Abstract
Purpose
Many practices are moving away from cleavage-stage transfer in favor of blastocyst transfer. The purpose of this study is to evaluate how the overall live birth rate for fresh IVF cycles may increase by optimizing the day of transfer for each patient.
Methods
This is a retrospective cohort study of 1225 first fresh autologous IVF cycles performed between May 2012 and November 2013. Stepwise logistic regression was used to determine characteristics associated with live birth following cleavage-stage versus blastocyst transfer. The optimal transfer day (i.e., the day that maximized the odds of live birth) was determined for each patient, and the actual live birth rate was compared with the projected rate had each patient undergone transfer on her optimal day.
Results
With transfer on the optimal day for each patient, the overall birth rate would have increased from its actual value of 34.8 % to a projected 43.0 %, a 24 % increase. The majority of this increase (21 %) was due to optimization of patients who underwent cleavage-stage transfer but had a higher projected birth rate from blastocyst transfer. These patients were older (37.8 versus 36.0 years, p < 0.01) and had more follicles ≥18 mm than patients who should have remained with a cleavage-stage transfer.
Conclusions
A model can be built enabling patient-specific identification of optimal transfer day; within this discovery cohort, such optimization was estimated to increase live birth following a fresh transfer by 24 %. This study suggests blastocyst transfer should be more widely offered; however, there remain patients for whom a cleavage-stage transfer may yield better outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selecting the optimal day of embryo transfer remains a challenge for in vitro fertilization (IVF). In recent years, advances in embryology techniques and laboratory conditions have improved the survival of embryos in culture, with many practices moving away from fresh cleavage-stage transfer on days 2/3 in favor of blastocyst transfer on days 5/6 [1–5]. Numerous studies have found that blastocyst transfer allows for improved selection of the highest quality embryos, thereby increasing the likelihood of successful implantation, pregnancy, and live birth [1, 6–9]. However, other randomized controlled trials have failed to show such benefit [10–12], and there may be some risks associated with proceeding to blastocyst transfer. First, embryos potentially viable in vivo may not survive to become blastocysts in vitro. A recent Cochrane review demonstrated that while couples who undergo a blastocyst transfer have a higher chance of live birth per cycle, they have lower cumulative pregnancy rates over time (fresh + thaw cycles), they are more likely to have no transferrable embryos available, and they are less likely to have supernumerary embryos for cryopreservation [1]. Second, there are some concerns from observational studies that blastocyst transfer may be associated with increased risks of preterm birth, very preterm birth, and birth defects [13, 14].
Infertility clinics typically develop their own protocols regarding which patients are eligible for extended culture and when the decision is made to perform a cleavage-stage versus blastocyst transfer. Some practices perform blastocyst transfers for all patients regardless of individual cycle characteristics, while others restrict blastocyst transfer to the best-prognosis patients. Ideally, each patient could be counseled, based on her specific demographic and cycle characteristics, as to whether her likelihood of a live birth would be greater with a cleavage-stage or blastocyst transfer.
The purposes of this study were therefore (1) to determine the probability of live birth following a cleavage-stage versus blastocyst fresh embryo transfer based on individual patient and cycle characteristics, (2) to calculate how the overall live birth rate may be increased by selecting the optimal day of transfer based on prediction models developed in our population, and (3) to determine characteristics that may be used to enhance selection of transfer day.
Materials and methods
IVF cycles
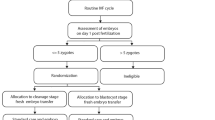
We conducted a retrospective analysis of 1225 first fresh IVF cycles performed at Brigham and Women’s Hospital from May 2012 to December 2013 after obtaining approval from the Partners’ HealthCare Institutional Review Board. This time frame was chosen because laboratory parameters such as media type, incubator oxygen tension, and embryo selection algorithms were uniform, limiting the possibility of confounding factors. Only the first autologous cycles for each patient were included. PGD/PGS cases were excluded. Cycles were grouped based on day of transfer: cleavage-stage transfer always occurred on day 3 (n = 882) and blastocyst transfer always occurred on day 5 (n = 343). The day of transfer for each patient was decided on day 3 following egg retrieval according to our protocol, which was based on the following criteria: On the first IVF cycle, patients <38 years of age with ≥6 zygotes on day 1 and ≥3 embryos of good quality on day 3 were eligible for a blastocyst transfer (n = 440). Nonetheless, some patients did not undergo their recommended day of transfer based on patient and physician preference: there were 159 patients eligible for blastocyst transfer but who underwent cleavage stage transfer, and 62 patients who did not meet eligibility criteria for extended culture but still underwent blastocyst transfer.
