Abstract
This study addresses for the first time the chemistry and biological activity of polysaccharides isolated from Didymosphenia geminata. Fourier-transform infrared spectroscopy (FTIR) and high-performance thin-layer chromatography (HPTLC) techniques were used to characterize the crude and acidic polysaccharides of D. geminata; galactose and xylose were found to be the main monomer components. The antioxidant activity as well as the effect on the expression of IL-6 and TNF-α in RAW 264.7 murine cell lines were determined for both types of polysaccharides. The antioxidant activity of crude polysaccharides proved to be stronger than acidic polysaccharides by means of quenching experiments of ABTS·+ and Trolox equivalent antioxidant capacity (TEAC) (p < 0.05). Crude polysaccharides induced an increase in both IL-6 and TNF-α contents. Acidic polysaccharides produced a similar effect on these cytokines, but to a lesser extent. These results suggest that polysaccharides from D. geminata could be explored as natural antioxidant and immunomodulatory agents in medicine and the nutraceutical industry. This investigation suggests an opportunity to turn the invasive mats of D. geminata, which currently cause environmental problems, into a source of polysaccharides with nutraceutical application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invading organisms are introduced species that have managed to successfully establish themselves outside their habitat. In aquatic ecosystems, the spread of invading organisms has detrimental effects, both on ecology and the economy (Alpert et al. 2000; Simberloff et al. 2013). In recent years, the invasive diatom Didymosphenia geminata has caused worldwide concern in lotic systems due to its ability to form long and branched polysaccharides filaments. These structures allow D. geminata to adhere to substrates, forming large macroscopic mats that can negatively affect benthic aquatic communities (Aboal et al. 2012; Figueroa et al. 2018; Ladrera et al. 2018). In addition, the proliferation and shedding of D. geminata mats generate economic losses for the tourist industry, mainly due to bad odor, and for the energy sector through the obstruction of pipes in hydroelectric plants (Kilroy 2004). Also, the aesthetic of the bodies of water is affected generating further economic losses in coastal communities, which depend on tourism associated with sport fishing and other recreational activities (Beville et al. 2012).
Many diatom species are notable for secreting extracellular polymeric substances including high molecular weight polysaccharides, sulfated polysaccharides, proteins, and uronic acids (Staats et al. 1999; Chiovitti et al. 2003; Magaletti et al. 2004; Urbani et al. 2005; Bahulikar and Kroth 2007). Unfortunately, despite the environmental relevance of D. geminata, the chemistry and biological properties of this diatom have been largely unexplored. To our knowledge, the only chemical study of D. geminata showed a composition of sulfated polysaccharides, uronic acids, and proteins (Gretz et al. 2006).
Polysaccharides together with oligosaccharides are the most abundant group of biopolymers. They have been found to play crucial roles in a range of biological processes including cell–cell interaction, embryonic development, bacteria and virus infection, and humoral and cellular immunity (Liu et al. 2015). The biological plasticity of polysaccharides is closely related to their chemical composition, specifically the monomeric constitution and degree of sulfation (Meng et al. 2015; Patel 2012). Changes in these variables can tune the physicochemical and structural properties of polysaccharides affecting the interaction of these biopolymers with enzymes and cell-surface receptors, resulting in various biological activities (Raposo et al. 2013; Fimbres-Olivarría et al. 2016). Due to the broad biological activities and low toxicities associated with polysaccharides, such biopolymers have gathered the attention of the nutraceutical, chemical, and biological community (Hoagland et al. 1993; Leung et al. 2006; Shao et al. 2013; Tanna and Mishra 2019). Recent efforts have shown that polysaccharides display a myriad of bioactivities including antiviral, anti-inflammatory, anticoagulant, antitumor, and immunomodulatory properties (Berteau and Mulloy 2003; Kim and Joo 2008; Queiroz et al. 2008; Lauritano et al. 2016). Additionally, these biopolymers can exhibit strong antioxidant activity due to their free radical scavenging ability, acting as protection systems against oxidative stress (Tannin-Spitz et al. 2005; Barahona et al. 2011; Raposo et al. 2013; Chen et al. 2016). Other studies have also demonstrated that polysaccharides display remarkable immunological properties, modifying macrophage activity leading to an immune response in macrophage cell lines (Celada and Nathan 1994; Surayot et al. 2015). In vitro studies have shown that polysaccharides extracted from algae induce the secretion of tumor necrosis factor alpha (TNF-α), a cytokine involved in physiological and pathological host processes. Among the roles of TNF-α are the regulation of leukocytes, cell death by inflammation and viral replication, and triggering the production of IL-6, a multifunctional cytokine that regulates various immune responses and participates in the mediation of the inflammatory response (Van Snick 1990; Wollenberg et al. 1993; Abdala-Díaz et al. 2011; Parages et al. 2012).
In spite of this array of possible polysaccharide functionalities and that unlike other diatoms, the thick mats of polysaccharides produced by D. geminata cause major ecological and environmental problems; to date, there is no information on the bioactive attributes of the polysaccharides produced by this diatom. Therefore, we set out to investigate the biological activity of polysaccharides isolated from D. geminata in order to identify any potential nutraceutical applications of this invasive diatom (Zgłobicka 2013). In this study, we have chemically characterized mats and polysaccharides from D. geminata by elemental analysis, FTIR, and HPTLC. We further evaluated antioxidant capacity by TEAC. Finally, the immunomodulatory activity of these polysaccharides was tested measuring the induction of IL-6 and TNF-α on RAW 264.7 macrophage cell lines.
