Abstract
Background
Human leukocyte antigen G (HLA-G) is thought to play a key role in implantation by modulating cytokine secretion to control trophopblastic cell invasion and to maintain a local immunotolerance.
Method of study
The literature is reviewed to provide a description of the genetic background, properties of the protein, and the function of HLA-G. Data are presented on potential clinical applications of HLA-G including the use of evaluation of HLA-G gene polymorphisms in the diagnosis of patients experiencing recurrent pregnancy loss and evaluation and testing of soluble HLA-G (sHLA-G) in embryo culture media for the selection of embryos for transfer after in vitro fertilization (IVF).
Results
The literature supports a central role of HLA-G for successful implantation. Of couples experiencing recurrent pregnancy loss, 32% demonstrated the -1725G HLA-G polymorphism. Our data showed that when embryos were selected for transfer after IVF based on culture media concentrations of sHLA-G ≥ 2 U/ml and good morphologic grade, a 65% pregnancy rate compared with a 0% pregnancy rate in those with <2 U/ml sHLA-G.
Conclusions
HLA-G is important for successful implantation in human beings. The HLA-G -725 promoter polymorphism is a risk factor for recurrent miscarriage. Measurement of sHLA-G in embryo culture media can help select embryos for transfer after IVF allowing fewer embryos to be transferred in an attempt to lower multiple gestation rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A central question in pregnancy is how the fetal–placental unit avoids maternal immune rejection. Although fetal and maternal cells interact throughout pregnancy, the fetus typically remains a privileged site, not subject to rejection. It is likely that the particular nature of the cells at the fetal–maternal interface and their products help prevent rejection of the fetus by the maternal immune system. Attention has been focused on human leukocyte antigen G (HLA-G) as is thought to play a key role in implantation by modulating cytokine secretion to control trophoblastic cell invasion and to maintain a local immunosuppressive state [1, 2]. Trophoblastic cells are the prime source of HLA-G [3]. Interaction between soluble forms of HLA-G (sHLA-G) secreted by the trophoblasts and uterine lymphocytes in the decidual tissues is a major player in the induction of immunotolerance for the invading blastocyst. This review will discuss the genetic background of HLA-G, properties of HLA-G protein including sHLA-G, the function of HLA-G, and potential clinical applications of HLA-G.
HLA-G: genetic background
A novel gene, HLA-G, was cloned in 1987 and was first described as a major histocompatability complex (MHC) class Ib gene whose structure is homologous to the other HLA class I genes [4]. The gene has an intron/exon organization identical to that in the class Ia genes (HLA-A, HLA-B, and HLA-C). Within the 5′ flanking region of the gene, the HLA-G promoter has elements (e.g., AP-1, NFkB) similar to sequences found in class 1a genes, but lacks an interferon response element, suggesting novel transcriptional regulatory mechanisms. The primary HLA-G RNA transcript is also differentially spliced; in addition to the full length mRNA, transcripts are produced that lack either exon two, both exons two and three [5], or exon four [6]. A soluble form of HLA-G encoded by an mRNA containing intron 4 was described by Fujii et al. [7].
Analysis of its transcription of the HLA-G gene has led to the identification of specific alternative mRNA splicing products. The HLA-G primary transcript has been shown to generate seven alternative mRNAs able to encode four membrane-bound (HLA-G1, G2, G3, and G4) and three secreted (HLA-G5, G6, and G7) protein isoforms (Fig. 1a,b) [5–8].