Laboratory protocols
Gametes and embryos were cultured in a humidified incubator maintained at 37 °C under an atmosphere of 5–6 % CO2, 5 % O2, and the balance of N2. IVF or ICSI was performed 4–6 h, or 3–5 h, respectively, after egg retrieval. The fertilization check was performed 16–18 h after insemination. Zygotes with 2 pronuclei (2PN) were cultured in 25 uL microdrops of a single-step medium (Global Total, IVFOnLine, Guelph, Ontario, Canada) under mineral oil.
Embryos were evaluated on day 3 between 66 and 69 h post-insemination. Morphological grading was based on (1) number of blastomeres, (2) extent of fragmentation, and (3) extent of asymmetry, as previously described [15]. Fragmentation was scored on a 0–4 scale based on the percentage of fragmentation as follows: 0, 0 %; 1, 1–9 %; 2, 10–25 %; 3, 26–50 %; and 4, >50 %. Symmetry was graded on a scale of 1–3, corresponding with perfectly symmetric, moderately symmetric, and severely asymmetric blastomeres, respectively. Good quality day 3 embryos were defined as having ≥8 cells, with <10 % fragmentation.
Blastocyst morphology was evaluated on day 5 and scored according to the stage of development, and to the quality of the inner cell mass (ICM) and trophectoderm (TE) [16, 17]. Good quality embryos on day 5 were defined as hatching/hatched blastocysts with “A” or “B” grades for both the ICM and TE. Fair quality embryos were defined as expanding, full, or expanded blastocysts with “A” or “B” grades for both the ICM and TE, or hatching/hatched blastocysts with at least one “C” grade for the ICM or TE. Poor quality embryos were considered as (1) any morula or blastocyst stage with a “D” grade for the ICM or TE, (2) any early blastocyst, or (3) any full, expanding, or expanded blastocyst with at least one “C” grade for the ICM or TE.
Statistical methods
Model building
We performed stepwise logistic regressions (cutoff: p < 0.10) to create models for estimating live birth among patients following both cleavage-stage and blastocyst transfers. All characteristics known by day 3 were included: age (and age2), body mass index (BMI), day 3 FSH, primary infertility diagnosis (DOR, endometriosis, male factor, ovulatory dysfunction, tubal factor, “unknown/idiopathic,” and uterine factor), ICSI vs. IVF, stimulation protocol (microflare, antagonist, or down-regulated), gonadotropin dose, peak estradiol, number of follicles (≥12, 16, or 18 mm), day of trigger, total number of eggs retrieved, number of mature oocytes, number of 2PNs, number of ≥8-cell embryos on day 3 (GT8C), GT8C with fragmentation <10 % on day 3 (GT8CF1), and number of embryos transferred. Due to the nonlinear relationships between age and number of zygotes on live birth, all ages ≤35 years were considered equivalent and any number of 2PN embryos ≥6 were considered equivalent.
For the blastocyst transfer model, additional regressions were performed that also included characteristics known only after day 3: number of embryos frozen, number of good/fair or poor quality embryos on day 5, and number of hours in culture. The number of good/fair embryos on day 5 was then estimated from parameters known by day 3 using a Poisson regression, as this was the only parameter found to additionally be significant in the models. The Poisson regression was calculated with truncation at age >41 years due to scarcity of data above this age (n = 10). All statistical analyses were performed using Matlab (version R2015a, MathWorks, Natick MA).
Model application
We estimated the probability of live birth for all patients using both the cleavage-stage and blastocyst transfer models, regardless of their actual transfer day. We then calculated the optimized overall live birth rate, had each patient undergone transfer on the day that maximized her chances of live birth, based on the models. We also projected the live birth rate had every patient undergone blastocyst transfer.
Among the cleavage-stage transfer group, the characteristics of patients who were projected to have a higher live birth rate following cleavage-stage versus blastocyst transfer were compared using Student’s t test; p value <0.05 defined significance. Lastly, sensitivity analyses were performed to determine true and projected live birth rates using only patients “eligible” for blastocyst in the blastocyst transfer model, and patients “ineligible” for blastocyst transfer in the cleavage-stage transfer model.