Materials and methods
Sampling for this study was carried out at the Biobío River (38° 10′21, 4″ S–71° 18′43, 5″ W) in the Araucanía Region of Chile. The collection of Didymosphenia geminata mats from the river was performed according to biosecurity protocols established by Díaz et al. (2013). Each fresh sample was rinsed with constantly flowing distilled water, lyophilized and cut. Chemicals used in the experiments, including ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide), trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), DMSO (dimethylsulfoxide), acetanilide, fructose, glucose, galactose, arabinose, ramose, mannose, ribose, and xylose standards, were from Sigma-Aldrich (USA). All reagents in this experiment were analytical grade. RAW 264.7 murine macrophage cell lines were acquired from American Type Culture Collection (ATCC), USA.
Scanning electron microscopy (SEM)
The method described by Hasle and Fryxell (1970) was used to identify mat samples. First, 2 mL of concentrated sulfuric acid (H2SO4) was added to 2 g of sample while applying constant agitation. Then, potassium permanganate (KMnO4) drops were added, giving the sample a purple tone, and drops of a saturated oxalic acid solution (H2C2O4) were also added. Samples were washed to remove acid and centrifuged for 5 min at 2500 rpm. Finally, the supernatant was removed. This procedure was repeated five times in order to completely remove residual acid. Taxonomic species identification was then performed using scanning electron microscopy according to what was described by Metzeltin and Lange-Bertalot (2014).
Chemical composition analysis
Elemental analysis of carbon, hydrogen, nitrogen, and sulfur content of polysaccharides of D. geminata was performed by using the Perkin-Elmer 2400 CHNS analyzer at 600 °C using acetanilide as a standard. The quantification of the soluble and insoluble carbohydrates was performed using the phenol-sulfuric acid colorimetric method described by Kochert (1993) with D-glucose as the standard. The total lipid content was calculated as described by Folch et al. (1957), with 200 mg of dry biomass homogenized in 10 mL of chloroform-methanol (2:1 v/v) that contained 0.01% BHT for 5 min; the lipid fraction was then separated by centrifugation and the total lipid content was calculated by gravimetry. The organic phase that contained the lipids was completely evaporated under a continuous flow of N2. The dry matter content was determined by drying the sample in an oven at 110 °C until a constant weight was reached. Ash was determined by burning the sample in a muffle furnace at 600 °C for 12 h; the samples were then cooled in a desiccator until a constant weight was reached.
Polysaccharide extraction and isolation
Five grams of lyophilized sample was treated with 500 mL of 85% ethanol with constant stirring for 12 h at room temperature in order to remove pigments. Then, the sample was dried at room temperature. Subsequently, using distilled water, samples were heated and kept boiling under constant agitation and allowed to sit for 1 h. Next, the samples were centrifuged, the supernatant was removed, and crude polysaccharides (CPs) precipitation was performed by adding 96% ethanol (v/v). Simultaneously, selective precipitation of acid polysaccharides (APs) was performed according to the method described by Parages et al. (2012), utilizing N-cetylpyridinium bromide (Cetavlon) 2% (w/v). Finally, the polysaccharides were purified with a 4 M NaCl solution, dialyzed with a 2 M NaCl solution for 12 h at 4 °C, and subsequently lyophilized.
Fourier transform infrared spectroscopy (FTIR)
Infrared spectra were determined using a FTIR. Analysis of the CPs and APs fractions was carried out by generating 16-mm disks prepared with a polysaccharide and potassium bromide (1% w/w) mixture pressed at 15.0 t hydrostatic pressure for 5 min. Subsequently, the disks were measured with an infrared spectrophotometer in the frequency range of 4000–400 cm−1. Baseline adjustment was conducted using the spectrophotometer (Thermo Nicolet OMNIC) program to flatten baselines in each spectrum.
Monosaccharide composition
The polysaccharides extracted from D. geminata were subjected to acidic hydrolysis (1:10 p/v) at 70 °C for 48 h using an aqueous solution of 15% HCl (v/v). After hydrolysis, 500 μL of the sample was diluted to 1 mL with HPLC-grade methanol.
Subsequently, the monosaccharides (monomers) were identified using the HPTLC technique and the samples and standard solutions were analyzed with an automatic TLC sampler (ATS 4, CAMAG Muttenz, Switzerland) equipped with a spray-on band applicator, automatic camera (ADC2), spectrophotodensitometer (Scanner 3), and photodocumentation system (Reprostar 3). The chromatography was performed on silica gel 60 F254 HPTLC plates. The plates were prewashed with methanol, treated by immersion in a 0.1 M solution of K2HPO4 in methanol (Camag immersion device), and activated for 30 min at 120 °C (Aranda et al. 2005).
All the instruments were controlled using WinCats 1.4.1 Planar Chromatography Manager (CAMAG). Finally, the monosaccharides were derivatized in situ via chromatographic separation with the retention factor (RF) compared to fructose, glucose, galactose, arabinose, rhamnose, mannose, ribose, and xylose standards.
