Abstract
Objective
To compare early vs. mid-follicular exposure to LH in patients with poor ovarian responsiveness undergoing in vitro fertilization (IVF).
Design
Prospective, randomized, controlled trial.
Setting
University Hospital, University-affiliated private Clinic.
Patients
Five hundred-thirty women with poor ovarian responsiveness during the first IVF cycle, undergoing their second IVF attempt.
Interventions
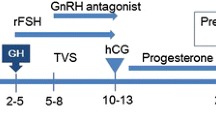
In a GnRH-analogue long protocol, ovarian stimulation with recombinant FSH (300 IU/day) plus randomly assigned addition of recombinant LH (150 IU/day) from day 1 (early LH exposure; n = 264) or from day 7 (late LH exposure; n = 266).
Main outcome measure(s)
Primary outcome was the number of oocytes retrieved. Secondary outcomes were: cancellation rate, total gonadotropin dose, duration of ovarian stimulation, number of embryos available for transfer, pregnancy rate per started cycle, per OPU and per embryo transfer, implantation rate, delivered/ongoing pregnancy rate.
Results
Apart from the totally administered LH dose, that was significantly higher in the group receiving it from day 1, all parameters related to IVF outcome were non significantly different in the two groups.
Conclusions
Adding LH to FSH from day 1 or from day 7 of ovarian stimulation in a GnRH-agonist long protocol exerts comparable effects on IVF outcome in poor responders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Luteinizing hormone (LH) displays several physiological effects on the developing follicles, including the stimulation of theca cells to enhance the production of androgens, subsequently aromatized to E, and the stimulation of the growth of large antral follicles [1, 2], paralleled by the inhibition of the growth of small antral follicles [3, 4].
Some medications with LH activity have been made available for ovulation induction and in vitro fertilization (IVF): (a) recombinant LH (rLH); (b) the 2:1 combination of recombinant FSH (rFSH) and rLH; (c) human chorionic gonadotropin (hCG), either urinary or recombinant; (d) hMG (human menopausal gonadotropin), a combination or urinary FSH and urinary hCG.
Some evidence suggests that administering medications with LH activity to patients undergoing IVF could improve IVF outcome with respect to using FSH alone. In some studies, ovarian stimulation performed with hMG after pituitary suppression with a GnRH-agonist resulted in a slightly increased (3–4 %) clinical pregnancy rate [5, 6] and in a higher embryo ploidy rate [7, 8] compared to FSH alone. In a recent multicenter, prospective, randomized trial, hMG and rLH were shown to have similar effects on IVF outcome [9].
On the other side, two extensive metanalysis showed that the administration of LH to unselected IVF patients was not able to improve IVF results [10, 11]. One of them, however, reported a better pregnancy rate when LH was given in addition to FSH to the so-called “poor responders”, women having a scarce ovarian reserve and a poor responsiveness to stimulation [10]. The usefulness of LH addition to poor responders, however, is still controversial, as other prospective [12–14] or retrospective [15, 16] studies failed to demonstrate any advantage derived from LH administration even in such patients, whereas Bosch [17] prospectively observed increased pregnancy and implantation rates in patients of advanced reproductive age receiving rLH in addition to rFSH.
The optimal timing for LH administration during ovarian stimulation is another not yet clarified point. It is better to give LH from the beginning of ovarian stimulation or to add it from the mid-follicular phase?
The satisfactory effectiveness of hMG in IVF suggests to give LH- or hCG-containing drugs since the beginning of the follicular phase. Some studies, however, reported a reduced IVF success rate when endogenous serum LH levels were high in the early follicular phase [18] or low in the mid-follicular phase [19], and high LH levels on days 7/8 of stimulation were associated with higher oocyte yield [19].
A rather small, retrospective study comparing patients receiving rFSH + hMG from day 1 or rFSH alone in the early follicular phase plus hMG from day 5–6 found no significant differences in IVF outcome [20].
