Abstract
Purpose
The aim of this study is to compare implantation and live birth rates (LBR) between fresh euploid embryo transfers versus cryo-all cycles with a subsequent embryo transfer into a prepared endometrium.
Material and Methods
This is a retrospective cohort study. Patients who underwent an IVF cycle with PGS with trophectoderm biopsy from January 2011 to July 2015 were included. Patients were divided into three groups: “Fresh Only,” “Frozen Embryo Transfer ('FET) Only,” and “Fresh ET then FET.” For “Fresh Only” group (n = 345), PGS results were received within 24 h. For “FET Only” group (n = 514), results were expected after 24 h, and embryos were cryopreserved after biopsy; only FET was performed in this group (no fresh transfer). For “FET with a previous fresh ET” (n = 139) group, patients underwent a fresh ET with a subsequent FET, in which the same cohort of embryos was utilized. The main outcome measures were pregnancy rate (PR), clinical PR, implantation rate (IR), LBR, and early pregnancy loss rate.
Results
IRs were statistically higher in the “FET Only” group when compared to the “Fresh Only” group (59.5 vs. 50.6 %, p < 0.01) and the “FET with a previous fresh ET” (59.5 vs. 50.6 %, p < 0.05). LBR was statistically significant in the “FET Only” group when compared to the “Fresh Only” group (57.6 vs. 46.5 %, p < 0.005) but not when compared to “FET with a previous fresh ET” group (57.6 vs. 47.7 %, p = 0.07).
Conclusions
This analysis suggests euploid embryos to be more likely to implant and achieve a LBR in a synthetic FET cycle than in a fresh cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Embryonic implantation into a synchronous endometrium is a critical step in achieving pregnancy. During cycles of controlled ovarian hyperstimulation (COH), the traditional focus to maximize oocyte yield may inadvertently diminish expected outcomes by creating a suboptimal endometrial environment. While the clinician’s approach is built on a knowledge base that has evolved over decades and integrates parameters currently available within the reproductive medicine community [1, 2], a growing body of evidence suggests that patients undergoing COH can potentially experience suboptimal endometrial development [3, 4]. Such effects would unavoidably diminish the likelihood of embryo implantation [5, 6] and consequently lower pregnancy rates. It is theorized that possible mechanisms that may alter embryo-endometrium synchrony may include a premature elevation of progesterone that could alter the normal endometrial window of implantation [3, 5].
Frozen thawed embryo transfer (FET) cycles have traditionally been associated with the utilization of “left over” embryos, as the morphologically superior embryos were selected for fresh transfer. This inherent bias may have affected former analyses of FET cycle outcomes and could have diminished early study’s FET cycles’ pregnancy rate(s) (PR). In this nature, it would be unfavorable to compare the quality of “second-best” embryos to their morphologically “superior” siblings. Therefore, previous studies comparing fresh embryo transfer (ET) with FET are limited due to the morphological differences in their study cohorts. Despite this fact, some studies have reported even higher pregnancy rates following a FET compared to fresh transfers [3, 6, 7]. In addition, with the advances and optimization made in cryopreservation methods [8], the quality of the frozen embryos and their reproductive potential are at least similar to those observed with fresh embryos [9]. Current techniques of cryopreservation are efficient, reliable, and documented as safe, and evidence is accumulating that freezing and rewarming embryos may result in outcomes equivalent if not superior to transfer in fresh cycles [8, 9].
Commensurate with cryopreservation advances, refinement of preimplantation screening (PGS) [10–12] offers an accurate means to determine embryo ploidy. Applying comprehensive chromosome screening (CCS) to high-quality embryos provides a reliable mechanism for ploidy evaluation prior to ET and an unprecedented ability to determine the optimal embryo(s) in any given cohort. However, in some cases, the turn-around time before genetic results are received is highly variable depending on the technology used (i.e., single-gene disorders, specific balanced translocations). Additionally, not all embryos reach proper development for day 5 biopsy and thus require extended culture until day 6. Therefore, in an effort to maximize embryo cohort size for biopsy and selection purposes, patients are encouraged to undergo day 5 and day 6 biopsy and subsequent cryopreservation of the entire cohort (as opposed to biopsy on day 5 and transfer on day 6). In either course, these delays prohibit the possibility for a fresh ET.
Prior to the introduction of PGS and a freeze-all strategy, it would have been unethical to advise patients to withhold from fresh ETs in order to analyze the use of these same “superior” embryos under a FET cycle(s). By standardizing embryo selection based on ploidy and monitoring patients’ endometrial environment, our study removes confounding factors observed in previous studies. Given the concern regarding the window of implantation, we sought to identify the optimal implantation environment for healthy euploid embryos by examining outcomes of cryopreserved euploid embryos transferred into non-COH stimulated endometrium as compared to euploid embryos transferred into COH-stimulated endometrium. Furthermore, we sought to eliminate any bias introduced by embryo selection by analyzing clinical outcomes of morphologically equivalent euploid embryos obtained from intended fresh IVF cycles as compared to intended cryo-all IVF cycles.
Material and methods
Study design
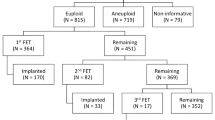
A single-center retrospective cohort analysis was performed on patients who completed an IVF cycle with PGS from January 2011 to December 2014. Study groups were identified from an electronic medical records database. All couples with viable blastocysts screened by PGS for aneuploidy and that had ≥1 euploid embryo(s) available for ET were included in the study. Only euploid embryos were transferred. Patients’ IVF cycle are not canceled if a thin endometrium (<5 mm) is observed at the study’s site; for this retrospective study, patients with an endometrium of <5 mm were excluded.