Results
Patient and cycle characteristics
The demographic characteristics of our patient population can be found in Supplemental Table 1. The average ages (year ± SD) of patients in the cleavage-stage and blastocyst transfer groups were 37.2 ± 4.1 and 34.2 ± 4.0, respectively. In the cleavage-stage transfer group, 25 % of patients carried a diagnosis of diminished ovarian reserve, 10 % had PCOS or ovulatory dysfunction, and 37 % had unexplained infertility. In the blastocyst transfer group, 11 % had a diagnosis of diminished ovarian reserve, 17 % had PCOS or ovulatory dysfunction, and 31 % had unexplained infertility. The average number of embryos transferred at the cleavage stage was 2.5, and 73 % of transferred cleavage-stage embryos were of good quality. The average number of embryos transferred at the blastocyst stage was 1.5, of which 74 % were considered good or fair quality.
Model building
Stepwise logistic regressions for live birth following cleavage-stage and blastocyst transfers were first performed using only characteristics known on or before day 3. Therefore, patient demographics, ovarian reserve response characteristics, and laboratory parameters available on day 3 were used. The receiver-operator characteristic (ROC) curves for these two regressions are shown in Fig. 1a, b. The area under the curve (AUC) was greater for cleavage-stage versus blastocyst transfers (0.68 versus 0.62, respectively). As expected, the probability of live birth agreed with the observed live birth rates as shown in Fig. 1a, b. Variables that remained significant in the final models are shown in Table 1a, b.
ROC curves and the projected vs. actual live birth rate for the logistic regression models. a Model for live birth following cleavage-stage ET, using only characteristics available by day 3 (Table 1A). b Model for live birth following blastocyst ET, using only characteristics available by day 3 (Table 1B). c Model for live birth following blastocyst ET, incorporating the projected numbers of embryos on day 5 (Table 1C–D), using only characteristics available by day 3
While age was significantly associated with live birth following cleavage-stage transfers, it was not significantly associated with live birth following blastocyst transfer. For cleavage-stage transfers, live birth rate began to decrease significantly around age 38 years.
The number of 2PN embryos was significantly associated with live birth following cleavage-stage transfers, but not for blastocyst transfers. To explore whether including laboratory characteristics on day 5 might improve the blastocyst model, another regression was performed that resulted in a marginally improved fit compared with that obtained from the regression using only factors on or before day 3 (AUC = 0.64 versus 0.62, respectively). The only factor in this improved regression not known by day 3 was the number of good/fair embryos on day 5. Age remained statistically insignificant for live birth.
To determine whether the number of good/fair embryos on day 5 could be estimated from characteristics known by day 3, we performed an additional Poisson regression. We specifically sought to calculate the fraction of embryos that developed into good/fair embryos on day 5; patient age was the only significantly associated covariate (Table 1d). We performed a similar regression for the number of poor embryos on day 5; this had no significantly associated factors.
The estimates of good/fair embryos on day 5 were incorporated into the model for live birth following blastocyst transfer. This final model then exclusively used factors known by day 3, including age. The ROC curve for the final blastocyst transfer model is shown in Fig. 1c (AUC = 0.64).
Model application
For our patient population, the actual live birth rates following cleavage-stage and blastocyst transfer were 29.6 and 48.1 %, respectively, resulting in an overall live birth rate of 34.8 %. The projected live birth rates for cleavage-stage and blastocyst transfers were calculated among the 1147 patients for whom all the required input parameters were available. Using the above prediction models, we then compared the projected live birth rates following cleavage-stage versus blastocyst transfer for each patient individually to determine the transfer day that would result in a higher chance of live birth. Of the 831 patients who underwent cleavage-stage transfer, 562 (67.6 %) should have undergone blastocyst transfer according to our models. Of the 316 patients who underwent blastocyst transfer, 113 (35.8 %) were found to have a higher live birth rate had they undergone cleavage-stage transfer. Had each patient undergone transfer on her optimal day, the projected overall birth rate would have risen from its actual value of 34.8 to 43.0 %, a statistically significant increase of 24 %.