a HLA-G isoforms [Three messages encode membrane isoforms (HLA-G1, -G2, -G3) and two encode soluble isoforms (HLA-G5 and -G6, also known as sG1 and sG2, respectively). HLA-G1 and -G5 associate with light chain. Isoforms HLA-G4 and -G7 remain poorly defined and are not included]. b The HLA gene is composed of 8 exons arranged in the same sequence as other HLA class I genes. The gene is alternatively spliced to yield 7 transcripts. In two of these, a stop sequence in intron 4 results in soluble isoforms. A 14 bp insertion/deletion is present in exon 8 in the 3′ UTR. al, a2, a3 extra-cellular domains
HLA-G: protein expression
HLA-G gene encodes proteins which are quite different from classical HLA class I antigens (A, B, and C) in that it is paucipolymorphic and the site of expression is extremely limited. Although it is expressed as a membrane-bound exhibiting a very restricted tissue distribution limited to extravillous cytotrophoblast cells in the placenta, as well as in maternal spiral arteries, endothelial cells of fetal vessels in the chorionic villi, amnion cells, thymus, and on interferon-γ-stimulated blood monocytes [9–14]. The HLA-G protein product has 86% sequence identity to the class I consensus sequence [15]. HLA-G has a lower molecular mass (37–39 kDa) than class 1a molecules due to a stop codon in exon 6 that results in the deletion of all but 6 amino acids in the cytoplasmic tail [16]. HLA-G1, which derives from the translation of the complete HLA-G transcript, has a structure similar to that of classical HLA class I molecules: a heavy chain constituted of three globular extra-cellular alpha-l, alpha-2, alpha-3 domains, non-covalently associated with β2microglobulin and a nonapeptide. HLA-G1 can be shed or proteolytically cleaved from the cell surface, as has been described for the classical HLA class I molecules [17]. The other membrane-bound HLA-G isoforms lack one or two globular domains, but all contain the alpha 1 domain. HLA-G truncated isoforms are known not to associate with β2-microglobulin or peptides. Secreted isoforms of HLA-G, i.e. HLA-G5, -G6, -G7 are the soluble counterparts of HLA-Gl, -G2, -G3, respectively. Membrane-bound and secreted pairs share the same extracellular structure and they differ only at their C-ends: whereas the membrane-bound isoforms have a trans-membrane region (encoded by exon 5) and an intra-cytoplasmic tail (encoded by exons 6–8), these are replaced in secreted isoforms by a short hydrophilic tail that is encoded by the 5′ sequences of intron 4 (HLA-G5 and HLA-G6) or intron 2 (HLA-G7) [5–8]. The presence of intron-encoded amino acids allows for the discrimination between shed or proteolytic cleaved HLA-G molecules and secreted HLA-G isoforms.
sHLA class I molecules have been recognized since 1970 [18, 19], but only recently they have become the subject of intense research because of their presumed importance in the immune response [6] and in the modulation of the maternal–fetal immune relationship during pregnancy [10]. sHLA-G molecules have been shown to display six alternative splicing products, four of which encode different truncated extracellular domains, as two products, soluble sHLA-Gl and -G2 which lacks exons 5 and 6, but contain intron 4. The resulting isoforms are likely to be expressed in soluble form, since they lack the transmembrane and intracellular domains. One of these transcripts encodes the full-length HLA-Gl soluble form (sHLA-G) and yields a 37-kDa soluble protein that lead to a purified form of sHLA-G and monoclonal antibodies, respectively.
HLA-G: function
Initially, HLA-G was thought to prevent allorecognition by maternal cytotoxic lymphocytes and to protect against natural killer (NK)-cell-mediated cytolysis of target cells [20]. More recently, evidence points toward immunomodulation through induction of secretion of cytokines creating a “chemical dialogue” between the embryo on the one hand and the maternal immune tolerance mechanisms on the other.
Recent studies using HLA-G proteins from transfected cells and recombinent sHLA-G indicated that these proteins regulate immune cells including T cells [21], antigen presenting cells and NK cells [22, 23] (Fig. 2). When recombinant sHLA-G was co-cultured with IL-15-stimulated uterine mononuclear cells, proliferation of CD4+ T cells was inhibited; however, interferon-γ and tumor necrosis factor-α production by NK cells were both increased. In contrast to membrane-bound HLA-G, sHLA-G did not affect the natural cytolytic activity of uterine mononuclear cells [24]. Recombinant sHLA-G strongly stimulates production of TGF-β1 by activated antigen presenting cells [25]. In vitro studies have demonstrated an ability of soluble and membrane-bound HLA-G to modulate cytokine release from human allogeneic peripheral blood mononuclear cells [26] and to have a concentration-dependent effect on generation of an allo-CTL response [27]. Soluble HLA-G suppresses the functional activity of NK cells [28] and inhibits NK cell-mediated cytotoxicity [29]. Taken together, these observations suggest that the function of HLA-G is to modulate cytokine secretion to induce immunotolerance, control trophoblast invasion and contribute to vascular remodeling of spiral arteries to allow for successful embryo implantation and pregnancy maintenance.