Participants
Stimulation protocol
Patients underwent standard COH for IVF either with a down-regulation protocol with leuprolide acetate (Lupron®, AbbVie Inc., North Chicago, IL), an antagonist protocol (Ganirelix Acetate®, Organon USA Inc., Roseland, NJ or Cetrotide®, EMD Serono, Rockland, MA), or a Microflare protocol (Lupron®, AbbVie Inc., North Chicago, IL). Final oocyte maturation was induced with r-hCG alone (Ovidrel®, EMD Serono, Rockland, MA) or, in patients with high ovarian response and/or in risk of ovarian hyperstimulation syndrome (OHSS) undergoing an antagonist protocol, with 40 UI of leuprolide acetate (Lupron®, AbbVie Laboratories, Chicago, IL) concomitant with 1000–1500 IU of hCG (Novarel®, Ferring Pharmaceuticals, Parsippany, NJ). Vaginal oocyte retrieval (VOR) was performed by using transvaginal ultrasound guidance 36 h later.
Embryo culture
After retrieval, embryos were cultured in Sage Quinn’s Advantage® Cleavage Medium (Cooper Surgical, Trumbull, CT) from day 0 to day 3. Media supplementation consisted of 5 % human serum albumin with 100 mg/mL (HSA-Solution™, Vitrolife, Göteborg Sweden) on day 0, and 10 % of synthetic serum substitute (SSS) with 6 % protein components consisting of 84 % pharmaceutical grade hSA (50 mg/mL) (SSS, Irvine Scientific, Santa Ana, CA) from day 1 to 6 of development. Low-oxygen conditions were maintained: from day 1 to 3 under 5 % oxygen, 5.5 % carbon dioxide, 89.5 % nitrogen and from day 3 to 6 under 5 % oxygen, 6 % carbon dioxide, 89 % nitrogen, provided by solid-state, ultra-stable, mini-incubators (Panasonic Sterisonic GxP incubator, Sanyo North America, Wood Dale, IL) using Nunclon 60-mm dishes with ten microdrops of 50 μL drops for up to one embryo per drop under 100 % paraffin oil (Ovooil™, Vitrolife, Göteborg Sweden). On day 3 after fertilization, the embryos were transferred from Sage Quinn’s Advantage® Cleavage Medium (zero glucose, pyruvate-dominant) to (glucose-rich) G-2.5™ Vitrolife Blastocyst Media (Göteborg Sweden) and supplement protein (10 % SSS, Irvine Scientific, Santa Ana, CA). On day 3 of embryo development, all the embryos were assisted “hatched” by a small 25–30 μm opening in the zona pellucida with a 10-μs pulse from a 400-μs pulse from a Zilos-tk laser (Hamilton Thorne Biosciences, Beverly, MA) to boost herniation of an emerging trophectoderm.
Embryo biopsy
On the morning of day 5, embryo’s zona pellucida was examined for a protruding trophectoderm. If discernible, the embryo was marked for biopsy; if not, the embryo was cultured for another 8–24 h and reassessed. We conducted biopsies under oil in Falcon 1006 Petri dishes (Becton Dickinson, Franklin Lakes, NJ) in 10 μL drops of Enhance WG—Vitrolife HTF/HEPES. With an Olympus IX70 microscope equipped with Narishige micromanipulators (East Meadow, NY), the blastocyst was secured with a thick-walled, blunt glass holding pipette (internal diameter, 20–30 μm), so that the protruding trophectoderm is stabilized at the 3 o’clock position. An estimated four to seven trophectoderm cells are then drawn into the lumen of a sharp, thin-walled biopsy pipette with an internal diameter of 30 μm and pulled gently away from the blastocyst. Trophectoderm cells detachment were achieved from 500 μs of near-infrared pulsations. Simultaneous during this process, the biopsy pipette was drawn away from the embryo until the cells separated from the blastocyst. The trophectoderm cells, generally five to six cells (but ranging from two to nine cells), were processed for analysis utilizing 24-chromosome aneuploidy screening by qPCR or aCGH. With either technique, the biopsied embryos were washed in blastocyst medium and transferred to individually numbered 10 μL droplets under oil; they were checked one day after the biopsy or at completion of the analysis for evidence of reexpansion, indicative of continuing viability. Since not all embryos hatch by day 5 which deems them ineligible for biopsy, it is not always possible to acquire genomic results in time for a fresh ET. Therefore, patients are encouraged to undergo cryo-all cycles in which day 5 and day 6 biopsies are available. These circumstances were known in advance, and IVF cycles were planned accordingly (intended fresh IVF cycle or intended cryo-all cycle).
Cryopreservation—rewarming
The Cryotop method for embryo vitrification was that described by Kuwayama et al. [13], with slight modifications. Early cleavage- and blastocyst-stage embryos were equilibrated in a single 10–12 min step at room temperature in 7.5 % (v/v) ethylene glycol (EG) þ 7.5 % dimethylsulfoxide (DMSO) in TCM199 medium + 20 % synthetic serum substitute (SSS). The equilibration time was defined by the reexpansion of the embryos. Typically, it took ∼12 min for blastocysts to fully re-expand. The vitrification step was performed in a solution containing 15 % EG + 15 % DMSO + 0.5 mol/L sucrose. Embryos were “washed” continuously in this solution for 45 s, at which point embryo collapsing was checked. Afterward, embryos were taken up into the pipette and placed at the end while making sure it contained the lowest possible volume of vitrification solution ahead of the embryo. Embryos were placed on the Cryotop sheet, and the excess solution was removed by aspiration. After checking the minimum volume, the Cryotop was plunged into liquid nitrogen (LN). This step was not longer than 10 s. The Cryotop was loaded with no more than one blastocyst. Although all of a patient’s embryos could have been equilibrated at the same time (in separate wells), the vitrification step was always performed strictly for the number of embryos designated to be loaded onto the Cryotop.
