Abstract
In the present study, diets prepared to contain 0% (D0), 3% (D3), 6% (D6), 9% (D9), 12% (D12), and 15% (D15) of the red alga Gracilaria lemaneiformis were used to investigate the effects of G. lemaneiformis on the growth, carcass composition, and activities of enzymes related to nutritional metabolism of juvenile red sea bream (Pagrosomus major). The weight gain (WG), specific growth rate (SGR), apparent digestibility coefficients (ADC), feed efficiency (FE), protein efficiency ratio (PER), and protein retention (PR) were significantly higher in D3 than in the other treatment groups (P < 0.05). Although the feed intake (FI) of D15 was greatly higher than that of the D0, the ADC of crude lipid, hepatic lipid level, and hepatosomatic index (HSI) of D15 (P > 0.05) were significantly reduced. Compared to the D0, hepatic glycogen and serum aspartate aminotransferase (AST) activity in the liver as well as serum total cholesterol (TC) of the fish on D3 and D6 were significantly higher (P < 0.05). Significantly higher activity of lipoprotein lipase (LPL) in the abdominal adipose tissues of fish fed on D3 was also observed (P < 0.05). The activities of lipase in anterior intestines appear decreased when the G. lemaneiformis supplementation level was over 12%. These results indicate that incorporate 3% G. lemaneiformis in diet could improve the growth performance and feed utilization of juvenile red sea bream. Incorporation of G. lemaneiformis at 15% level in the diet was also feasible for juvenile red sea bream, as it had no influence on growth performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prices for traditional aquacultural feed ingredients have been rising due to increasing demand (FAO 2016). Fish meal is the major dietary protein source for the aquaculture industry; hence, the price of fish meal directly affects the cost of feed. For sustainability of the aquaculture industry, it is crucial to find new or alternative aquaculture feed ingredients that are locally and readily available (Tschirner and Kloas 2017). In an intensive culture system composition of the cultured fish may be affected by high-fat artificial diets and with the composition and deposition of the lipid further influencing the nutritional value and organoleptic properties of the fish (Peres and Oliva-Teles 1999).
The beneficial effects of algae on the improvement of growth performance and lipid metabolism of fish have been demonstrated (Araújo et al. 2016; Lozano et al. 2016; Cian et al. 2018; Guerreiro et al. 2018; Moutinho et al. 2018). There is therefore an increasing interest in the use of algae sources for animal nutrition, although the mechanism of its benefits as a feed additive is not clear. Among the different species of macroalgae, red algae have relatively high protein content, which makes them most suitable feed source for animal nutrition. The red alga Gracilaria lemaneiformis is an important raw material for agar-production and possesses various bioactive functions such as anti-viral, antioxidant, anti-influenza, and immunomodulation effects (Chen et al. 2005a, 2005b). Due to its large-scale production in China, G. lemaneiformis could be used as a feed ingredient for commercially important animals from the economic point of view (Xuan et al. 2013).
Adult red sea bream (Pagrosomus major) is a euryhaline and omnivorous bottom-dwelling fish which is known to ingest algae (Mustafa et al. 1995; Nakagawa 1997). In recent years, because of its market value and high demand, the red sea bream has become one of the important culture fish species in China (Dawood et al. 2015).
To the best of our knowledge, few studies have been carried out on the effects of algae on the nutritional metabolism of fish. The objective of the present study was to evaluate the feasibility of using G. lemaneiformis as a feed ingredient on the juvenile red sea bream by assessing its growth performance, body composition, serum biochemical indexes, and the activities of enzymes related to the digestive–absorptive function.
Materials and methods
Experimental diets
Fresh Gracilaria lemaneiformis was purchased from a seaweed farm (Zhanjiang, Guangdong Province, China). After being washed in seawater, G. lemaneiformis was air-dried in the shade for 2 weeks and then finely ground using a laboratory mill. Other dietary ingredients were purchased from a feed company (DoYoo Industrial Co., Ltd., Zhengzhou, China). Proximate analysis of major dietary ingredients was performed prior to formulation of the experimental diets (Table 1).
