of embryos transferred. Birmingham, AL: American Society for Assisted Reproductive Medicine; 1998.
American Society for Reproductive Medicine. Practice Committee opinion: guidelines on number of embryos transferred. Birmingham, AL: American Society for Assisted Reproductive Medicine; 1999.
Practice Committee of the Society for Assisted Reproductive Technology, the American Society for Reproductive Medicine. Guidelines on the number of embryos transferred. Fertil Steril. 2004;82:773–84.
Stern JE, Ceders MI, Jain T. Assisted reproduction technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril. 2007;88:275–82.
Practice Committee of the Society for Assisted Reproductive Technology, Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86:S51–2.
Practice Committee of Society for Assisted Reproductive Technology, Practice Committee of American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2008;90:S163–4.
Stillman RJ. The Suleman octuplets: what can an aberration teach us? Fertil Steril. 2010;93:341–3.
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–9.
Practice Committee of American Society for Reproductive Medicine, Practice Committee of Society for Assisted Reproductive Technology. Criteria for number of embryos to transfer: a committee opinion. Fertil Steril. 2013;99:44–6.
Kissin DM, Kulkarni AD, Mneimneh A, Warner L, Boulet SL, Crawford S, et al. Embryo transfer practices and multiple births resulting from assisted reproductive technology: an opportunity for prevention. Fertil Steril. 2015;103:954–61.
Practice Committee of Society for Assisted Reproductive Technology, and Practice Committee of American Society for Reproductive Medicine. Elective single-embryo transfer. Fertil Steril. 2012;97:835–42.
Kulkarni AD, Jamieson DJ, Jones Jr HW, Kissin DM, Gallo MF, Macaluso M, et al. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369:2218–25.
Hamilton BE. Martin JA. Osterman MJK. Curtin SC. Mathews TJ. Births: final data for 2014. National Vital Statistics Reports 2015; 66. Centers for Disease Control and Prevention.
Tymstra T. At least we tried everything: about binary thinking, anticipated decision regret, and the imperative character of medical technology. J Psychosom Obstet Gynecol. 2007;28:131.
Twisk M, van der Veen F, Repping S, Heineman M-J, Korevaar JC, Bossuyt PMM. Preferences of sub-fertile women regarding elective single embryo transfer: additional in vitro fertilization cycles are acceptable, lower pregnancy rates are not. Fertil Steril. 2007;88:1006–9.
Leese B, Denton J. Attitudes towards single embryo transfer, twin and higher order pregnancies in patients undergoing infertility treatment: a review. Hum Fertil. 2010;13:28–34.
Kovacs P. Commentary: will patients accept fewer embryo transfers? Medscape. July 21, 2015.
Teman E. Birthing a mother. Los Angles: University of California Press; 2010.
Ashenden S. Reproblematising relations of agency and coercion: surrogacy. In Gender, agency and coercion. In S. Madhock S. Phillips A. Wilson K. 2013. Bassingstoke: Palgrave Macmillan. 2013, pp. 195–218.
Radin M. Contested commodities. 1996. Cambridge, Mass. Harvard University Press.
Shapiro J. For a feminist considering surrogacy, is compensation really the key question? Wash Law Rev. 2014;89:1345–73.
Ainsworth S. Bearing children, bearing risks: feminist leadership for progressive regulation of compensated surrogacy in the United States. Wash Law Rev. 2014;89:1077–123.
Phillips A. Our bodies: whose property? Princeton: Princeton University Press; 2013.
Child–parent Security Act, Assemb. B. 4319, 2015 Assemb., Reg. Sess. (N.Y. 2016)
D’Alton-Harrison R. Mater semper incertus est: who’s your mummy? Medical Law Review 2014. 22, no.3: 357–83.
Hinson DS. McBrien M. Surrogacy across America. Family Advocate 2011–12; 34: 32–6.
Finkelstein A. MacDougall S. Kintominas A. Olsen A. Surrogacy law and policy in the U.S.: a national conversation informed by global lawmaking. Report of the Columbia Law School, Sexuality & Gender Law Clinic. 2016.
American Society for Reproductive Medicine. 2013. Consideration of the gestational carrier: a committee opinion. www.arsm.org.
Practice Committee of the Society for Assisted Reproductive Technology and the American Society for Reproductive Medicine. Recommendations for practices utilizing gestational carriers: a committee opinion. Fertil Steril. 2015;103:e1–e18.
American College of Obstetricians and Gynecologists Committee Opinion. Family building through gestational surrogacy. Am College Obstet Gynecologists. 2016;660:1–7.
Bernstein G. Unintended consequences: prohibitions on gamete donor anonymity and the fragile practice of surrogacy. Indiana Health Law Rev. 2013;10:291–324.