For warming, the Cryotop was removed from the LN and instantly placed in 1.0 mol/L sucrose in TCM199 + 20 % SSS at 37 °C. Special care was taken to avoid any manipulation of the embryos and thus protect them from mechanical stress. After 1 min, embryos were placed in 0.5 mol/L sucrose in TCM199 + 20 % SSS at room temperature for 3 min and were not subjected to any further manipulation. Finally, embryos were washed for 5 min and then for 1 min with TCM199 þ 20 % SSS at room temperature. The embryos were cultured at 37 °C for R2 h before ET. All vitrification materials were obtained from Kitazato. Immediately after warming, embryo survival was determined according to the appearance of the blastomeres and ZP. Blastocyst survival was evaluated according to morphologic appearance after warming and the ability of the blastocele to re-expand before transfer. If embryos had degenerated by the time of ET, they were catalogued as “dead embryos,” which represented a change to their original classification as “surviving embryos.”
Study groups
Three study groups were identified.
Group 1: Fresh only
A fresh IVF cycle, CCS, and a fresh ET
Patients underwent an IVF cycle with CCS and fresh ET, with embryos biopsied on day 5 and results available the morning of day 6. Patients included in this group were those who had planned to undergo a fresh transfer. Prior to PGS result evaluation, embryos were morphologically reassessed. Embryos were selected for ET according to (1) ploidy result and (2) morphology. Luteal phase support (LPS) was administered with micronized progesterone vaginally (either Endometrin®, Ferring Pharmaceuticals Inc., Parsippany, NJ, or Crinone®, Actavis Pharma, Parsippany, NJ) and orally (Prometrium®, AbbVie Inc., North Chicago, IL) beginning the day after the VOR.
Group 2: FET only
A fresh IVF cycle, CCS, no fresh ET, all embryos cryopreserved, FET in the subsequent cycle
Patients underwent a cryo-all IVF cycle with CCS, where all biopsied embryos were cryopreserved on day 5 or 6. No embryos were transferred during the initial, fresh IVF cycle. Once the genetic results were obtained, a subsequent FET cycle was scheduled. Patients included in this group were those who planned to undergo cryo-all cycles. Study patients underwent a cryo-all strategy because of the following reasons: (1) patients were counseled with the intention of increasing their biopsy cohort, and (2) patients underwent PGS for single-gene defect on top of aneuploidy screening, therefore results were unattainable in time for a fresh transfer. Patients started oral estradiol (E2) (Estrace®, Teva Pharmaceuticals, Sellersville, PA) 2 mg twice daily for 1 week, then 2 mg three times daily. Endometrial thickness was assessed weekly until a thickness of ≥8 mm was observed. Immediately thereafter, intramuscular progesterone (Progesterone injection®, Watson Pharma Inc., Parsippany, NJ) was added. Thawing and transferring of the embryo(s) was performed after 5 days of progesterone supplementation. Embryos were selected for ET according to (1) ploidy result and (2) morphology.
Group 3: FET with a previous Fresh ET
A fresh IVF cycle, CCS, a fresh ET, remaining embryos cryopreserved, a FET in a subsequent cycle
Patients underwent an IVF cycle with CCS with a fresh euploid ET, where the surplus euploid embryos were cryopreserved. Patients included in this group sought either to achieve another pregnancy after a successful fresh ET or to become pregnant after a failed fresh ET by using their remaining “second-best” euploid embryos. Patients started oral E2 (Estrace®, Teva Pharmaceuticals, Sellersville, PA) 2 mg twice daily for 1 week, then 2 mg three times daily. Endometrial thickness was assessed weekly until a thickness of ≥8 mm was observed. Immediately thereafter, intramuscular progesterone (Progesterone injection®, Watson Pharma Inc., Parsippany, NJ) was added. Thawing and transferring of the embryo(s) was performed 5 days after progesterone supplementation was started. Embryos were selected for ET according to (1) ploidy result and (2) morphology. This group was compared to the “FET Only” group only.
Outcome variables
The primary outcome variable was implantation rate (IR). The IR was calculated as the ratio of the number of gestational sacs (GS) to the number of transferred euploid embryos. Monozygotic twins were considered as one sac in this analysis. Secondary outcomes were pregnancy rate (PR), clinical PR, live birth rate (LBR), early pregnancy loss rate, and multiple PR. A clinical pregnancy was defined as the detection of a GS on an ultrasound (US) examination 22–25 days after retrieval. A pregnancy was defined as the detection of β-hCG ≥5 mUI/mL 14 days after the VOR. Early pregnancy losses were defined as a positive pregnancy test and/or a GS with or without fetal heart (FH) activity that did not pass the 20th week of gestation. PR and clinical PR were calculated as the ratio of total pregnancies and ongoing clinical pregnancies, respectively, to the number of assisted reproductive technology (ART) cycles entailing an ET. The LBR was calculated as the ratio of the number of live births to the number of patients that delivered. For this calculation, only patients transferred before January 1, 2015 were included. Early pregnancy loss rate was calculated as the ratio of early pregnancy losses to the number of patients with a positive pregnancy. Multiple PR was calculated as the ratio of clinical pregnancies with ≥2 GSs to the number of patients with a clinical pregnancy.