HLA-G: clinical applications
Since expression of HLA-G by embryonic cells plays an essential role in pregnancy development [30], recent attention has been focused on potential clinical applications of HLA-G, both diagnostic and therapeutic. Diagnostic applications have included evaluating couples with recurrent miscarriage for mutations of the HLA-G gene and analyzing embryo culture media for sHLA-G protein concentrations. Serum sHLA-G levels during pregnancy have been measured, but their diagnostic efficacy has not as yet been established.
Evaluation of HLA-G gene polymorophisms
Compared with the classical class I genes (the most polymorphic genes in the human genome), HLA-G has relatively little polymorphism in its coding region. The polymorphisms that alter the protein sequence include 5 alleles: G*0101, G*0103, G*0104, G*0105N and G*0106. A polymorphic 1 bp deletion of a cytosine residue at codon 130 results in a null allele (G*0105N), which does not encode functional HLA-G1 or-G5 protein isoforms [31]. This null mutation has been associated with an increased risk for recurrent miscarriage [32, 33]. Female carriers of the mutations*01013or*0105N comprised 4% of the fertile controls, 11% of women with 3–4 losses and 29% of women with greater than 5 losses suggesting a correlation between HLA-G mutations and number of pregnancy losses [34]. Variations have also been identified in the 5′-upstream regulatory region of HLA-G [34, 35]. One variant, -725G, changes the methylation status of CpG dinucleotide in the promoter region and has been associated with an increased risk of miscarriages. Among a population of Hutterites, a 10% abortion rate was noted in mating couples with-725C/C × C/C matings. The abortion rate was increased by 7% in matings where 50% to 100% of the embryos would be C/G and by 18% where half of the embryos would be expected to be G/G. Figure 3 displays the results of our study in which 50 patients (25 couples) with a history for recurrent spontaneous abortion who had no evidence of anatomic, immunology or thrombophilic risk factors were tested for HLA-G 0104, 0105N, 01013 and -725G variants. Thirty-two percent (8/25) of couples demonstrated the -725G variation of which 75% (6/8) had one partner who was heterozygous and 25% (2/8) both partners were heterozygous for the polymorphism. We concluded that the HLA-G -725 promoter polymorphism is a risk factor for recurrent miscarriages. Taken together, these observations suggest that a single HLA-G gene mutation while it may contribute is not a major cause of recurrent pregnancy loss by itself. The challenge is to find the correct combination of factors that define the risk for recurrence of pregnancy loss.
Evaluation of sHLA-G in embryo culture media
A critical period of fetal development for survival is that of the early pre-implantation embryo and therefore determining whether HLA-G is expressed during this period is important for understanding the possible role of the embryo to send a protective signal to maternal immune cells at the time of implantation. Jurisicova et al. reported that it is possible to detect HLA-G heavy chain mRNA in 40% of blastocysts, in some embryos at earlier pre-blastocyst cleavage stages of development (2–4 cell, 5–8 cell, and morula) and in some unfertilized oocytes [36]. In concordance with mRNA data, a similar proportion of embryos stained positive for HLA-G immunohistochemistry. In addition, it was also found that patients, who became pregnant and did not have a fetal loss, had a significantly higher proportion of HLA-G positive sibling blastocysts than patients who did not conceive. These studies represented the first report demonstrating the presence of protein and mRNA for the heavy chain of HLA-G, a non-classical class I MHC antigen, and for β2m throughout the whole course of human pre-implantation development from the oocyte to blastocyst stages [37]. In mice, the HLA-G homolog, Qa-2, the Ped gene product, is associated with more rapid embryo development and “painting” preimplantation embryos with Qa-2 makes them grow faster [38]. Subsequent studies have isolated sHLA-G from the culture media surrounding embryos and blastocysts undergoing in vitro fertilization (IVF). The absence of sHLA-G from human embryo culture media is associated with reduced embryo development and pregnancy rates. Soluble HLA-G was first identified in embryo culture media by Jurisicova et al. [36] and later in culture media from multiple embryos on day 3 [39]. It was shown by Fuzzi et al. [39], that the concentration of sHLA-G in the culture media from groups of 3-day-old embryos correlates positively with both cleavage rate and with subsequent implantation potential. Subsequent studies examined sHLA-G concentrations in day 2–3 culture media droplets of individual embryos selected for transfer. An increased pregnancy rate of 63–64% and implantation rate of 32% was reported for embryo transfers in which at least one embryo selected for embryo transfer was positive for sHLA-G compared with lower pregnancy rates of 36% decreased implantation rates of 19% in patients receiving only embryos whose culture media was negative for sHLA-G. Expression of sHLA-G was also related to increasing cell stage [40, 41].