Aim of the present study was to compare early vs. mid-follicular exposure to LH in a prospective, randomized trial on a high number of IVF patients with proven poor ovarian responsiveness.
Materials and methods
Patients
Five hundred thirty patients who underwent a second IVF attempt in our IVF Unit in the years 2003–2010 were included in the study. The study was authorized by the local ethical committee and all patients gave their written informed consent.
Patients were recruited because they matched the definition of “proven poor responders” (PPR) that was given on the basis of the ovarian sensitivity index (OSI) calculated during the first IVF attempt.
OSI is obtained dividing the totally administered gonadotropin dose by the number of retrieved oocytes, and represents the gonadotropin amount needed to obtain each retrieved oocyte [21]. OSI has been shown to be highly correlated to AMH, and to be more accurate than the total number of oocytes or the total gonadotropin dose to express ovarian responsiveness to stimulation [21]. During a preliminary evaluation of OSI on more than 4000 IVF cycles, it sorted out that the OSI threshold of 900 IU represents the 10th centile of OSI distribution in our IVF population: in other words, patients requiring more than 900 IU gonadotropins/retrieved oocyte are the 10 % less responsive patients in our IVF population, and thus were defined as PPR.
Ovarian stimulation
Pituitary suppression was achieved by administering i.n. Buserelin (Suprefact, Hoechst, Germany; 1000 mcg/d) from the 21st day of the previous menstrual cycle. Superovulation was then induced using either rFSH (Gonal F, Merck-Serono, Switzerland; 300 IU/d) plus rLH (Luveris, Merck-Serono, Switwerland; 150 IU/d) from day 1 (early LH group) or rFSH (300 IU/d) alone from day 1 plus rLH (150 IU/d) from day 7 (late LH group).
In order to monitor ovarian response to stimulation and eventually adjust rFSH dose, serial serum estradiol measurements and transvaginal US assessments were performed every second day from day 7 of stimulation. When appropriate, rFSH dose was individually adjusted from day 7, never exceeding 450 IU/day.
The cycle was cancelled when no response to stimulation was recorded (no follicles above 6 mm diameter and serum estradiol <80 pg/ml on day 7 of gonadotropin administration).
Urinary hCG (Gonasi HP, Ibsa, Switzerland; 10,000 IU) was administered subcutaneously when the leading follicle reached 18 mm diameter, with appropriate serum estradiol levels. Oocyte pick-up (OPU) was scheduled approximately 36 h after hCG administration.
Even if our study was performed in a time period (2003–2010) during which the strict Italian rules on IVF forbidding to produce more that three embryos per IVF cycle were applied (it was from 2004 to 2009), Italian regulation did not affect results because PPR patients produced a few oocytes, often with a suboptimal quality. Consequently, we always inseminated all retrieved oocytes without obtaining more than 3 embryos and transferring in utero all obtained embryos, as prescribed by the Italian law on IVF. Since IVF and ICSI had comparable results in our program throughout the study time period, patients undergoing IVF and ICSI were considered together.
Embryo transfer was scheduled after 48–72 h of in vitro embryo culture and performed using a soft catheter (Sydney, Cook, Australia). Vaginal progesterone (Crinone 8, Merck-Serono, Switzerland; 400 mg/d) was given for 15 days after embryo transfer to sustain the luteal phase.
Blood hCG measurement was performed 15 days after embryo transfer and in case of a positive hCG test, transvaginal ultrasound examination was scheduled after further 15 days to confirm pregnancy and assess the number of implanted embryos. Only the US evidence of a gestational sac was defined as pregnancy and counted when calculating the pregnancy rates.
Pregnancy outcome was registered even if the medical pregnancy care program was performed elsewhere.
Statistical analysis
The power of the study was calculated according to the primary outcome, that was the number of oocytes retrieved at OPU. The power calculation analysis showed that at least 200 cases per study arm were needed to detect a difference of 15 % in the primary outcome with 85 % statistical power (beta error 15 %) and a significance level at p < 0.05 . Since a total number of at least 400 OPUs with oocyte retrieval were needed, we included in the study 530 patients because a maximal theorical cancellation rate of 20 % was a priori estimated in this kind of patients.