Antioxidant activity (ABTS·+)
The antioxidant activity of CPs and APs extracted D. geminata was determined using the method described by Re et al. (1999). Radical cation ABTS·+ (2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) reaction was generated using a 7 mM ABTS solution with 2.45 mM potassium persulfate. Prior to using ABTS·+ it was diluted with PBS, pH 7.4, to an absorbance of 0.700 ± 0.02 at 413 nm. A total of 500 μg mL−1 of CPs and APs was used for testing, dissolved in DMSO, and mixed with ABTS·+. Absorbance was recorded in order to measure the kinetics of the reaction. As a standard reference, Trolox solutions were used at different concentrations (0–20 μM).
Macrophage cell culture
Cell cultures were grown in DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units mL−1 sodium penicillin, 0.1 mg mL−1 streptomycin sulfate and 0.25 μg mL−1 amphotericin B at a temperature of 37 ± 2 °C with humidified air containing 5% CO2. In order to increase the cell density of these units, cultures were subcultured every 3 days at a 1:5 ratio by gently scraping them. Cells were used when confluence reached 75%.
MTT assay
An MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide) stain assay was performed according to the method described by Mosmann (1983), with some modifications by Cárdenas et al. (2011). RAW 264.7 (6 × 103 cells well−1) murine macrophage cells were incubated with different polysaccharide concentrations in micro plates for 72 h at 37 °C with 5% CO2. Afterward, 10 μL MTT solution was added in a phosphate buffer and incubated for 4 h. Then, 150 μL of isopropanol acid (0.04 N) was added and absorbance was determined at 550 nm. The results were expressed as the average percentage of viable cells compared to untreated cells and inhibitory concentration was estimated as the compound concentration that allows for 50% cell population survival (IC50).
Determination of IL-6 and TNF-α in RAW 264.7 macrophage cell lines
Raw 264.7 macrophage cell lines were cultivated at different polysaccharide concentrations (0–100 μg mL−1). Bacterial LPS (50 ng mL−1) was used as a positive control for activation of macrophages and were then incubated for 48 h. After incubation, the supernatant was collected and used to determine the production of TNF-α or IL-6 following polysaccharide stimulation by ELISA, a protocol described by Abdala-Díaz et al. (2011). After washing and blocking with phosphate-buffered saline (PBS) containing 3% bovine serum albumin, cultured supernatants were added to each of the 12 cells for 12 h at 4 °C. The unbound material was washed and monoclonal biotinized anti-mouse TNF-α or IL-6 (0.5 mg, BD Pharmingen) antibodies were added at a 2 mg mL−1 concentration for 2 h. Fixed antibodies were detected by adding avidin-peroxidase (Sigma) for 30 min and then adding an ABTS substrate solution. Absorbance was read at 405 nm. In parallel, a standard curve was constructed using several dilutions of TNF-α or recombinant murine IL-6 in PBS containing 10% fetal calf serum (FCS). Quantification of cytokines was determined by interpolating the absorbance on the standard line.
Statistical analysis
To evaluate changes over time of the antioxidant activity of the CPs and APs, a one-way analysis of variance (ANOVA) with repeated measures over time was performed. To compare differences between each cytokine treatment with polysaccharides, a one-way ANOVA was run, followed by a post hoc Tukey’s test. Prior to these analyses, the assumptions of normality and variance homogeneity were evaluated. All statistical analyses were performed with R version 3.2.3 (Core Team 2016). Differences were considered statistically significant when P < 0.05.
Results
Scanning electron microscopy (SEM) to identify Didymosphenia geminata
SEM was employed to identify biological material (Fig. 1). SEM images of the collected mats showed an average thickness of 12 ± 1.5 mm. In the specimens collected (n = 10), mucilaginous branching peduncles were observed with a diameter that varied between 13 and 25 μm (Fig. 1a and c). The average valvar size was 135 ± 0.55 μm long and 40 ± 0.41 μm wide with capitate ends, where the apical end was larger (30 ± 0.38 μm) than the basal end (23 ± 0.47 μm). In addition, a central raphe, uniseriate striae extending to the center of the valvar mantle and in the central area with 3–4 stigmas was observed (Fig. 1a–c). With these data in hand, it was determined that the biological material collected corresponded to D. geminata in accordance with previous reports of these features (Metzeltin and Lange-Bertalot 2014).
Elemental analysis and chemical composition analysis
Elemental analysis and chemical composition of polysaccharides of D. geminata mats are displayed in Table 1. These analyses showed that D. geminata mats were made up of 25.06 ± 1.16% total carbon, 0.43 ± 0.13% total nitrogen, 4.60 ± 0.40% total hydrogen, and 1.41 ± 0.16% total sulfur. Chemical composition showed 3.38 ± 0.57% of soluble carbohydrates and 14.52 ± 0.84% of insoluble carbohydrates. Lipids were scarce, showing 1.3 ± 0.18%, along with 2.75 ± 0.52% of proteins and 7.82 ± 0.36% of moisture. In addition, it was observed that approximately 70% of the mass formed by D. geminata corresponded to ash.