Statistical analysis
Statistical analysis was performed using Statistic Applied Software (SAS) version 9.4 (by SAS Institute Inc., Cary, NC, USA). Measurement levels of descriptive data were compared by unpaired two-sided t test with significance at p < 0.05; results are expressed as mean ± standard deviation with 95 % confidence intervals. Distributions between outcomes were assessed by Chi-square test. Fisher exact test was computed on all contingency tables with significance at p < 0.05 for samples less than 10. The Clopper-Pearson interval was used to calculate binomial confidence intervals. Adjusted odds ratios (OR) and their 95 % confidence intervals (CI) for implantation rate, PR, clinical PR, LBR, early pregnancy loss rate, and multiple PR were calculated to evaluate the relative odds of each event compared with the reference group. Study was designed with 80 % power to detect the difference of 11 % in IR between “Only IVF” versus “Only FET” groups with a reference proportion of 50 % and a two-tailed 5 % significance level. The required sample size was thus computed to be 320 patients per group. We conducted a stepwise multiple regression analysis to verify interaction of the main outcome measure with age, basal antral follicle count (AFC), and body mass index (BMI) to determine equivalence within the study groups. Because a strong correlation between age, day 3 FSH, and AMH was observed, we only included age as a covariate in the model.
This research was approved by the Western Institutional Review Board (WIRB). Because of its retrospective nature, a formal consent was not required.
Results
A total of 837 patients underwent 998 cycles and an embryo transfer between January 2011 and July 2015. From those, we identified 859 cycles scheduled to undergo a solely fresh (n = 345) or solely frozen (n = 514) transfer, and 139 underwent a fresh ET followed by a FET cycle. All demographic and laboratory characteristics are shown in Tables 1 and 2 and Supplemental Figs. 1 to 6. When age, basal AFC, and BMI were included into a step-forward regression analysis, no significant contribution of these variables was observed.
Comparison of “fresh only” versus “FET only”
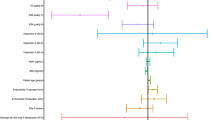
Patients in the “Fresh Only” group had a higher average number of follicles >14 mm at surge in the stimulation cycle (14.0 vs. 12.8, p < 0.01), a thicker endometrium at surge day in the ET cycle (9.9 (range 5–16 mm) vs. 9.0 (range 5–13 mm), p < 0.001), a higher number of oocytes retrieved after the IVF cycle (18.3 vs. 16.8, p < 0.05), a higher number of ongoing embryos on day 1 (11.7 vs. 10.5, p < 0.05), day 3 (11.2 vs. 10.0, p < 0.05), day 5 (8.0 vs. 6.5, p < 0.0001), and day 6 (8.5 vs. 4.8, p < 0.0001). Group 1 also had a higher proportion of euploid embryos (61.3 vs. 57.8 %, p < 0.05), a higher number of embryos transferred (1.3 vs. 1.1, p < 0.01), and a higher number of vitrified embryos (3.6 vs. 5.4, p < 0.0001) when compared to group 2 (Table 1 and Supplemental Figs. 1 and 2).
Patients who underwent a Fresh ET were observed to have 30 % less probability of implantation than those who waited (OR 0.7 (95 % CI 0.54–0.89)); IR in group 1 (50.6 %) was significantly lower than group 2 (59.5 %) (p < 0.01). Additionally, group 1 patients had a 30 % less probability of a positive pregnancy test (66.7 vs. 74.3 %, p < 0.05; OR 0.7 (95 % CI 0.51–0.93)), 30 % less probability of a clinical pregnancy (53.9 vs. 63.2 %, p < 0.01; OR 0.7 (95 % CI 0.51–0.88)), and 2.9 times higher probability of a multiple pregnancy (17.2 vs. 6.7 %, p < 0.01; OR 2.9 (95 % CI 1.62–5.17)). Early pregnancy loss rate was similar in both groups (Table 1). Lastly, patients that underwent a cryo-all cycle had 1.6 times higher probability of having a live birth when compared to patients that underwent a fresh ET (OR 1.6 (95 % CI 1.14–2.13)). The LBR in the “Fresh Only” group was 46.5 % versus. 57.6 % of the “FET Only” group.
Comparison between “FET only” versus “FET with a previous ET”
When comparing outcomes of patients from group 2 versus group 3, we observed that patients who did not have an ET after the COH cycle were older both at the time of the stimulation cycle (36.2 vs. 34.9, p < 0.05) and at the ET cycle (36.5 vs. 35.4, p < 0.05); patients in this group also had a lower average peak E2 level (2418.6 vs. 2826.2, p < 0.001), a lower number of follicles >14 mm in the stimulation cycle (12.8 vs. 16.5, p < 0.001), and a higher amount of total gonadotropins used (3376.6 vs. 3093.8, p < 0.05). For the laboratory variables analyzed, we observed a significantly lower average number of eggs retrieved (16.8 vs. 20.8, p < 0.0001), a lower average number of eggs inseminated (13.1 vs. 15.9, p < 0.001), a lower average number of ongoing embryos on day 1 (10.5 vs. 14.0, p < 0.0001), day 3 (10.0 vs. 13.6, p < 0.0001), day 5 (6.5 vs. 9.8, p < 0.0001), and day 6 (4.8 vs. 10.5, p < 0.0001). Patients in the “FET Only” also had a lower average total number of biopsied embryos (5.4 vs. 8.4, p < 0.0001), a lower proportion of euploid embryos (57.8 vs. 64.8 %, p < 0.001; OR 0.8 (95 % CI 0.65–0.86)), and a lower average number of embryos transferred (1.1 vs. 1.3, p < 0.001) than patients in the “FET with a previous ET” group (Table 2 and Supplemental Figs. 3 and 4).