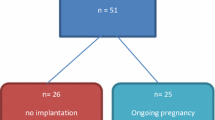
Figures 4 and 5 show the pregnancy outcomes of 326 embryos from 49 couples undergoing IVF and embryo transfer in whom embryos were selected for transfer based on both embryo culture sHLA-G concentrations and embryo morphology [42]. sHLA-G concentrations were measured 2 days after fertilization by intracytoplasmic sperm injection using the sHLA-G ELISA kit from ExBio/BioVendor (Praha, Czech Rep.) Half of the embryo culture media tested had sHLA-G concentrations ≥ 2 U/ml and half <2 U/ml. All patients who became pregnant had at least one embryo whose culture media had sHLA-G ≥ 2 U/ml and no patients who had transfers of all embryos with sHLA-G concentration <2 U/ml became pregnant (Fig. 4). The embryo morphological scores do not always correspond to concentration of sHLA-G. However, when “good grade” embryos (7–8 cell, grade 1–2) were transferred, those with ≥2 U/ml were associated with a 65% pregnancy rate compared with 0% pregnancy rate in those with <2 U/ml sHLA-G concentration (Fig. 5). That not all embryos whose culture media had sHLA-G concentrations ≥2 U/ml became pregnant suggest the sHLA-G is essential for but not sufficient for implantation to occur. Nonetheless, selecting embryos for transfer after IVF based on HLA-G testing provides an opportunity to selectively transfer a fewer number of embryos, minimizing the risk of multiple gestations. Not all investigators had been able to confirm the presence of sHLA-G in embryo culture media or at least have been skeptical concerning detectable amount of secreted sHLA-G [43, 44]. These differences have been ascribed to the different methodologies used to measure sHLA-G.
Testing for sHLA-G
The titration of sHLA-G levels in body fluids has been achieved by several laboratories using 9 different ELISA formats which were set up with various capture and detection antibody pairs (Table 1). These ELISA formats detect either sHLA-G1 + G5, HLA-G5 specifically, or multiple HLA-G isoforms including truncated ones. Of note, capture and detection antibody pairs are routinely used according to locally adapted ELISA procedures. Furthermore, when setting up a sHLA-G ELISA test, the thorny issue of the standard protein arises. Here again, purified sHLA-G proteins to be used as standard in ELISA testing range from biological sample-derived protein [45, 46] to crude or purified supernatants or lysates of various cells transfected with HLA-G1 or HLA-G5 [46–50] none of these standard proteins claiming to be perfect. The bottom line is that even though these methodologies are all-valid and yield semi-quantitative results that accurately reflect sHLA-G concentration differences, the diversity of methodologies prevents result comparisons between teams.
The goals of the 2nd International Workshop on HLA-G (Paris, France, 2003) and especially the Wet-Workshop for quantification of sHLA-G (Essen, Germany, 2004) were to validate an ELISA test capable of measuring sHLA-G1 and HLA-G5 molecules, and an other ELISA test specific for HLA-G5, that can both be used on a variety of sample types and that can be set up easily using commercially available reagents. The reason for these efforts was that even though (a) HLA-G expression is relevant in a variety of pathological situations; (b) amongst the various HLA-G isoforms, soluble ones are the most accessible and the easiest to quantitate; and (c) several methods have been set up to measure sHLA-G levels, there is no consensus on a reference method that would allow standardization and comparison of results.