Secondary outcomes were the following: cancellation rate, total gonadotropin dose, duration of ovarian stimulation, OSI, number of embryos available for transfer, pregnancy rate per started cycle, per OPU and per embryo transfer, implantation rate, delivered/ongoing pregnancy rate (at least 14 weeks of gestational age).
As the variables in the two study groups were not normally distributed, they were compared using the Wilcoxon rank test for unpaired data.
Randomization
All patients meeting the inclusion criteria (n = 587) were offered to participate in the study (Fig. 1). Among them, n = 53 refused randomization and were excluded from the study, whereas other four were excluded because in the weeks they asked for to decide if accepting to join the study, a spontaneous pregnancy occurred.
Randomization was performed using a computerized algorhythm without any restriction. No blocks were used since the size of the study groups was estimated to be big enough to ensure a balanced distribution of patients between groups. Allocation concealment was obtained using sequentially-numbered dark envelopes: until they were opened at the time of allocation, both physicians and patients were blinded to the study.
Patients whose ovarian stimulation cycle was cancelled and did not undergo OPU were considered as lost to follow-up and not included in the analysis of the primary outcome (number of retrieved oocytes).
Results
Patients receiving early LH exposure (early LH group, n = 264) were compared to patients to whom LH was given from the mid-follicular phase (day 7) of the stimulation cycle (late LH group, n = 266). Patients in either groups did not significantly differ for age, BMI and variables related to ovarian reserve (antral follicle count and day 3 FSH level). They were selected and included in the study because they had a proven poor ovarian responsiveness to gonadotropins, thus it is not surprising that their mean age was rather high (around 39 years) and the ovarian reserve predictors were suggestive of a poor chance to produce oocytes (mean basal FSH above 10 IU/L, mean antral follicle count around 5) (Table 1).
The specific type of studied population can explain why the overall results (pregnancy rate/ET 18.7 %, implantation rate 12.9 %) are rather poor in the present study, and in the meanwhile the overall miscarriage rate (26.7 %) VV.
The cancellation rate (11 % in the early LH group, 10.1 % in the late LH group) was overall lower than a priori expected, and the number of OPUs with oocyte retrieval was consequently higher than expected. This allowed to increase the statistical power of the study with respect to the primary ourcome, that was oocyte yield at OPU: the number of retrieved oocytes was comparable in the early LH group (3.7 ± 2.1) and in the late LH group (3.5 ± 2.4), the difference being very subtle, much lower than the one that was considered relevant in the power calculation analysis (15 %).
Apart from the totally administered rLH dose, that was obviously significantly higher in the group receiving rLH from day 1 (2163 ± 285 vs.1042 ± 348 IU, p < 0.01), all other variables, including all secondary outcomes were very similar in the two groups (Table 2).
Due to a slightly higher pregnancy rate and to a slightly lower miscarriage rate, the delivered/ongoing pregnancy rate was a little higher in patients receiving rLH from the mid-follicular phase (14.9 % in late LH exposure group vs. 12.6 % in the early LH exposure group), but this difference was not significant both statistically and clinically (Table 2).
Adverse effects were overall both infrequent and not severe, as no patient needed hospitalization (Table 3). They included nausea and weight gain (exceeding 4 kg) during ovarian stimulation, discomfort and bleeding at OPU, and were equally distributed in the two study groups. All twin pregnancies were twins, no triplet pregnancy occurred.
Overall, our results suggest that the administration of 150 IU/day of rLH from day 1 or from day 7 of ovarian stimulation to poor responders has the same effect on IVF outcome.
Discussion
Several medications containing LH activity are available for inducing superovulation in IVF, but the optimal way to use them is still matter of debate. In particular, it is not yet clear if LH activity could be more useful if given from the early follicular phase or it would be better to start it during the mid-follicular phase.