The primary outcome of IR was significantly higher in patients who did not have an embryo(s) transferred after the COH cycle (59.5 vs. 50.6 %, p < 0.05); cryo-all cycles had 1.4 times higher probability of implantation (OR 1.4 (95 % CI 1.03–2.01)) when compared to patients that underwent a fresh ET after the COH cycle. Additionally, we also observed a 60 % less probability of a multiple pregnancy (OR 0.4 (95 % CI 0.18–0.79)), which was a significantly lower multiple PR than in group 2 (6.7 %) compared to group 3 (16.1 %, p < 0.05). The PR, clinical PR, and MR where similar between groups. Lastly, the LBR in group 2 was 57.6 versus 47.7 % in group 3, although this was not statistically significant (Table 2).
We additionally performed a secondary sub-analysis for group 3 in which we segregated all patients according if the outcome of the fresh cycle was successful. Therefore, subgroup 3A included patients who had a positive pregnancy test after an ET in the COH cycle and subgroup 3B those with a negative pregnancy test. All demographic characteristics and laboratory variables were similar between groups except a lower number of embryos transferred (1.2 vs. 1.4, p < 0.05), and a higher proportion of patients who experienced an early pregnancy loss (36.4 vs. 17.9 %, p < 0.05; OR 2.6 (95 % CI 1.20–5.75)) in the subgroup in which patients resulted pregnant in the fresh cycle, which is interpreted as 2.6 times more likely to have a miscarriage (Table 3 and Supplemental Figs. 5 and 6).
Discussion
Controversy surrounds the impact of COH on uterine receptivity and its effect on the implantation of healthy embryos. Previous studies that examined such impact may not have fully accounted for the confounding variable of embryo quality, especially ploidy status. Current techniques of embryo biopsy and genetic analysis allow accurate selection of euploid embryos for transfer and to more precisely determine the role of COH in ART and to understand its impact on pregnancy rates [1–3]. This study analyzed the difference in implantation rate in euploid embryos transferred under gonadotropin-stimulated fresh cycle versus outcomes of embryos transferred in a FET cycle in which no embryos were transferred after COH. Patients with embryos transferred only in a fresh cycle had statistically lower implantation and live birth rates than those transferred during FET (Fig. 1 and Table 1). These findings suggest the transfer of the best available embryo under a synthetically prepared endometrium is more recommended that transferring fresh.
The intent of transferring fresh embryos still remains the norm in ART procedures, while FETs are considered for secondary indications such as preserving surplus embryos generated from an initial fresh cycle. But as cryopreservation techniques have been refined [8, 9], more studies have reported improved outcomes after FET cycles when compared to fresh ET [7, 14, 15]. Traditionally, embryo cryopreservation was undertaken in patients with surplus embryos or patients with contraindications to transfer such as risk of OHSS or poor endometrial development and in some instances for fertility preservation. Recently, several groups have compared PR between embryos transferred during fresh—gonadotropin-stimulated—cycles versus FETs in a subsequent estrogen-stimulated (synthetic) cycle [3, 16] or under a natural cycle [14, 17, 18] with comparable conclusions. For example, a meta-analysis by Roque et al. [7] included 3 trials accounting for 633 women. The authors observed no difference in the early pregnancy loss rate (RR 0.83; 95 % CI 0.43–1.60) and a significant increase in clinical (RR 1.31, 95 % CI 1.10–1.56) and ongoing pregnancy rates (RR 1.32, 95 % CI 1.10–1.59) in favor of FET, which is consistent with our observations. Another randomized controlled trial performed by Zhu et al. [19] compared vitrified-warmed blastocyst versus fresh transfer, and also found increased clinical PR and IR in the thaw group (IR 37 vs. 25.2 %, clinical PR 55.1 vs. 36.4 %, frozen vs. fresh, respectively). It should be highlighted that most of the studies included in these meta-analyses were observational in nature, and that the FET cycles utilized morphologically inferior embryos owing to the preferential transfer of the more morphologically advanced embryos during the fresh cycle. Prior studies may have shown even greater differences between fresh COH and FET cycles if the morphologically “superior” or “first choice” siblings were all cryopreserved. Moreover, in our opinion, optimal outcomes are achieved by transfer of a euploid morphologically optimal embryo during a FET cycle.
ET under a synchronically prepared endometrium may convey an advantage over fresh IVF/ET cycles for a number of reasons. Firstly, a freeze-all strategy that plans to utilize subsequent FET cycle(s) offers the opportunity to control the window of implantation [20] and possibly improve embryo implantation. Generally, ovarian multi-follicular development with exogenous hormones for an IVF exposes the endometrium to supraphysiological concentrations of estrogen and progesterone [21], which can dramatically impact the timing of endometrial development and/or the achievement of receptivity. It has been clinically demonstrated that patients with high E2 concentrations produce significantly more oocytes and also have elevated progesterone concentrations [22]. Nevertheless, when abnormal steroid hormone concentrations appear, it can be detrimental to endometrial morphology and hence deter receptivity [23]. Alterations in the timing of endometrial development during each menstrual cycle or the quality of endometrial receptivity during the window of implantation are highly implicated in IVF failures [24]. These alterations can close the window of implantation too early or late with respect to the embryo developmental stage and present a barrier to blastocyst implantation.
Endometrial exposure to progesterone is critical in determining the window of implantation, but controlling progesterone exposure during COH is challenging. Premature elevations in progesterone concentration have been associated with adverse IVF outcomes [25–28]. Yet, recent studies stress the importance of the timing of the window of implantation as much as its duration. A 2013 abstract by Franasiak et al. presented at ASRM’s annual meeting showed a statistical reduction in outcomes from ET with delayed blastulation (morula or Gardner 1) compared with normal blastulation (Gardner 2–6). The authors further showed that the PRs of the same two blastulation groups, when handled during a FET cycle, were closely similar (patients ≥35 years old: 37 vs. 42 %, p = 0.3) [29]. Poor implantation may not be a result of embryo quality but rather a result of altered embryonic-endometrial synchrony.