As shown in Fig. 2, the majority of this increase was due to optimization of those patients who actually underwent cleavage-stage transfer. Had only patients who underwent cleavage-stage transfer been optimized, the projected live birth rate among those patients would have increased from its actual value of 29.6 to 39.6 %. This would correspondingly increase the overall live birth rate from 34.8 to 42.0 %, representing a statistically significant increase of 21 % over the true value. Conversely, had the transfer day of only blastocyst patients been optimized, the overall live birth rate would have been 35.8 %, only a 3 % increase (not significant). For reference, had every patient undergone blastocyst transfer, the projected overall birth rate would be 39.5 %, a significant 14 % rise over the actual value.
Projection of live birth rate with both cleavage-stage transfer (the horizontal axis) and blastocyst transfer (the vertical axis) for all 1147 patients for whom all the required input parameters were available. Each point represents a unique patient. Patients who actually underwent a cleavage-stage transfer are labeled red, while patients who actually underwent a blastocyst transfer are labeled blue. Points to the right of the line y = x have a higher projected birth rate following cleavage-stage transfers, while points to the left of the line y = x have a higher projected birth rate following blastocyst transfers. The projected live birth rate following cleavage-stage transfer was computed using the model shown in Table 1A, while the projected live birth rate following blastocyst transfer was computed using the model shown in Table 1C, incorporating the projected fraction of good/fair and poor embryos on day 5 (Table 1D)
The characteristics of patients who underwent cleavage-stage transfer but would have ideally undergone blastocyst transfer are shown in Table 2. These patients were significantly older and had an increased number of follicles ≥18 mm compared with those patients who would not have benefited from blastocyst transfer.
In sensitivity analyses, we restricted our data to eligible patients, using only patients eligible for a blastocyst transfer in the blastocyst model, and only patients ineligible for blastocyst transfer in the cleavage-stage model. In these analyses, the true live birth rate following cleavage-stage transfer was 26 %, and the live birth rate following blastocyst transfer was 50.9 %. Optimization of embryo transfer day for these eligible patients increased their overall live birth rate from its actual value of 33 to 42 %, a 27 % rise over the true live birth rate. This value is similar to the 24 % seen in the primary analysis. Optimizing only patients who underwent a cleavage-stage transfer increased the overall live birth rate to 41.5 %, again accounting for most of the observed increase.
Discussion
The principal finding of this study was that the overall live birth rate following a first fresh transfer may be increased by optimizing the transfer day for each patient. After applying our models to achieve this goal, the overall live birth rate for this study population would have been increased by 24 %; this increase was driven almost entirely by increasing the number of blastocyst transfers in select women who underwent cleavage-stage transfer.
The etiology of improved live birth rate following blastocyst transfer is thought to be multifactorial. The increased implantation potential of blastocysts may be due in large part to the higher likelihood of euploid embryos to blastulate by day 5 and then survive post-transfer [18, 19]. Moreover, evidence suggests that there may be improved synchronicity between embryo development and the endometrium on day 5. There are also data to suggest that the day 5 uterus is less pulsatile than that on day 3, which may decrease the likelihood of embryo expulsion following transfer [20, 21].
Since the introduction of extended culture media to support human embryo development beyond day 3 [5, 22], along with more recent advancements [23, 24], the prevalence of blastocyst embryo transfer has been increasing [1–5, 22]. While previously utilized only for patients with the best prognosis, many practices have expanded their use of blastocyst culture to patients with poorer prognoses, with some clinics exclusively offering blastocyst transfer. However, it is difficult to predict whether a particular embryo or cohort of embryos will survive to become blastocysts, which makes the decision to extend culture to day 5 challenging. Moreover, the third day after egg retrieval is the latest point at which to decide whether to perform a cleavage stage transfer.
In this study, we developed a regression model to project whether each patient would have a higher chance of live birth following cleavage-stage or blastocyst transfer for the first IVF cycle. The number of available embryos was significantly associated with live birth following cleavage-stage transfer, but not blastocyst transfer. This was likely due to a strong bias in the patient population: in our dataset, women with <6 zygotes on day 1, or ≤3 GT8C embryos on day 3, almost exclusively underwent cleavage-stage transfer. Thus, there was virtually no outcome data for patients with ≤3 GT8C embryos who underwent blastocyst transfer. As a result, the number of available embryos on day 1 or 3 was not found to be a significant predictor in the blastocyst model, although it is possible that they affect the odds of live birth for these patients.