Fourier-transform infrared spectroscopy (FTIR) analysis of polysaccharides (CPs and APs)
CPs and APs were analyzed using infrared spectroscopy (see Fig. 2 for the most relevant peaks). Band 1, at 3407 cm−1 intensity, was associated with hydroxyl groups and corresponded to O–H tension. The weaker band 2, at 2934 cm−1, distinct of alkanes, corresponded to C–H tension. The most intense band of CPs, at 1609 cm−1 (band 3), corresponded to C=O tension. The spectral band appearing between 1426 and 1334 cm−1 corresponded to alkyl groups C–H tension (bands 4 and 5). Band 6, with 1242 cm−1 absorption represents sulfate groups (sulfate esters) with S=O tension. Bands 7 and 8 corresponded to C–O tension of primary alcohols.
Monomer composition of polysaccharides determined by high-performance thin-layer chromatography (HPTLC)
After hydrolysis of polysaccharides, monosaccharides were derivatized in situ and then analyzed using HPTLC. The retention factor (Rf) was used to compare samples with known standards. According to this analysis, galactose and xylose are the main monomer components in both CPs and APs (Fig. 3).
HPTLC analysis of the monosaccharide constituents of the CPs (track 1) and APs (track 2) of Didymosphenia geminata (5 μL each). Standards are located between tracks 3 and 10, with fructose in track 3, glucose in track 4, galactose in track 5, arabinose in track 6, rhamnose in track 7, mannose in track 8, ribose in track 9, and xylose standards in track 10. CPs were loaded in track 1 and APs were loaded in track 2
Polysaccharide antioxidant activity quantified
The ability of CPs and APs from D. geminata to quench ABTS·+ radicals was evaluated (Fig. 4). CPs neutralized ABTS·+ radicals more efficiently over time than APs. In addition, the antioxidant activities of D. geminata polysaccharides were studied by means of the Trolox Equivalent Antioxidant Capacity (TEAC) calculated from ABTS·+ (Fig. 5). The variations in TEAC for APs and CPs presented significant differences (p < 0.05) over time. TEAC values for CPs doubled from the initial number, ranging from 4.70 ± 0.87 μmol g−1 DW to 11.37 ± 1.68 μmol g−1 DW. APs TEAC values ranged from 3.71 ± 1.26 μmol g−1 DW to 5.85 ± 0.12 μmol g−1. CPs TEAC values were overall higher than the antioxidant activities displayed by APs.
Cytotoxicity effect of polysaccharides on RAW 264.7 macrophage cell lines
CPs presented cytotoxic activity on RAW 264.7 macrophage cell lines. Addition of CPs produced significant differences in the survival rates of macrophage cell lines (p < 0.05), with 22% inhibition observed at a concentration of approximately 50 μg mL−1, meaning a fivefold decrease from the initial value of 0.19 μg mL−1. When cells were exposed to a 100 μg mL−1 concentration, no cells survived. The 50% inhibitory concentration (IC50) indicates that concentrations above 35 μg mL−1 have a cytotoxic effect on macrophage cell lines. In contrast, after exposure of cell lines to various concentrations of APs, proliferation of macrophages was not affected and no significant cytotoxic activity was observed (0 to 100 μg mL−1) (Fig. 6).
Cytotoxicity effect of polysaccharides on RAW 264.7 macrophage cell lines. Plot shows cell survival ± standard deviation after exposure to various concentrations of polysaccharides extracted from Didymosphenia geminata mats (n = 3). The same letter above two points indicates no significant difference between levels (Tukey’s test)
Polysaccharides isolated induce the production of IL-6 and TNF-α in RAW 264.7 macrophages cell lines
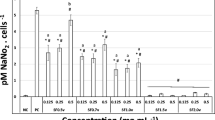
Subjecting RAW 264.7 cells to different polysaccharide concentrations after 24 h produced a dose-response effect for IL-6 and TNF-α secretion. CPs induced a significant increase in IL-6 secretion (p < 0.05; Fig. 7). The average initial content observed was 4.65 ± 1.11 pg mL−1, increasing to 53.89 ± 0.42 pg mL−1 when the cells were exposed to 100 μg mL−1 of CPs. When the cells were exposed to different concentrations of APs, no significant increases in IL-6 production were observed (p ˃ 0.05; Fig. 7).
A significant change in TNF-α was also observed in macrophages exposed to different levels of CPs (p < 0.05; Fig. 8). Treatment with 100 μg mL−1 of CPs caused a significant increase in the production of TNF-α reaching an average value of 76.17 ± 3.7 pg mL−1. APs also induced an increase in TNF-α secretion (p < 0.05), although it was not as strong as the effect produced by CPs (Fig. 8).
Discussion
Over the last decades, problems associated with invasive mats of D. geminata have grown significantly leading to detrimental effects on benthic aquatic communities (SanMiguel et al. 2016; Figueroa et al. 2018; Ladrera et al. 2018). Damage caused by this diatom has reached the tourism and energy sectors (Kilroy 2004). A number of governments and institutions concerned with environmental policies, biodiversity, and natural resources have made major efforts to combat this by launching programs dedicated to controlling the spread of D. geminata. Despite such initiatives, invasive mats of D. geminata continue to negatively affect aquatic ecosystems and still cause economic losses in the industry sector. Yet, very little knowledge about the bioactive molecules and mats produced by D. geminata has been gained. We envision that this could have strong nutraceutical prospects as well as potential in circular economic strategies to transform an environmental problem into a valuable product (Clark et al. 2016; Sosa-Hernández et al. 2019).