The 3-day Wet-Workshop on sHLA-G involved 24 investigators from 13 laboratories (teams) and led to the validation of two ELISA procedures and a standard protein [51]. The ELISA procedure to measure sHLA-G1 and secreted HLA-G5 indiscriminately involved a capture by the anti-sHLA-Gl + G5 mAb MEM-G/9 and detection by anti-β2-microglobulin. The ELISA procedure to measure secreted HLA-G5 specifically involved a capture with the anti-intron-4 of HLA-G mAb 5A6G7 and detection by anti-pan-HLA class I mAb W6/32. The standard curves for these ELISA tests were generated using recombinant HLA-G5 protein extracted from HLA-G5 + β2m co-transfected insect cells.
The results obtained with both ELISA formats using the recombinant HLA-G5 standard protein that they both recognize, indicate that these two ELISA measure HLA-G5 levels up to 80 ng/ml with the same efficiency, since super-imposable standard curves were obtained. The results obtained with biological samples show that both ELISA formats are specific and do not cross-react with other molecules including other HLA that might be contained in biological samples, and that these tests allow for the quantification of sHLA-G1 and HLA-G5 if used in combination. Finally, even though each investigator may have obtained different standard curves, this did not affect the absolute values obtained for the samples measured, indicating that both tests generate reproducible results. Two of the participants (R. Roussev and R. Rizzo) brought embryo culture media samples which both reacted positively with MEM-G/9 as capture and anti-β2-microglobulin as detecting antibodies but not with other combinations, suggesting that embryo secretes sHLA-G1 isoform. Overall, these two ELISA formats reached expectations of the participants and the opinion was they could serve as reference assays to measure for sHLA-G molecules in culture supernatants and biological fluids. Thus, the Wet-Workshop for quantification of sHLA-G in Essen, Germany has validated ELISA formats to measure sHLA-Gl + G5 molecules or HLA-G5 exclusively. In addition the results of the workshop gave strong evidence for at least one unreported structure for HLA-G5, and possibly for sHLA-G1 as well, that can be detected by the combined use of both ELISA formats.
Evaluation of sHLA-G in pregnancy serum
HLA-G is not generally expressed in non-pregnant adults, making it a suitable marker for the diagnosis and monitoring of pregnancy. Soluble HLA-G circulates in maternal blood during pregnancy [50]. It has been shown that women with preovulatory low sHLA-G levels appear to be on risk for early abortion after IVF [52]. In addition, sHLA-G levels in the maternal blood are indicative of the vigor of cytotrophoblast invasion and the corresponding health of the placental–maternal interface. One complication of pregnancy in which an abnormal trophoblastic cell invasion has been observed is pre-eclampsia [53]. The commonly associated finding of incomplete cytotrophoblast invasion of the spiral arteries suggests impairment of placentation [54]. This lack of trophoblast invasion in pre-eclampsia could be either a primary event or a secondary response to an abnormal immune reaction to the fetus. Several features of this disease suggest that an abnormal response of the mother to the fetus may be the basic defect in this disease [55]. Pre-eclampsia is most likely to occur in primagravidas, and is usually less severe or absent in subsequent pregnancies, but it may reoccur in the same mother if she changes sexual partner. This suggests that an inappropriate response to some paternally derived antigen, possibly associated with HLA-G, may result in pre-eclampsia. HLA-G expression has been found to be decreased in the placental bed of women with pre-eclampsia [53], and sHLA-G protein levels are decreased in both maternal serum and fetal placental tissues in women with preeclampsia [56, 57]. However, there is currently no good predictive test for the development of pre-eclampsia, even though shallow invasion in the first trimester sets up conditions for the clinical signs that manifest later in pregnancy, most commonly late in the third trimester.
The results from some early experiments with anti-HLA-G monoclonal antibodies 1B8 and 3F6, for use in the detection of HLA-G, have shown that these antibodies cannot be used in the detection of pre-eclampsia. Both of these antibodies bind to a subsequence in the αl domain of HLA-G. Because these antibodies detect the same region of HLA-G, there is no synergy to be derived from their combined use in detecting HLA-G. There is a need for an improved detection method for HLA-G in which more than one antibody is used, and in which the antibodies used do not compete for the same binding region. Further, there is a need for an HLA-G detection method, such as an ELISA, which has a high binding selectivity for HLA-G and low binding selectivity for the other HLA class I antigens (see “Testing for sHLA-G” above).