Despite advances in understanding endometrial physiology, gynecologists still lack a definitive endometrial test to identify each patient’s window of implantation. It is presumed to occur between days 6 and 10 after ovulation across the mid-secretory phase of a natural menstrual cycle [30]. Moreover, endometrial dating for the past 60 years has relied primarily on histologic evaluation postulated by Noyes et al. who designed a series of morphological criteria to date the endometrium and distinguish the different stages throughout the menstrual cycle [31]. Although these criteria did not prove to be accurate or precise enough to diagnose luteal phase deficiency with validity, there is a continued search for new markers including ultrasonographic measurements, ultrastructural examination by electron microscopy, immunological markers, steroid hormones and receptors, growth factors, and other proteins and secreted factors [32]. Some of these biomarkers are integrated into specific tests, such as the E-tegrity [33] or the Endometrial Function Test [34]. More recently, with the development of the new “-omics” era, new methods continue to be developed. For instance, the Endometrial Receptivity Array (ERA), a diagnostic tool that consists of a customized array that analyses the expression levels of 238 genes that are up-regulated in more “receptive” cycles, identifies a transcriptomic signature for the endometrium [35] and identifies a receptive environment.
Another benefit of FET cycles is its ability to increase the number of biopsied embryos for patients utilizing PGS and streamlines the clinical processes by scheduling single embryo transfer in subsequent cycles until a patient achieves a pregnancy or exhaust available euploid embryos. A freeze-all strategy allows embryologists to biopsy and cryopreserve embryos the moment they expand and hatch, therefore transferring the best embryo from the entire cohort and not only the one that was ready. This avoids the cryopreservation and thaw of fully hatched blastocysts, which we have correlated to decreased clinical outcomes in our population (unpublished data). This approach also reduces uncertainty in scheduling procedures and prevents the unavoidable disappointment that patients undergo when informed that there are no suitable embryos for transfer. According to our data, roughly only 40 % of embryos are suitable for biopsy on day 5 post-insemination and, given the limits of the implantation window, cannot be candidates for transfer in fresh gonadotropin cycles. Furthermore, at least 50 % of these patients will have to transfer in a subsequent FET cycle.
A third important point is a decrease in multiple PRs. This is made possible by the higher single euploid ET rate in an FET cycles. Multiple gestation increases maternal morbidity and both fetal and neonatal morbidity and mortality. The most important maternal complications associated with multiple gestations are preeclampsia, gestational diabetes, and preterm labor and delivery [36]. In IVF, multiple embryos may be transferred, and regardless of which treatment is performed, the objective is the same: to maximize the probability of pregnancy while minimizing the risk of a multiple gestation. Hence, the most direct way to limit the risk of multiple pregnancies is to transfer as few embryos as possible per cycle. Therefore, an additional benefit of tools such as PGS and cryopreservation with active management of the window of implantation is that clinicians are able to suggest, with more confidence, the option of elective single ET. This study’s results show that the multiple PR is significantly lower in the “FET Only” group when compared with the “Fresh Only” group (6.7 vs. 17.2 %, p < 0.01), although a significantly lower average number of embryos were transferred in the former group (1.3 ± 0.4 (95 % CI 1.2–1.3) vs. 1.1 ± 0.4 (95 % CI 1.1–1.1), p < 0.01).
Fourth, interestingly, we found that IRs from patients in group “FET with a previous fresh ET” were the same to those in the “Fresh Only” group (50.9 vs. 50.9 %), but significantly lower than “FET Only” (50.9 vs. 59.5 %), highlighting the fact that outcomes are similar even when utilizing “second-best” embryos. Some patients’ best embryos develop first and are utilized during the fresh cycle. Waiting until an environmentally ideal FET cycle would benefit the decision process of embryo selection prior to ET. With a cryo-all approach and subsequent FET, we would be consistently transferring the best euploid embryo under the best uterine environment first rather than mishandling superior embryos at perceivably less advantageous opportunities of endometrial developmental development.
Lastly, elective embryo cryopreservation has been described as a potential prevention/risk-reducing approach for ovarian hyperstimulation syndrome (OHSS) [37, 38]. OHSS is the most serious, potentially lethal and a major complication during COH for ART. It occurs in approximately 1–14 % of cycles [39], and up to 33 % of IVF cycles have reported to be associated with mild forms [40]. While a GnRH agonist might be used instead of the gold standard hCG trigger to induce final oocyte maturation as a strategy to decrease early onset OHSS [41], it does not prevent the late-onset form. By freezing all embryos and postponing the ET, clinicians can evade the late-onset form that often presents in an ongoing pregnant woman due to a revival of the multiple corpora lutea by early embryonic endogenous hCG secretion.
FET may convey additional advantages. At some point, concern was raised toward the long-term consequences of ET undergoing a synthetic cycle, particularly obstetric and perinatal morbidity. Several investigators have addressed this issue and most have agreed that children born after FET demonstrated similar [42] or even improved outcomes compared with those born in fresh cycles [43, 44]. A systematic review and a meta-analysis performed in 2012 published by Maheshwari et al. included 11 observational studies. They concluded that singleton pregnancies following frozen versus fresh transfers were associated with better perinatal outcomes (antepartum hemorrhage RR 0.67, 95 % CI 0.55–0.81; preterm birth RR 0.84, 95 % CI 0.78–0.90; small for gestational age RR 0.45, 95 % CI 0.30–0.66; low-birth weight RR 0.69, 95 % CI 0.62–0.76; and perinatal mortality RR 0.68, 95 % CI 0.48–0.96) [45]. When comparing the risk of major congenital anomalies, no difference between the techniques was shown [46]. On the other hand, there is a report of an increased risk of macrosomia in singletons born after FET comparing with fresh embryo transfer [47]. Overall, these findings indicated that birth outcomes after FET are, at better, comparable with fresh embryo transfers. Nevertheless, such studies are observational and include only “second-best” embryos.