In this study, field samples were collected and taxonomically identified as D. geminata using SEM, as proposed by Rivera et al. (2013), by considering cell form, striate disposition, areola shape, stigma quantity variation, and terminal raphe fissure. An apical pore at the narrowed capitated end was observed, through which the muco-polysaccharide filament grows out. Furthermore, identification of samples included taxonomic characters described by Metzeltin and Lange-Bertalot (2014), including cell length, width, and spacing between areolas characteristic of D. geminata.
The chemical composition of algae varies depending on the species and its metabolic requirements, which is reflected in protein, carbohydrate, and lipid content (Brown et al. 1997). In the present study, the D. geminata mats showed low protein, lipid, and carbohydrate content compared with that reported for the brown alga Sargassum cristaefolium (Wang et al. 2015). This low organic composition was mainly due to the fact that D. geminata mats are made primarily of extracellular polysaccharides filaments that constitute up to 90% of the biomass (Whitton et al. 2009). The high percentage of ash observed was similar to that reported by Courtois de Viçose et al. (2012) in the benthic diatoms Amphora sp., Navicula incerta, Nitzschia sp., and Proschkinia sp.
Infrared (IR) spectra of CPs and APs displayed similar trends in their signals, showing the presence of –OH, –CH, –C=O, and –S=O groups. The band associated with the sulfate groups found in this study was similar to that reported by Pereira et al. (2009), who observed sulfate group presence between 1240 and 1260 cm−1 in polysaccharides extracted from the red seaweed species Gelidium corneum, Pterocladiella capillacea, Sargassum vulgare and Padina pavonica. Similarly, Fimbres-Olivarría et al. (2016) reported the IR spectrum of polysaccharides extracted from the diatom Navicula sp. in which a band around 1244 cm−1 absorption associated with the presence of S=O groups was observed. Furthermore, Parages et al. (2012) reported that vibrations associated with sulfate groups of polysaccharides extracted from Arthrospira (Spirulina) platensis presented immuno-regulatory effects in macrophage cell lines. It should be considered that sulfate groups have a wide range of biological and physiological activity (Na et al. 2010). The infrared spectroscopy results obtained in this study strongly suggest the presence of sulfate groups in CPs with a higher signal compared to those found in APs. Current efforts in our group are focused on fully characterizing these sulfated polysaccharides, which will be part of a different study and published in due course.
Establishing a relationship between the monomeric composition of polysaccharides and their biological activity might be challenging due to the complexity of these substrates (Wang et al. 2016). Our study shows that the polysaccharides isolated from D. geminata were primarily made from galactose and xylose. This finding is in accordance with the study carried out by Wustman et al. (1998), who reported that the polysaccharide filaments formed by the diatoms Cymbella cistutala and Cymbella mexicana were built mainly from galactose. The presence of xylose was also noted.
Polysaccharides are molecules that display antioxidant properties and thus can delay or prevent oxidative damage (Zhang et al. 2010). Our investigation shows that antioxidant activity, obtained from the reaction kinetic with ABTS·+, present a greater drop for CPs than for APs. The kinetic profiles for the reaction of ABTS·+ with polysaccharides display fast initial reaction kinetics, followed by a reduction. These results are comparable with those described by Barahona et al. (2011), where antioxidant activity for different algal polysaccharides was evaluated by comparing ABTS·+ kinetic reactions, with absorbance dropping rapidly at 1 min and stabilizing with the passage of time.
Trolox equivalent antioxidant capacity (TEAC) may provide a more accurate estimate of the capacity of a sample to scavenge radicals and better quantification of antioxidant activity (Floegel et al. 2011). We found that CPs extracted from mats display higher TEAC antioxidant activity than APs. Similarly, Wei et al. (2010) demonstrated that APs purified from Prunella vulgaris had significantly lower antioxidant activity compared to CPs, which they attributed to the possible presence of other antioxidant compounds in CPs such as pigments, flavones, peptides, proteins, and polyphenol (Wu et al. 2014).
It has been proposed that polysaccharides extracted from algae induce macrophage immune responses in three ways (Schepetkin and Quinn 2006): (i) enhancing macrophage phagocytic activity, (ii) inducing ROS and NO production, and (iii) secreting TNF-α, IL-1, and IL-6 cytokines. In fact, polysaccharides extracted from microalgae present antitumor and anti-metastatic activity and enhanced pro-inflammatory transcription (Mishima et al. 1998; Pugh and Pasco 2001; Abdala-Díaz et al. 2011). For example, Leiro et al. (2007) demonstrate that polysaccharides extracted from Ulva rigida activate RAW macrophage cell lines. This result directly correlated with the degree of sulfation of polysaccharides, suggesting that the presence of sulfate groups is important for macrophage activation and induction of its immunomodulatory response (Castro et al. 2006).
This study demonstrates that increasing the concentration of CPs from D. geminata mats has highly cytotoxic effects on the RAW 264.7 macrophage cell line compared to the polysaccharides of A. platensis (Parages et al. 2012). In contrast, when exposing macrophage cell lines to different concentrations of APs, no cytotoxic effects were observed. This finding is in accordance with what was previously described by Abdala-Díaz et al. (2011), who observed that the APs extracted from H. spinella do not generate cytotoxic effects when exposed to macrophage cell lines. Here, we also observed that the CPs stimulate the secretion of IL-6 and TNF-α, while the APs only induced secretion of the TNF-α cytokine. Previous reports have shown that APs extracted from A. platensis induced the production of TNF-α (Parages et al. 2012).