The usefulness of LH since the early follicular phase is suggested by some studies reporting a higher embryo ploidy rate [7, 8] and a slightly higher pregnancy rate [5, 6] when hMG was used for ovarian stimulation instead of rFSH.
On the other side, early studies showed that LH receptors are expressed on theca cells since the beginning of follicular development, but appear on granulosa cells only in the midfollicular phase [22], an observation suggesting that LH could be active (and useful) only from day 5–6 of the cycle onward.
A randomized, controlled study performed on GnRH-antagonist (flexible protocol) IVF cycles reported a significantly lower pregnancy rate for patients having high endogenous serum LH levels in the early follicular phase, from day 1 of the cycle to the day of GnRH-antagonist start [18]. Moreover, in long GnRH-agonist protocol, early rLH exposure was found unable to increase the number of oocytes and embryos when compared to rFSH alone [23]. The lack of LH effect observed by Kovacs [23] was not dependent on the use of rLH instead of hMG, since a multicenter, prospective, randomized trial demonstrated hMG and rLH to have similar effects on IVF outcome [9].
Interestingly, on the other side the addition of rLH during the late follicular phase was reported to enhance follicular insulin sensitivity and to decrease intrafollicular androgen levels, possibly improving the late stages of oocyte maturation [24]. Moreover, a high endogenous LH concentration on days 7/8 of a long protocol stimulation was found to lead to significantly higher oocyte yield and number of available embryos, whereas a sharp drop of serum LH level during the mid-follicular phase was associated with a significantly lower live birth rate in IVF [19]. Similarly, a study on IVF patients undergoing mild ovarian stimulation protocol with GnRH-antagonist reported that the pregnancy and implantation rates were significantly lower if the serum LH levels were low in the late follicular phase [25].
We designed the present study to prospectively analyse IVF outcome in a large series of patients randomized to receive exogenous LH from day 1 or from day 7 of an ovarian stimulation cycle for IVF. We aimed at selecting a group of patients who (a) could be as homogenous as possible, and (b) could theorically benefit from LH activity.
A comprehensive metanalisys (three studies, 310 patients) showed that the only subset of patients receiving some benefit from LH exposure were the so-called “poor responders”, in which a significantly higher ongoing pregnancy rate was observed when rFSH + rLH were administered vs. rFSH alone (odd ratio 1.85; CI 1,10–3,11) [10]. The usefulness of LH addition to poor responders was questioned by some other prospective [12–14] or retrospective [15, 16] studies not included in the metanalysis, but was recently confirmed by a large randomized controlled study by Bosch [17], that reported a significantly higher implantation rate and a trendly higher pregnancy rate in patients of advanced reproductive age receiving rFSH plus rLH vs. patients receiving rFSH alone.
We chose to select a study population of “poor responders” according to the ovarian sensitivity index (OSI, [21]), that was calculated using data registered during the first IVF cycle. OSI (the total gonadotropin dose/retrieved oocyte ratio) accurately reflects ovarian responsiveness to gonadotropins, is highly related to AMH circulating levels [21], and represents a reliable way to identify “poor responders”. To this purpose it is preferable to prognostic predictors (age, basal FSH levels, AMH, antral follicle count) because it is actually based on what already happened with a previous, similar stimulation protocol. The use of OSI allowed us to identify a quite homogeneous population of “proven poor responders” (PPRs) that underwent the second IVF attempt at our IVF Unit. The specific type of population enrolled in the study can explain why the overall results (pregnancy and implantation rates) are rather poor, and in the meanwhile the miscarriage rate is quite high.
The primary end point was chosen to be the number of oocytes retrieved at OPU since this variable was likely to be affected by LH administration. In fact, De Placido [26] showed that rLH administration was useful to increase oocyte yield in patients responding poorly to rFSH.
In our study, patients included in either groups (early or late LH exposure) were very homogeneous for age, BMI and predictors of ovarian reserve. Overall, we observed that the administration of rLH from day 1 or from day 7 of a GnRH-agonist long protocol of ovarian stimulation did not change IVF outcome, and that all the considered variables (with the obvious exception of the amount of LH given), were quite similar in both groups.