Interestingly, maternal age was not significantly associated with live birth following blastocyst transfer, which may have been due to the selected, overall good prognosis population in this patient group. Of note, the number of good/fair embryos on day 5 was associated with live birth, although this parameter could not be used in the final model as it is not available on day 3, when the decision of transfer day was made in our patient population. To account for this, a separate regression was used to determine which factors known by day 3 could predict the number of good/fair embryos on day 5. In this regression, only age was found to be significant and was incorporated into the final blastocyst model.
Figure 2 reveals a significant overlap in the projected versus optimal transfer day. This suggests that optimizing the transfer day for individual patients can improve the live birth rate, which, in our population, was reflected in an increase of 24 %. This increase was largely driven by the majority (67.6 %) of patients who underwent cleavage-stage transfer, but who were projected to have a higher live birth rate had they undergone blastocyst transfer. Indeed, among patients undergoing cleavage-stage transfers, the number needed to treat (i.e., how many patients should be optimized to result in an additional live birth) was 10. Approximately 1/3 of patients who underwent blastocyst transfer were found to have a higher chance of live birth had they undergone cleavage-stage transfer; however, the projected increase in live birth rate was insignificant, at only 3 % (34.8 vs. 35.8 %). Therefore, the probability of live birth for these patients was quite similar following blastocyst or cleavage-stage transfer. On average, patients who actually underwent blastocyst transfer had greater chances of live birth than those who underwent cleavage-stage transfer, for both models. For reference, if all patients had exclusively undergone blastocyst transfer, the live birth rate for this study population would have increased by 14 %.
The final analysis in our study focused on patients who actually underwent cleavage-stage transfer. Patients in this group who had a higher chance of live birth with blastocyst transfer were, interestingly, older (37.8 vs. 36.0 years) and had more follicles ≥18 mm than patients who had a higher chance of live birth with cleavage-stage transfer. These findings suggest that blastocyst transfer should be more widely offered to older patients who may be better responders. Nevertheless, there are still some patients for whom cleavage-stage transfer remains the better option for optimizing live birth rate; most notably, these patients tend to have fewer follicles ≥18 mm. Based on the patient population analyzed in this study and the results obtained, we recommend offering blastocyst transfer to all women <38 years of age with ≥6 zygotes on day 1 and ≥3 embryos of good quality on day 3, which are the current guidelines at our institution. It is interesting to note that while our initial goal was to use parameters known by day 3 to predict live birth outcome, our models found that some of the best outcome predictors, such as age, number of follicles ≥18 mm, and number of 2PN embryos, are known as early as day 1.
Limitations to this study include the biases inherent to our retrospective design and to the models themselves. Specifically, the cleavage-stage model was developed using patients who actually underwent cleavage-stage transfer, and the blastocyst model was built using patients who actually underwent blastocyst transfer. However, we applied both models to all patients, regardless of actual transfer day, to project their chances of live birth following either day of transfer. The patient populations used to develop the models were inherently different from one another, based on our clinical practice. Thus, it is possible that the variables found to be significant among women for one model would no longer be significant when applied to the other population. This model could not be properly validated in another population of patients due to changes in laboratory and clinical protocols that could have biased results. Furthermore, this model is not designed to predict cumulative live birth rates following more than one transfer. There may be limitations in the generalizability of our findings, as they arise from a single institution in an insurance-mandated state.
In sensitivity, analyses restricted to eligible patients for blastocyst transfer in the blastocyst-transfer model and only ineligible patients in the cleavage-stage transfer model made the groups more homogenous and gave comparable results to our original models. Heterogeneity of our original patient groups (i.e., having both eligible/ineligible patients in the cleavage and blast transfer groups) may result in groups that are more similar to each other. This similarity between groups may reduce potential selection bias and confounding by indication.
In conclusion, this study suggests that increasing the availability of blastocyst transfer to a higher proportion of patients would increase the overall live birth rate for our patient population. Future research should further investigate the specific populations among whom a cleavage-stage transfer would be more beneficial than a blastocyst transfer, and investigate cumulative pregnancy rates following fresh transfer on each optimal day.
References
Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118.
Marsh CA, Farr SL, Chang J, Kissin DM, Grainger DA, Posner SF, et al. Trends and factors associated with the day 5 embryo transfer, assisted reproductive technology surveillance, USA, 2001–2009. Hum Reprod. 2012;27:2325–31.