Our results show the strong difference in biological activity between CPs and APs, with the former signicantly more potent than the latter. While such dissimilarity could be attributed to the presence of other bioactive substances in CPs (Wei et al. 2010), the difference in degree of sulfation between CPs and APs could also be a critical factor (Jiao et al. 2011; Wang et al. 2015). There have been a number of studies suggesting that the degree of sulfation in polysaccharides can strongly increase the biological activities of biopolymers (Manlusoc et al. 2019). Our IR studies showed a strong intensity of signals associated to sulfate groups, but further experiments are needed and currently underway to assess this.
In conclusion, the antioxidant and immunomodulatory effects of polysaccharides (CPs and APs) extracted from mats of the invasive diatom D. geminata are characterized for the first time. In general terms, CPs proved to be more active than APs in both antioxidant and immunoregulatory assays. We believe that this difference could potentially be attributed to a higher degree of sulfation of CPs compared to APs; however, this remains to be confirmed in future studies. CPs showed remarkable antioxidant activity by means of fast-initial reaction kinetics of ABTS·+ and TEAC. We also showed that these polysaccharides, in particular CPs, displayed a strong immunomodularoty effect on macrophage cell lines, driving increases in cytokines IL-6 and TNF-α.
Given the findings here and that the bioactivity of polysaccharides is strongly related with their structural properties and sulfation levels (Jiao et al. 2011; Wang et al. 2015), future work to quantifiy the degree of sulfation of polysaccharides from D. geminata and standarize methods to isolate them efficiently could serve as the basis for nutraceutical applications with the goal of producing large quantities of sulfated polysaccharides displaying useful bioactivity from the invasive mats of D. geminata.
References
Abdala-Díaz R, Chabrillón M, Cabello-Pasini A, Gómez-Pinchetti JL, Figueroa F (2011) Characterization of polysaccharides from Hypnea spinella (Gigartinales) and Halopithys incurva (Ceramiales) and their effect on RAW 264.7 macrophage activity. J Appl Phycol 23:523–528
Aboal M, Marco S, Chaves E, Mulero I, García-Ayala A (2012) Ultrastructure and function of stalks of the diatom Didymosphenia geminata. Hydrobiologia 695:17–24
Alpert P, Bone E, Holzpfel C (2000) Invasiveness, invasibility and the role of environmental stress in the spread of nonnative plants. Perspect Plant Ecol Syst 3:52–66
Aranda M, Vega M, Villegas F (2005) Routine method for quantification of starch by planar chromatography (HPTLC). J Planar Chromat 18:285–289
Bahulikar RA, Kroth PG (2007) Localization of EPS components secreted by freshwater diatoms using differential staining with fluorophore-conjugated lectins and other fluorochromes. Eur J Phycol 42:199–208
Barahona T, Chandía NP, Encinas MV, Matsuhiro B, Zúñiga EA (2011) Antioxidant capacity of sulfated polysaccharides from seaweeds. A kinetic approach. Food Hydrocoll 25:529–535
Berteau O, Mulloy B (2003) Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 13:29R–40R
Beville ST, Kerr GN, Hughey KFD (2012) Valuing impacts of the invasive alga Didymosphenia geminata on recreational angling. Ecol Econ 82:1–10
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Cárdenas C, Quesada AR, Medina MA (2011) Anti-angiogenic and anti-inflammatory properties of kahweol, a coffee diterpene. PloS One 6:e23407
Castro R, Piazzon MC, Zarra I, Leiro J, Noya M, Lamas J (2006) Stimulation of turbot phagocytes by Ulva rigida C. Agardh polysaccharides. Aquaculture 254:9–20
Celada A, Nathan C (1994) Macrophage activation revisited. Immunol Today 15:100–102
Chen Y-X, Liu X-Y, Xiao Z, Huang Y-F, Liu B (2016) Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int J Biol Macromol 91:505–509
Chiovitti A, Higgins MJ, Harper RE, Wetherbee R, Bacic A (2003) The complex polysaccharides of the raphid diatom Pinnularia viridis (Bacillariophyceae). J Phycol 39:543–554
Clark JH, Farmer TJ, Herrero-Davila L, Sherwood J (2016) Circular economy design considerations for research and process development in the chemical sciences. Green Chem 18:3914–3934
Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Courtois de Viçose G, Porta A, Viera MP, Fernández-Palacios H, Izquierdo MS (2012) Effects of density on growth rates of four benthic diatoms and variations in biochemical composition associated with growth phase. J Appl Phycol 24:1427–1437
Díaz C, Molina X, Montecino V (2013) Manual para el monitoreo e identificación de la microalga bentónica Didymosphenia geminata. Subpesca, Chile
Figueroa F, Pedreros P, Cruces F, Abdala-Díaz R, Hernández V, Becerra J, Urrutia R (2018) Effect of Didymosphenia geminata coverage on the phytobenthic community in an Andean basin of Chile. Rev Chil Hist Nat 91:10