It is worth to mention that we observed a surprisingly high rate of early pregnancy loss across groups, considering that these were genetically screened embryos (“Only Fresh”: 19.7 % (68/345) vs. “Only FET”: 19.8 % (102/514); FET with a previous fresh ET: 25.2 % (35/139)). Although a significant number of women will suffer an early pregnancy loss because of chance alone and will not have any identifiable abnormality [48], there are other non-genetic settings responsible for a pregnancy loss [49]. Also, this finding could be explained by the presence of polyploidic abnormalities probably undetected by current PGS technology.
We acknowledge the potential weaknesses of this study. First, the retrospective nature of the study creates a selection bias and raises the possibility of unmeasured confounding differences between cohorts. Second, although most of the patients that underwent an IVF/PGS cycle were characteristically “normal” or “good” responders, this study is not limited to them. However, “low responders,” i.e., patients with abnormal ovarian reserve markers, were not likely to make it to transfer and be included in this study. Third, we recognize that not all patients, regardless of COH response, develop high-quality blastocysts. Therefore, this approach is not suitable to every patient. Fourth, an advantage of undergoing a cryo-all cycle is the embryologist’s ability to biopsy the entire embryo cohort, whether embryos expanded on day 5 or day 6. In Fresh ET cycles, only day 5 expanded embryos are eligible for biopsy. Therefore, a potential limitation is presented when comparing “Fresh Only” versus “FET Only.” Fifth, the reason for undergoing PGS cycle has evolved in “Fresh Only” and “FET Only” groups. The initial impetus for PGS in patients undergoing a fresh transfer was diminished fertility with most utilizing aneuploidy screening to improve PRs, while for cryo-all cycles PGS was focused on a genetic defect. With more recent knowledge of the advantages of FET cycles, patients are now counseled to undergo cryo-all cycles without compromising pregnancy outcomes. Moreover, such differences in PGS indication could impact aneuploidy rates and/or cancelation rates. Pregnancy outcomes would not be expected to waver as all patients included had at least one euploid embryo available for ET. Lastly, a selection of patients who were initially included in group 1 (Fresh Only) pursued a subsequent FET, making them eligible for group 3. Therefore, this subset of patients was included in two analyses, creating another potential limitation.
In summary, great efforts have been taken to achieve optimal embryonic and endometrial synchronization. This study’s intention is to discern how endometrial preparation may affect receptivity and, in turn, implantation rates. To our knowledge, this is the largest study of its kind to compare optimal ET of fresh and frozen euploid embryos in COH and FET cycles. We suggest a strategy of close monitoring and synchronization of the window of implantation that is based on FET and strengthened with PGS-based embryo selection, as we seek to optimize reproductive potential.
References
Fatemi HM, Popovic-Todorovic B. Implantation in assisted reproduction: a look at endometrial receptivity. Reprod Biomed Online. 2013;27:530–8.
Koot Y, Maklon N. Embryo implantation: biology, evaluation and enhancement. Curr Opin Obstet Gynecol. 2013;25(4):274–9.
Queenan Jr JT, Veeck LL, Seltman HJ, Muasher SJ. Transfer of cryopreserved-thawed pre-embryos in a natural cycle or a programmed cycle with exogenous hormonal replacement yields similar pregnancy results. Fertil Steril. 1994;62:545–50.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–8.
Levi AJ, Drews MR, Bergh PA, Miller BT, Scott Jr RT. Controlled ovarian hyperstimulation does not adversely affect endometrial receptivity in in vitro fertilization cycles. Fertil Steril. 2001;76:670–4.
von Grothusen C, Lalitkumar S, Rao Boggavarapu N, Gemzell-Danielsson K, Lalitkumar PG. Recent advances in understanding endometrial receptivity: molecular basis and clinical applications. Am J Reprod Immunol. 2014;72(2):148–57.
Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99(1):156–62.
Cobo A, Santos MJ d l, Castello D, Gamiz P, Campos P, Remohi J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150warming cycles. Fertil Steril. 2012;98:1138–46. e1131.
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–85.
Wilton L. Preimplantation genetic diagnosis for aneuploidy screening in early human embryos: a review. Prenat Diagn. 2002;22:512–8.
Hardy K, Martin K, Leese H, Winston R, Handyside A. Human preimplantation development in vitro is not adversely affected by biopsy at the 8-cell stage. Hum Reprod. 1990;5:708–14.
Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015;30(2):473–83.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80.
Fatemi HM, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. 2010;94(6):2054–8.
Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet. 2010;27(7):357–63.
Chang EM, Han JE, Won HJ, Kim YS, Yoon TK, Lee WS. Effect of estrogen priming through luteal phase and stimulation phase in poor responders in in-vitro fertilization. J Assist Reprod Genet. 2011;29:225–30.
El Bahja D, Hertz P, Schweitzer T, Lestrade F, Ragage JP. Frozen embryo transfer protocol: does spontaneous cycle give good results? Gynecol Obstet Fertil. 2013;41:648–52.
Xiao Z, Zhou X, Xu W, Yang J, Xie Q. Natural cycle is superior to hormone replacement therapy cycle for vitrificated-preserved frozen-thawed embryo transfer. Syst Biol Reprod Med. 2012;58:107–12.
Zhu D, Zhang J, Cao S, Zhang J, Chin Heng B, Huang M, et al. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles –time for a new embryo transfer strategy? Fertil Steril. 2011;95(5):1691–5.
Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796–9.
Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14:236–42.
Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril. 2009;91:749–66.