Evaluation of sHLA-G in other scenarios
Recently, it was found that malignancy of ovarian cancers is associated with high sHLA-G titers in tumor ascites [58]. In some melanoma patients the role of HLA-G in immune escape has been emphasized by its involvement in the resistance to IFN therapies [59]. HLA-G expression is significantly associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia and is a better independent prognostic factor than the currently used ZAP-70 or CD38 status [60]. HLA-G in situ expression by heart and liver allo-grafts, and high serum levels of HLA-G5 and sHLA-G1 were significantly associated with no acute and chronic graft rejection [61, 62], and positively associated with prolonged graft survival [63].
Summary
HLA-G has become a relevant marker for diagnosis in a number of reproductive and pathologic situations. Methodology to precisely measure sHLA-G levels is increasingly in demand. Furthermore, if such assessments are found to be reliable, recombinant HLA-G might be a powerful tool for inducing a desirable state of immunotolerance to facilitate pregnancy or graft acceptance and its antagonist for treatment of cancers.
References
Hunt JS. HLA and maternal–fetal relationship. Austin: RG Landes Co.; 1996.
Puppo F, Ghio M, Contini P, Brenci S, Bignardi D, Filaci G, et al. Immunoregulatory role of soluble HLA molecules: a new skin for an old subject? Arch Immunol Ther Exp 1998;46:157–60.
Chu W, Fant ME, Geraghty DE, Hunt JS. Soluble HLA-G in human placentas: synthesis in trophoblasts and in interferon-gamma-activated macrophages but not placental fibroblasts. Hum Immunol 1998;59:435–42.
Ellis SA, Sargent IL, Redman CW, McMichael AJ. Evidence for a novel HLA antigen found on human extravillous trophoblast and a choriocarcinoma cell line. Immunology 1986;59:595–601.
Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci USA 1992;89:3947–51.
Kirszenbaum M, Moreau P, Gluckman E, Dausset J, Carosella E. An alternatively spliced form of HLA-G mRNA in human trophoblasts and evidence for the presence of HLA-G transcript in adult lymphocytes. Proc Natl Acad Sci USA 1994;91:4209–13.
Fujii T, Ishitani A, Geraghty DE. A soluble form of the HLA-G antigen is encoded by a messenger ribonucleic acid containing intron 4. J Immunol 1994;153:5516–24.
Paul P, Cabestre FA, Ibrahim EC, Lefebvre S, Khalil-Daher I, Vazeux G, et al. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol 2000;61:1138–49.
Loke YW, King A. Human implantation: cell biology and immunlogy. Cambridge: Cambridge University Press; 1995.
Hunt SJ, Petroff MG, McIntyre RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J 2005;5:681–93.
Blaschitz A, Lenfant F, Mallet V, Hartmann M, Bansusan A, Geraghty DE, et al. Endothelial cells in chorionic fetal vessels of first trimester placenta express HLA-G. Eur J Immunol 1997;27:3380–8.
McMaster M, Zhou Y, Shorter S, Kapasi K, Geraghty D, Lim K, et al. HLA-G isoforms produced by placental cytotrophoblast and found in amniotic fluid are due to unusual glycosylation. J Immunol 1998;160:5922–8.
Crisa L, McMaster M, Ishii JK, Fisher S, Salomon DR. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J Exp Med 1997;186:289–98.
Yang Y, Chu W, Geraghty DE, Hunt S. Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J Immunol 1996;156:4224–31.
Parham P, Lomen CE, Lawlor DA, Ways JP, Holmes N, Coppin HL, et al. Nature of polymorphism in HLA-A, -B, and -C molecules. Proc Natl Acad Sci USA 1988;85:4005–9.
Shimizu Y, Geraghty DE, Koller BH, Orr HT, DeMars R. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci USA 1988;85:227–31.
Dobbe LM, Stam NJ, Neefjes JJ, Giphart MJ. Biochemical complexity of serum HLA class I molecules. Immunogenetics 1988;27:203–10.
Van Rood JJ, Van Leeuwan A, Van Santen MCT. Anti-HLA-A2 inhibitor in normal human sera. Nature 1970;226:336–67.