Söderström-Anttila V. Wennerholm U-B. Loft A. Pinborg A. Aittomaki K. Romundstad LB. Berg C. Surrogacy: outcomes for surrogate mothers, children and the resulting families—a systematic review. Human Reproduction Update 22, 2016; 2: 260–76.
American Society for Reproductive Medicine. Multiple pregnancy and birth: twins, triplets, and high-order multiples: a guide for patients. 2012.
Practice Committee of the Society for Assisted Reproductive Technology and the American Society for Reproductive Medicine. Recommendations for practices utilizing gestational carriers: a committee opinion. Fertility and Sterility 2015;103: e1-8 at e3.
Shenfield F, Pennings G, Cohen J, Devroey P, de Wert G, Tarlatzis B. ESHRE task force on ethics and law 10: surrogacy. Hum Reprod. 2005;20:2705–7.
Shenfield F, Pennings G, De Mouzon J, Ferraretti AP, Goossens V. ESHRE’s good practice guide for cross-border reproductive care for centers and practitioners. Hum Reprod. 2011;26:1625–7.
Melissa Kay Cook, et al. v. Cynthia Anne Harding, et al., California Central District Court, 2:16-cv-00742 2016 WL, February 2, 2016 at 52.
National ART Surveillance System (NASS) tabular data provided on request by the Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, August 8, 2015, April 11, 2016, December 16 and 29, 2016.
MedCalc. https://www.medcalc.org/calc/
Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk? Int J Public Health. 2008;53:165–7.
American College of Obstetricians and Gynecologists Committee Opinion. Family building through gestational surrogacy. American College of Obstetricians and Gynecologists. March 2016; 660:3.
Practice Committee of the Society for Assisted Reproductive Technology and the American Society for Reproductive Medicine. Recommendations for practices utilizing gestational carriers: a committee opinion. Fertility and Sterility 2015; 103: e3 and e.5.
Melissa Kay Cook v. Cynthia Anne Harding. Case no. 2:16-cv-00742-ODW (AFM) Document 92,06.06.16. Order Granting Defendants Motions to Dismiss [44,46,54, 60] 2159 at 2165, lines 11–17.
2016 WL 424998 (C.D.Cal.) (Trial Pleading) United States District Court, C.D. California. Los Angeles Division Melissa Kay COOK et al. v. Cynthia Anne Harding M.P.H., et al. No. 2:16-CV-00742 at 62.
Gleicher N. The irrational attraction of elective single-embryo transfer (eSET). Hum Reprod. 2013;28:294–7.
Gleicher N, Kushnir VA, Barad DH. Risks of spontaneously an IVF-conceived singleton and twin pregnancies differ, requiring reassessment of statistics premises favoring elective single embryo transfer (eSET). Reprod Biol Endocrinol. 2016;14:25–32.
Beeson D, Darnovsky M, Lippman A. What’s in a name? Variations in terminology of third-party reproduction. Reprod Biomed Online. 2015;31:805–15.
Lupton D. Social worlds of the unborn. Basingstoke: Palgrave Macmillan; 2013.
Fuchs EL, Berenson AB. Screening of gestational carriers in the United States. Fertil Steril. 2016;106:1496–502.
Beck H. The legalization of emotion: managing risk by managing feelings in contracts for surrogate labor. Law Soc Rev. 2015;49:143–77.
Family Building Act H.R. 697, 111th Cong. (1st Sess. 2009).
RESOLVE, 2016. http://www.resolve.org/family-building-options/insurance_coverage/state-coverage.html.
Rosenthal Marie. Aetna follows best practices for IVF procedures: incentives lower multiple births. Managed Healthcare Executive. April 1. 2013.
Johnston J, Gusmano MK, Patrizio P. In search of real autonomy for fertility patients. Health Econ Policy Law. 2015;10:243–50.
Mid-South Insurance Co. vs. Doe, 2:02-1789-18, 274 F.Supp.2d 757 (D.S.C. 07/29/03).
California Family Code, Section 7962 (4).
Robert R. 2011. The common core standards and next chapter in American education. 2011. Cambridge Mass: Harvard Education Press.
Explicit reference to counselling: Virginia (§ 20–156 to 20–165); Texas TFC Chapter 160; Louisiana 2016 HB NO. 1102, Part III D.
Maine (19-A §§ 1931, 1932).
Texas (TFC Chapter 160).
Utah (§ 78B-15-801 to 78B-15-809).
Acknowledgements
Dr. Sheree L. Boulet, Centre for Disease Control and Prevention, Atlanta provided the data used in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
White, P.M. “One for Sorrow, Two for Joy?”: American embryo transfer guideline recommendations, practices, and outcomes for gestational surrogate patients. J Assist Reprod Genet 34, 431–443 (2017). https://doi.org/10.1007/s10815-017-0885-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0885-7