Our observations, prospectively obtained on a large number of PPRs, substantially confirm those previously reported by a small retrospective study, that compared patients receiving rFSH plus hMG (75 IU7day) from day 1 vs. patients treated with rFSH alone from day 1 and rFSH plus hMG from day 5–6, founding no significant differences as for fertilization, implantation or pregnancy rates [20].
In conclusion, our prospective randomized study demonstrates that adding rLH to rFSH in a 1:2 proportion either from day 1 or from day 7 of ovarian stimulation has a comparable effect on ovarian response and on IVF outcome in poor responders. Based on our findings, it seems that giving LH from day 7 would be advantageous at least because of the reduced cost and the fewer injections for the patient.
References
Filicori M, Cognigni GE, Gamberini E, Parmegiani L, Troilo E, Roset B. Efficacy of low-dose human chorionic gonadotropin alone to complete controlled ovarian stimulation. Fertil Steril. 2005;84:394–401.
Durnerin CI, Erb K, Fleming R, Hillier H, Hillier SG, Howles CM, Hugues JN, Lass A, Lyall H, Rasmussen P, Thong J, Traynor I, Westergaard L, Yates R, Luveris Pretreatment Group. Effects of recombinant LH treatment on folliculogenesis and responsiveness to FSH stimulation. Hum Reprod. 2008;23:421–6.
Filicori M, Cognigni GE, Samara A, Melappioni S, Perri T, Cantelli B, Parmegiani L, Pelusi G, DeAloysio D. The use of LH activity to drive folliculogenesis: exploring uncharted territories in ovulation induction. Hum Reprod Update. 2002;8:543–57.
Hugues JN, Soussis J, Calderon I, Balasch J, Anderson RA, Romeu A, Recombinant LH Study Group. Does the addition of recombinant LH in WHO group II anovulatory women over-responding to FSH treatment reduce the number of developing follicles? A dose-finding study. Hum Reprod. 2005;20:629–35.
Platteau P, Nyboe Andersen A, Loft A, Smitz J, Danglas P, Devroey P. Highly purified HMG versus recombinant FSH for ovarian stimulation in IVF cycles. Reprod Biomed Online. 2008;17:190–8.
Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI. Efficacy and safety of human menopausal gonadotrophins versus recombinant FSH: a meta-analysis. Reprod Biomed Online. 2008;16:81–8.
Weghofer A, Munné S, Brannath W, Chen S, Tomkin G, Cekleniak N, Garrisi M, Barad D, Cohen J, Gleicher N. The impact of LH-containing gonadotropins on diploidy rates in preimplantation embryos: long protocol stimulation. Hum Reprod. 2008;23:499–503.
Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, Kim JG, Moon SY. Tri-pronucleated zygotes may occur less frequently in luteinizing hormone activity-added cycles. Gynecol Endocrinol. 2011;27:458–63.
Pacchiarotti A, Sbracia M, Frega A, Selman H, Rinaldi L, Pacchiarotti A. Urinary hMG (Meropur) versus recombinant FSH plus recombinant LH (Pergoveris) in IVF: a multicenter, prospective, randomized controlled trial. Fertil Steril. 2010;94:2467–9.
Mochtar MH, Van der Veen, Ziech M, van Wely M. Recombinant Luteinizing Hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database Syst Rev. 2007;(2):CD005070.
Kolibianakis EM, Kalogeropoulou L, Griesinger G, Papanikolaou EG, Papadimas J, Bontis J, Tarlatzis BC. Among patients treated with FSH and GnRH analogues for in vitro fertilization, is the addition of recombinant LH associated with the probability of live birth? A systematic review and meta-analysis. Hum Reprod Update. 2007;13:445–52.
Fábregues F, Creus M, Peñarrubia J, Manau D, Vanrell JA, Balasch J. Effects of recombinant human luteinizing hormone supplementation on ovarian stimulation and the implantation rate in down-regulated women of advanced reproductive age. Fertil Steril. 2006;85:925–31.