Fimbres-Olivarría D, López-Elías AJ, Carvajal-Millán E, Márquez-Escalante AJ, Martínez-Córdova RL, Miranda-Baeza A, Enríquez-Ocaña F, Valdéz-Holguín EJ, Brown-Bojórquez F (2016) Navicula sp. sulfated polysaccharide gels induced by Fe(III): Rheology and microstructure. Int J Mol Sci 17:1238
Floegel A, Kim D-O, Chung S-J, Koo SI, Chun OK (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal 24:1043–1048
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Gretz M, Riccio M, Hungwe T, Burger H, Kiemle S, Apoya M, Spaulding S (2006) Extracellular polymers of the stalked diatom Didymosphenia geminata, Spaulding, S., R. Wiltshire & L. Elwell (conference organizers), Current Knowledge of Didymosphenia geminata: Developing a Research and Management Response. Federation of Fly Fishers and EPA Region, pp 15-16
Hasle G, Fryxell G (1970) Diatoms: Cleaning and mounting for light and electron microscopy. Trans Am Microsc Soc 89:469–474
Hoagland KD, Rosowski JR, Gretz MR, Roemer SC (1993) Diatom extracellular polymeric substances: function, fine structure, chemistry, and physiology. J Phycol 29:537–566
Jiao G, Yu G, Zhang J, Ewart HS (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 9:196–223
Kilroy C (2004) A new alien diatom, Didymosphenia geminata (Lyngbye) Schmidt: its biology, distribution, effects and potential risks for New Zealand fresh waters. Christchurch, National Institute of Water and Atmospheric Research Ltd., Auckland
Kim M-H, Joo H-G (2008) Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett 115:138–143
Kochert G (1993) Carbohydrate determination by the phenol-sulphuric acid method. In: Hellebust J, Craigie J (eds) Handbook of phycological methods. Physiological and biochemical methods. Cambridge University Press, Cambridge, pp 95–97
Ladrera R, Gomà J, Prat N (2018) Effects of Didymosphenia geminata massive growth on stream communities: Smaller organisms and simplified food web structure. PloS One 13:e0193545
Lauritano C, Andersen JH, Hansen E, Albrigtsen M, Escalera L, Esposito F, Helland K, Hanssen KØ, Romano G, Ianora A (2016) Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front Mar Sci 3:68
Leiro JM, Castro R, Arranz JA, Lamas J (2007) Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int Immunopharmacol 7:879–888
Leung MYK, Liu C, Koon JCM, Fung KP (2006) Polysaccharide biological response modifiers. Immunol Lett 105:101–114
Liu J, Willför S, Xu C (2015) A review of bioactive plant polysaccharides: biological activities, functionalization, and biomedical applications. Bioact Carbohydr Dietary Fibre 5:31–61
Magaletti E, Urbani R, Sist P, Ferrari CR, Cicero AM (2004) Abundance and chemical characterization of extracellular carbohydrates released by the marine diatom Cylindrotheca fusiformis under N- and P-limitation. Eur J Phycol 39:133–142
Manlusoc JKT, Hsieh C-L, Hsieh CY, Salac ESN, Lee Y-T, Tsai P-W (2019) Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers 11:1163
Meng L, Sun S, Li R, Shen Z, Wang P, Jiang X (2015) Antioxidant activity of polysaccharides produced by Hirsutella sp. and relation with their chemical characteristics. Carbohydr Polym 117:452–457
Metzeltin D, Lange-Bertalot H (2014) The genus Didymosphenia M. Schmidt. A critical evaluation of established and description of 11 new taxa. In: Lange-Bertalot H (ed) Iconographia Diatomologica. Koeltz Scientific Books, Konigstein, p 298
Mishima T, Murata J, Toyoshima M, Fujii H, Nakajima M, Hayashi T, Kato T, Saiki I (1998) Inhibition of tumor invasion and metastasis by calciumspirulan (Ca-SP), a novel sulfated polysaccharide derived from a blue-green alga, Spirulina platensis. Clin Exp Metastasis 16:541–550
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Meth 65:55–63
Na YS, Kim WJ, Kim SM, Park JK, Lee SM, Kim SO, Synytsya A, Park YI (2010) Purification, characterization and immunostimulating activity of water-soluble polysaccharide isolated from Capsosiphon fulvescens. Int J Immunopharmacol 10:364–370
Parages ML, Rico RM, Abdala-Díaz RT, Chabrillón M, Sotiroudis TG, Jiménez C (2012) Acidic polysaccharides of Arthrospira (Spirulina) platensis induce the synthesis of TNF-α in RAW macrophages. J Appl Phycol 24:1537–1546
Patel S (2012) Therapeutic importance of sulfated polysaccharides from seaweeds: updating the recent findings. Biotech 3:1–15
Pereira L, Amado AM, Critchley AT, van de Velde F, Ribeiro-Claro PJA (2009) Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll 23:1903–1909
Pugh N, Pasco DS (2001) Characterization of human monocyte activation by a water soluble preparation of Aphanizomenon flos-aquae. Phytomedicine 8:445–453
Queiroz KCS, Medeiros VP, Queiroz LS, Abreu LRD, Rocha HAO, Ferreira CV, Jucá MB, Aoyama H, Leite EL (2008) Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed Pharmacother 62:303–307