Haga JA, She JX, Kao KJ. Biochemical characterization of 39 kDa class I histocompatibility antigen plasma. A secretable membrane protein derived from transmembrane domain deletion. J Biol Chem 1991;266:3695–701.
LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen-presenting cells, NK cells, and T cells. FASEB J 2005;19:662–4.
Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol 2000;164:6100–4.
Morales PJ, Pace JL, Platt JS, Phillips TA, Morgan K, Fazleabas AT, et al. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J Immunol 2003;171:6215–24.
Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med 1999;189:1093–100.
van der Meer A, Lukassen HG, van Cranenbroek B, Weiss EH, Braat DD, van Lierop MJ, et al. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod 2007;13:123–33.
McIntire RH, Morales PJ, Petroff MG, Colonna M, Hunt JS. Recombinant HLA-G5 and -G6 drive U937 myelomonocytic cell production of TGF-beta-1. J Leukoc Biol 2004;76:1220–8.
Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, Kikuchi A, et al. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod 2001;7:195–200.
Kapasi K, Albert SE, Yie S, Zavazava N, Librach CL. HLA-G has concentration-dependent effect on the generation of an allo-CTL response. Immunology 2000;101:191–200.
Roussev RG, Ng SC, Coulam CB. Natural killer cell functional activity suppression by intravenous immunoglobulin, intralipid and soluble human leucocyte antigen-G. Am J Reprod Immunol 2007;57:262–9.
Marchal-Bras-Goncalves R, Rouas-Freiss N, Connan F, Choppin J, Dausset J, Carosella ED, et al. A soluble HLA-G protein that inhibits natural killer cell-mediated cytotoxicity. Transplant Proc 2001;33:2355–9.
Hackmon R, Hallak M, Krup M, Weitzman D, Sheiner E, Kaplan B, et al. HLA-G antigen and parturition: maternal serum, fetal serum and amniotic fluid levels during pregnancy. Fetal Diagn Ther 2004;19:404–9.
Ober C, Aldrich C, Rosinsky B, Robertson A, Walker MA, Willadsen S, et al. HLA-G1 protein expression is not essential for fetal survival. Placenta 1998;19:127–32.
Aldrich CL, Stephenson MD, Karrison T, Odem RR, Branch DW, Scott JR, et al. HLA-G genotypes and pregnancy outcome in couples with unexplained recurrent miscarriage. Mol Hum Reprod 2001;7:1167–72.
Pfeiffer KA, Fimmers R, Engels G, van der Ven H, van der Ven K. The HLA-G genotype is potentially associated with idiopathic recurrent spontaneous abortion. Mol Hum Reprod 2001;7:373–8.
Hviid TV, Sorensen S, Morling N. Polymorphism in the regulatory region located more than 1.1 kilobases 5′ to the start site of transcription, the promoter region, and exon 1 of the HLA-G gene. Hum Immunol 1999;60:1237–44.
Ober C, Aldrich CL, Chervoneva I, Billstrend C, Rahimov F, Gray HL, et al. Variation in the HLA-G promoter region influences miscarriage rates. Am J Hum Genet 2003;72:1425–35.
Jurisicova A, Casper RF, MacLusky NJ, Mills GB, Librach CL. HLA-G expression during pre-implantation human embryo development. Proc Natl Acad Sci USA 1996;93:161–5.
Menicucci A, Noci I, Fuzzi B, Criscuoli L, Scarselli G, Baricordi O, et al. Non-classic sHLA class I in human oocyte culture medium. Hum Immunol 1999;60:1054–7.
McElhinny AS, Exley GE, Warner CM. Painting Qa-2 onto Ped slow preimplantation embryos increases the rate of cleavage. Am J Reprod Immunol 2000;44:52–8.
Fuzzi B, Rizzo R, Criscuoli L, Noci I, Melchiorri L, Scarselli B, et al. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur J Immunol 2002;32:311–5.
Roussev RG, Keskintepe L, Coulam CB, Sher G, Maasarani G. Soluble HLA-G (sHLA-G) in human embryo culture media: a useful marker for assessing embryo quality and implantation potential for IVF. Fertil Steril 2003;80(Suppl 3):S12.