Berkkanoglu M, Isikoglu M, Aydin D, Ozgur K. Clinical effects of ovulation induction with recombinant follicle-stimulating hormone supplemented with recombinant luteinizing hormone or low-dose recombinant human chorionic gonadotropin in the midfollicular phase in microdose cycles in poor responders. Fertil Steril. 2007;88:665–9.
Barrenetxea G, Agirregoikoa JA, Jiménez MR, de Larruzea AL, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor-responder women: a randomized controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cycles. Fertil Steril. 2008;89:546–53.
Franco Jr JG, Baruffi RL, Oliveira JB, Mauri AL, Petersen CG, Contart P, Felipe V. Effects of recombinant LH supplementation to recombinant FSH during induced ovarian stimulation in the GnRH-agonist protocol: a matched case-control study. Reprod Biol Endocrinol. 2009;7:58–63.
Maguire M, Csokmay J, Segars J, Payson M, Armstrong A. Enough is enough! Patients who do not conceive on 600 IU/d of gonadotropins show no improvement from an additional 150 IU of LH activity. Fertil Steril. 2011;95:372–3.
Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: an age-adjusted analysis. Fertil Steril. 2011;95:1031–6.
Kolibianakis EM, Albano C, Kahn J, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is associated with a reduced chance of pregnancy. Fertil Steril. 2003;79:873–80.
Lahoud R, Al-Jefout M, Tyler J, Ryan J, Driscoll G. A relative reduction in mid-follicular LH concentrations during GnRH agonist IVF/ICSI cycles leads to lower live birth rates. Hum Reprod. 2006;21:2645–9.
Sönmezer M, Iltemir Duvan C, Ozmen B, Taşçi T, Ozkavukçu S, Atabekoğlu CS. Outcomes after early or midfollicular phase LH supplementation in previous inadequate responders. Reprod Biomed Online. 2010;20:350–7.
Biasoni V, Patriarca A, Dalmasso P, Bertagna A, Manieri C, Benedetto C, Revelli A. Ovarian sensitivity index is strongly related to circulating AMH and may be used to predict ovarian response to exogenous gonadotropins in IVF. Reprod Biol Endocrinol. 2011;9:112–8.
Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46.
Kovacs P, Kovats T, Kaali SG. Results with early follicular phase recombinant luteinizing hormone supplementation during stimulation for in vitro fertilization. Fertil Steril. 2010;93:475–9.
Gutman G, Barak V, Maslovitz S, Amit A, Lessing JB, Geva E. Recombinant luteinizing hormone induces increased production of ovarian follicular adiponectin in vivo: implications for enhanced insulin sensitivity. Fertil Steril. 2009;91:1837–41.
Yanaihara A, Yorimitsu T, Motoyama H, Ohara M, Kawamura T. The decrease of serum luteinizing hormone level by a gonadotropin-releasing hormone antagonist following the mild IVF stimulation protocol for IVF and its clinical outcome. J Assist Reprod Genet. 2008;25:115–8.
De Placido G, Alviggi C, Perino A, Strina I, Lisi F, Fasolino A, De Palo R, Ranieri A, Colacurci N, Mollo A, Italian Collaborative Group on Recombinant Human Luteinizing Hormone. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum Reprod. 2005;20:390–6.
Conflict of interest disclosure
Any of the authors had commercial interests that could represent a conflict of interest with what reported in the present manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Adding LH to FSH from day 1 or day 7 of ovarian stimulation in a GnRH-agonist long protocol exerts comparable effects on IVF outcome in patients with poor ovarian responsiveness.
Rights and permissions
About this article
Cite this article
Revelli, A., Chiado’, A., Guidetti, D. et al. Outcome of in vitro fertilization in patients with proven poor ovarian responsiveness after early vs. mid-follicular LH exposure: a prospective, randomized, controlled study. J Assist Reprod Genet 29, 869–875 (2012). https://doi.org/10.1007/s10815-012-9804-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9804-0