Raposo MF, Santos Costa de Morais RM, Miranda Bernardo de Morais AM (2013) Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs 11:233–252
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rivera P, Basualto S, Cruces F (2013) Acerca de la diatomea Didymosphenia geminata (Lyngbye) M. Schmidt: su morfología y distribución en Chile. Gayana Bot 70:373–400
Sanmiguel A, Blanco S, Álvarez-Blanco I, Cejudo-Figueiras C, Escudero A, Pérez ME, Noyón G, Bécares E (2016) Recovery of the algae and macroinvertebrate benthic community after Didymosphenia geminata mass growths in Spanish rivers. Biol Invasions 18:1467–1484
Schepetkin IA, Quinn MT (2006) Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int J Immunopharmacol 6:317–333
Shao P, Chen X, Sun P (2013) In vitro antioxidant and antitumor activities of different sulfated polysaccharides isolated from three algae. Int J Biol Macromol 62:155–161
Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pysek P, Sousa R, Tabacchi E, Vila M (2013) Impacts of biological invasions – what’s what and the way forward. Trends Ecol Evol 28:58–60
Sosa-Hernández JE, Romero-Castillo KD, Parra-Arroyo L, Aguilar-Aguila-Isaías MA, García-Reyes IE, Ahmed I, Parra-Saldivar R, Bilal M, Iqbal HMN (2019) Mexican microalgae biodiversity and state-of-the-art extraction strategies to meet sustainable circular economy challenges: high-value compounds and their applied perspectives. Mar Drugs 17:174
Staats N, De Winder B, Stal L, Mur L (1999) Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum. Eur J Phycol 34:161–169
Surayot U, Wang J, Lee JH, Kanongnuch C, Peerapornpisal Y, You S (2015) Characterization and immunomodulatory activities of polysaccharides from Spirogyra neglecta (Hassall) Kützing. Biosci Biotechnol Biochem 79:1644–1653
Tanna B, Mishra A (2019) Nutraceutical potential of seaweed polysaccharides: structure, bioactivity, safety, and toxicity. Compr Rev Food Sci Food Saf 18:817–831
Tannin-Spitz T, Bergman M, van Moppes D, Grossman S, Arad S (2005) Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J Appl Phycol 17:215–222
Urbani R, Magaletti E, Sist P, Cicero AM (2005) Extracellular carbohydrates released by the marine diatoms Cylindrotheca closterium, Thalassiosira pseudonana and Skeletonema costatum: Effect of P-depletion and growth status. Science Total Environ 353:300–306
Van Snick J (1990) Interleukin-6: An overview. Annu Rev Immunol 8:253–278
Wang C-Y, Wu T-C, Hsieh S-L, Tsai Y-H, Yeh C-W, Huang C-Y (2015) Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J Food Drug Anal 23:766–777
Wang JQ, Hu SZ, Nie SP, Yu Q, Xie MY (2016) Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev 64:1–13
Wei M, Xiong S, Jin H, Xu G-b, Xia C (2010) Isolation and antioxidant activity of water-soluble acidic polysaccharide from Prunella vulgaris Linn. Food Science 1:22
Whitton BA, Ellwood NTW, Kawecka B (2009) Biology of the freshwater diatom Didymosphenia: a review. Hydrobiologia 630:1–37
Wollenberg GK, DeForge LE, Bolgos G, Remick DG (1993) Differential expression of tumor necrosis factor and interleukin-6 by peritoneal macrophages in vivo and in culture. Am J Pathol 143:1121–1130
Wu C, Wang X, Wang H, Shen B, He X, Gu W, Wu Q (2014) Extraction optimization, isolation, preliminary structural characterization and antioxidant activities of the cell wall polysaccharides in the petioles and pedicels of Chinese herbal medicine Qian (Euryale ferox Salisb.). Int J Biol Macromol 64:458–467
Wustman BA, Lind J, Wetherbee R, Gretz MR (1998) Extracellular matrix assembly in diatoms (Bacillariophyceae). Organization of fucoglucuronogalactans within the adhesive stalks of Achnanthes longipes. Plant Physiol 116:1431–1441
Zgłobicka I (2013) Aspects of structural biology of Didymosphenia geminata (Lyngb.) M. Schmidt (Bacillariophyta). Int J Algae 15:291–310
Zhang Z, Wang F, Wang X, Liu X, Hou Y, Zhang Q (2010) Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr Polym 82:118–121
Acknowledgments
The authors are grateful for the support provided by Dr. Casimiro Cárdenas in the cultivation of macrophages and the Central Services and Research Support (SCAI), University of Malaga. Juan Mellado, for improving the resolution of SEM images. Authors thank Prof. Ellen Leffler for proofreading this article. Authors thank project EQM 170077. J.R. Cabrera-Pardo thanks Fondecyt Regular 1190652. J. Becerra thanks Conicyt PIA/apoyo CCTE AFBI7007.
Funding
This study was financially supported by CONICYT National Doctoral Grant No21130577 and CONICYT/FONDAP 15130015 Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Figueroa, F.A., Abdala-Díaz, R., Hernández, V. et al. Invasive diatom Didymosphenia geminata as a source of polysaccharides with antioxidant and immunomodulatory effects on macrophage cell lines. J Appl Phycol 32, 93–102 (2020). https://doi.org/10.1007/s10811-019-01976-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01976-6