Sher G, Keskintepe L, Nouriani M, Roussev R, Batzofin J. Expression of sHLA-G in supernatants of individually cultured 46-h embryos: a potentially valuable indicator of ‘embryo competency’ and IVF outcome. Reprod Biomed Online 2004;9:74–8.
Roussev RG, Coulam CB. Detection of soluble HLA-G levels in embryo culture media is predictive for embryo quality and successful pregnancy in IVF. Am J Reprod Immunol 2004;51:471–2.
Sergent IL. Does ‘soluble’ HLA-G really exist? Another twist to the tale. Mol Hum Reprod 2005;11:695–8.
Menezo Y, Elder K, Viville S. Soluble HLA-G release by the human embryo: an interesting artefact? Reprod Biomed Online 2006;13:763–4.
Yie SM, Li LH, Li YM, Librach C. HLA-G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. Am J Obstet Gynecol 2004;191:525–9.
Emmer PM, Steegers EA, van Lierop MJ, Steegers-Theunissen RP, Loke YW, Joosten I. Amniotic fluid soluble human leukocyte antigen G is markedly decreased in offspring with neural tube defects. Early Hum Dev 2002;66:101–5.
Fuzzi B, Rizzo R, Criscuoli L, Noci I, Melchiorri L, Scarselli B, et al. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur J Immunol 2002;32:311–5.
Rebmann V, Pfeiffer K, Passler M, Ferrone S, Maier S, Weiss E, et al. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens 1999;53:14–22.
Sebti Y, Le Friec G, Pangault C, Gros F, Drenou B, Guilloux V, et al. Soluble HLA-G molecules are increased in lymphoproliferative disorders. Hum Immunol 2003;64:1093–101.
Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am J Obstet Gynecol 2000;183:682–8.
Rebmann V, Lamaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Report of the wet workshop for quantification of soluble HLA-G in Essen, 2004. Hum Immunol 2005;66:853–63.
Pfeiffer KA, Rebman V, Passier M, van der Ven K, van der Ven H, Krebs D, et al. Soluble HLA levels in early pregnancy after in vitro fertilization. Hum Immunol 2000;61:559–64.
Van Lierop MJ, Wijnands F, Loke YW, Emmer PM, Lukassen HG, Braat DD, et al. Detection of HLA-G by a specific sandwich ELISA using monoclonal antibodies 6233 and 56B. Mol Hum Reprod 2002;8:776–84.
Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, et al. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol 1997;151:1809–18.
Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980;1:3–19.
Redman CW. Immunology of preeclampsia. Semin Perinatol 1991;15:257–62.
Hara N, Fujii T, Yamashita T, Kozuma S, Okai T, Taketani Y. Altered expression of human leukocyte antigen G (HLA-G) on extravillous trophoblasts in preeclampsia: immunohistological demonstration with anti-HLA-G specific antibody “87G” and anti-cytokeratin antibody “CAM5.2”. Am J Reprod Immunol 1996;36:349–58.
Singer G, Rebmann V, Chen YC, Liu HT, Ali SZ, Reinsberg J, et al. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res 2000;9:4460–4.
Ugurel S, Rebmann V, Ferrone S, Tilgen W, Grosse-Wilde H, Reinhold U. Soluble human leukocyte antigen-G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer 2001;92:369–76.
Nuckel H, Rebmann V, Durig J, Duhrsen U, Grosse-Wilde H. HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood 2005;105:1694–8.
Creput C, Durrbach A, Menier C, Guettier C, Samuel D, Dausset J, et al. Human leukocyte antigen-G (HLA-G) expression in biliary epithelial cells is associated with allograft acceptance in liver-kidney transplantation. J Hepatol 2003;39:587–94.
Lila N, Carpentier A, Amrein C, Khalil-Daher I, Dausset J, Carosella ED. Implication of HLA-G molecule in heart-graft acceptance. Lancet 2000;355:2138–42.
Lila N, Amrein C, Guillemain R, Chevalier P, Latremouille C, Fabiani JN, et al. Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation 2002;105:1949–54.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roussev, R.G., Coulam, C.B. HLA-G and its role in implantation (review). J Assist Reprod Genet 24, 288–295 (2007). https://doi.org/10.1007/s10815-007-9148-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-007-9148-3