Thomas K, Thomson AJ, Sephton V, Cowan C, Wood S, Vince G, et al. The effect of gonadotrophic stimulation on integrin expression in the endometrium. Hum Reprod. 2002;17:63–8.
Garrido-Gómez T1, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simón C. Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril. 2013;99(4):1078–85.
Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Embryo cryopreservation rescues cycles with premature luteinization. Fertil Steril. 2010;93:636–41.
Al-Azemi M, Kyrou D, Kolibianakis EM, Humaidan P, van Vaerenbergh I, Devroey P, et al. Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online. 2012;24:381–8.
Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update. 2007;13:343–55.
Franasiak J, Forman EJ, Hong KH, Werner MD, Upham KM, Scott RT. Investigating the impact of the timing of blastulation on implantation: active management of embryo-endometrial synchrony increases implantation rates. Fertil Steril. 2013;100(3):S97.
Navot D, Scott RT, Droesch K, Veeck LL, Liu HC, Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril. 1991;55:114–8.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25.
Blesa D, Ruiz-Alonso M, Simón C. Clinical management of endometrial receptivity. Semin Reprod Med. 2014;32(05):410–4.
Lessey BA, Castelbaum AJ, Sawin SW, SUN J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63(3):535–42.
Dubowy RL, Feinberg RF, Keefe DL, et al. Improved endometrial assessment using cyclin E and p27. Fertil Steril. 2003;80(1):146–56.
Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95(1):50–60. e1–e15.
Practice Committee of American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion. Fertil Steril. 2012;97(4):825–34.
Griesinger G, Berndt H, Schultz L, Depenbusch M, Schultze-Mosgau A. Cumulative live birth rates after GnRH-agonist triggering of final oocyte maturation in patients at risk of OHSS: a prospective, clinical cohort study. Eur J Obstet Gynecol Reprod Biol. 2010;149(2):190–4.
Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2003;80(5):1309–14.
Roque. Freeze-all policy: is it time for what? J Assist Reprod Genet. 2014 Nov 27.
Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in IVF cycles. Fertil Steril. 2006;85:112–20.
Lewitt N, Kol S, Manor D, Itskovitz-Eldor J. Comparison of gonadotrophin-releasing hormone analogues and human chorionic gonadotrophin for the induction of ovulation and prevention of ovarian hyperstimulation syndrome: a case–control study. Hum Reprod. 1996;11:1399–402.
Aytoz et al. Obstetric outcome of pregnancies after the transfer of cryopreserved and fresh embryos obtained by conventional in-vitro fertilization and intracytoplasmatic sperm injection. Human Reprod. 1999;14(10):2619–24.
Kansal Kalra S et al. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril. 2011;95(2):548–53.
Wennerholm UB et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Human Reprod. 2013;28(9):2545–53.
Maheshwari A et al. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98(2):368–377.e9.
Pelkonen S, Hartikainen AL, Ritvanen A, Koivunen R, Martikainen H, Gissler M, et al. Major congenital anomalies in children born after frozen embryo transfer: a cohort study 1995–2006. Hum Reprod. 2014;29(7):1552–7.
Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29(3):618–27.
Saravelos SH, Regan L. Unexplained recurrent pregnancy loss. Obstet Gynecol Clin North Am. 2014;41(1):157–66.
Christiansen OB, Nybo Andersen AM, Bosch E, Daya S, Delves PJ, Hviid TV, et al. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril. 2005;83(4):821–39. Review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Capsule This analysis suggests euploid embryos to be more likely to implant and achieve a LBR in a synthetic FET cycle than in a fresh cycle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Demographic characteristics of Only IVF vs. Only FET. Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Only IVF set against the quantiles of the Only FET data set testing the normality of each group (DOCX 1029 kb)
ESM 2
Laboratory outcomes of Only IVF vs. Only FET. Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Only IVF set against the quantiles of the Only FET data set testing the normality of each group (DOCX 780 kb)
ESM 3
Demographic characteristics of Only FET vs. Fresh then FET. Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Only FET set against the quantiles of the Fresh then FET data set testing the normality of each group (DOCX 855 kb)
ESM 4
Laboratory outcomes of Only FET vs. Fresh then FET. Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Only FET set against the quantiles of the Fresh then FET data set testing the normality of each group (DOCX 756 kb)
ESM 5
Demographic characteristics of patient s in the Fresh then FET group segregated according if they fresh cycle was successful (pregnant vs. non-pregnant). Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Pregnant set against the quantiles of the Non-Pregnant data set testing the normality of each group (DOCX 412 kb)
ESM 6
Laboratory outcomes of patient s in the Fresh then FET group segregated according if they fresh cycle was successful (pregnant vs. non-pregnant). Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Pregnant set against the quantiles of the Non-Pregnant data set testing the normality of each group (DOCX 401 kb)
ESM 7
Comparison of patients in the ‘Fresh ET then FET’ segregated according if they fresh cycle was successful (pregnant vs. non-pregnant). Results are expressed as mean ± standard deviation with 95 % confidence intervals (CI). Significance established at p < 0.05. For each group, pregnancy rates, clinical pregnancy rates, early pregnancy loss rates, multiple pregnancy rates, and live birth rates were calculated. Adjusted odds ratios (OR) and their 95 % CI by Clopper-Pearson method for implantation rate, PR, clinical PR, and early pregnancy loss rate were calculated to evaluate the relative odds of each event compared with the reference group (Fresh Only) (DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Rodriguez-Purata, J., Lee, J., Whitehouse, M. et al. Reproductive outcome is optimized by genomic embryo screening, vitrification, and subsequent transfer into a prepared synchronous endometrium. J Assist Reprod Genet 33, 401–412 (2016). https://doi.org/10.1007/s10815-016-0647-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0647-y