Jones GM, Trounson AO. Blastocyst stage transfer: pitfalls and benefits. The benefits of extended culture. Hum Reprod. 1999;14:1405–8.
Thomas MR, Sparks AE, Ryan GL, Van Voorhis BJ. Clinical predictors of human blastocyst formation and pregnancy after extended embryo culture and transfer. Fertil Steril. 2010;94:543–8.
Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–8.
Mangalraj AM, Muthukumar K, Aleyamma T, Kamath MS, George K. Blastocyst stage transfer vs cleavage stage embryo transfer. J Hum Reprod Sci. 2009;2:23–6.
Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–9.
Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–46.
Zech NH, Lejeune B, Puissant F, Vanderzwalmen S, Zech H, Vanderzwalmen P. Prospective evaluation of the optimal time for selecting a single embryo for transfer: day 3 versus day 5. Fertil Steril. 2007;88:244–6.
Rienzi L, Ubaldi F, Iacobelli M, Ferrero S, Minasi MG, Martinez F, et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Hum Reprod. 2002;17:1852–5.
Van der Auwera I, Debrock S, Spiessens C, Afschrift H, Bakelants E, Meuleman C, et al. A prospective randomized study: day 2 versus day 5 embryo transfer. Hum Reprod. 2002;17:1507–12.
Frattarelli JL, Leondires MP, McKeeby JL, Miller BT, Segars JH. Blastocyst transfer decreases multiple pregnancy rates in in vitro fertilization cycles: a randomized controlled trial. Fertil Steril. 2003;79:228–30.
Maheshwari A, Kalampokas T, Davidson J, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of blastocyst-stage versus cleavage-stage embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2013;100:1615–21.e1–10.
Dar S, Lazer T, Shah PS, Librach CL. Neonatal outcomes among singleton births after blastocyst versus cleavage stage embryo transfer: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:439–48.
Racowsky C, Combelles CMH, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod Biomed Online. 2003;6:323–31.
Ceyhan ST, Jackson K V., Racowsky C. Biennial Review of Infertility Voorhis BJ, Schlegel PN, Racowsky C, Carrell DT, editors. S. Temel Ceyhan, Katharine V. Jackson, Catherine Racowsky. Sel. Most Competent Embryo. Totowa, NJ: Humana Press; 2009.
Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. Towar. Reprod. Certain. Fertil. Genet. Beyond 1999 Plenary Proc. 11th World Congr. 1999. p. 378–88.
Rubio C, Rodrigo L, Mercader A, Mateu E, Buendía P, Pehlivan T, et al. Impact of chromosomal abnormalities on preimplantation embryo development. Prenat Diagn. 2007;27:748–56.
Zakharova EE, Zaletova VV, Krivokharchenko AS. Biopsy of human morula-stage embryos: outcome of 215 IVF/ICSI cycles with PGS. PLoS One. 2014;9:e106433.
Fanchin R, Ayoubi JM, Righini C, Olivennes F, Schönauer LM, Frydman R. Uterine contractility decreases at the time of blastocyst transfers. Hum Reprod. 2001;16:1115–9.
Gardner DK, Balaban B. Choosing between day 3 and day 5 embryo transfers. Clin Obstet Gynecol. 2006;49:85–92.
Biggers JD, Racowsky C. The development of fertilized human ova to the blastocyst stage in KSOM(AA) medium: is a two-step protocol necessary? Reprod. Biomed. Online;5:133–40.
Machtinger R, Racowsky C. Culture systems: single step. Methods Mol Biol. 2012;912:199–209.
Quinn P. Culture systems: sequential. Methods Mol Biol. 2012;912:211–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
LF is supported by T32HD060454 in reproductive, perinatal, and pediatric epidemiology from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health.
Ethical approval
For this type of study, formal consent is not required.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Capsule By optimizing the day of embryo transfer for individual patients, overall live birth rate can be significantly increased.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 37 kb)
Rights and permissions
About this article
Cite this article
Goldman, R.H., Kaser, D.J., Missmer, S.A. et al. Building a model to increase live birth rate through patient-specific optimization of embryo transfer day. J Assist Reprod Genet 33, 1525–1532 (2016). https://doi.org/10.1007/s10815-016-0803-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0803-4