The diet without G. lemaneiformis served as control (D0) diet, while five isonitrogenous (49% crude protein) and isolipid (12% crude lipid) experimental G. lemaneiformis meal-based diets were formulated to contain 3% (D3), 6% (D6), 9% (D9), 12% (D12), and 15% (D15) G. lemaneiformis by substituting fish meal on an equal protein basis (Kader et al. 2012; Ragaza et al. 2012). Starch from wheat was used as the primary ingredient responsible for the binding properties without influencing the growth of the red sea bream (Wilson 1994).
The ingredients of the six diets were thoroughly mixed and pelleted by passing through a laboratory feed-pelletizer equipped with a 2-mm die (SLP-45; Fishery Mechanical Facility Research Institute, Shanghai, China) at 65 ± 5 °C. The pellets were then air-dried using a fan at room temperature. The dry pellets were packed in sealed plastic bags and stored at − 20 °C until used. About 20 g of each diet was sampled in triplicates for the analysis of biochemical composition.
Experimental fish and feeding conditions
Red sea bream was obtained from a commercial producer (Zhangzhou, China). After rearing in a net cage for 2 weeks in the sea, the fish were then acclimated in laboratory tanks for 2 weeks prior to use in the experiment. A 56-day growth trial was carried out from May to July 2018 in cylindrical tanks. Fish of homogenous size (initial average weight 7.86 ± 0.03 g) were randomly distributed into 18 plastic buckets (diameter by height 80 × 70 cm, 400 L) with 25 fish per bucket. Each diet was randomly assigned to triplicate buckets. Fish in each bucket were collectively weighed on the initial day after being fasted for 24 h and anesthetized with eugenol at a concentration of 40 mg L−1. Fish were fed to visual apparent satiety, at 08:30 and 16:30 daily, and the amounts fed were recorded daily. Dead fish and uneaten feed were weighted and removed. The temperature was maintained at 27 ± 1.5 °C and the salinity, at 32 ± 1‰. Photoperiod was set at 12-h light:12-h dark. During the experimental period, half the aquarium water was changed twice a day (morning and evening) and water kept aerated.
Digestibility trial
The digestibility trial was conducted during the last 2 weeks of the feeding trial. Cr2O3 (1%) (99.9% trace metals basis, Shanghai Macklin Biochemical Co., Ltd.) was used as the external indicator in the control and five experimental diets. After a week acclimation of experimental diet, feces from each replicate were collected by siphoning 1–2 h after feeding. Briefly, once feces were observed, they were immediately collected by gently siphoning, dried for 5 h at 60 °C and stored at − 20 °C until analysis.
Sample collection
At the beginning of the feeding trial, 10 juvenile red sea bream were randomly weighed and stored at − 20 °C as the initial samples. At the end of the growth trial, fish were weighed after being anesthetized with eugenol and the total number of the fish in each tank was recorded after fasting for 24 h to calculate the weight gain (WG), survival, specific growth rate (SGR), feed efficiency (FE), feed intake (FI), protein efficiency ratio (PER), protein retention (PR), and the final mean weight. Five fish from each bucket were collected and stored frozen (− 20 °C) until being used for proximate analysis of the whole body. Another six fish were randomly collected from each bucket and sacrificed to determine hepatosomatic index (HSI), viscerosomatic index (VSI), and condition factor (CF). The muscles and livers were separated immediately and frozen at − 20 °C for proximate composition. Three fish per bucket were anesthetized using eugenol (40 mg L−1), and the blood samples were immediately collected with needle and syringe through the caudal vein into tubes. Blood was separated in a 4 °C centrifuge (1500 rpm, 15 min), and the serum was stored at − 80 °C for biochemical assays. Another five fish were dissected; liver, stomach, adipose, and anterior intestine tissues were removed onto ice, were washed using distilled water, and then immediately dipped into liquid nitrogen to snap freeze before being stored at − 80 °C.
Biochemical analysis
The protein (984.13, A–D), crude lipid (920.39, A), ash content (942.05), and dry matter (934.01) of diets or fish samples were analyzed using established methods according to AOAC (2006). Protein contents of diets and fish samples were determined using a Kjeldahl Auto Sampler System 1035 Analyzer (Foss, Sweden), while crude lipid content was determined with Soxhlet extraction. Ash content was analyzed through combustion of samples in a muffle furnace at 550 °C for 8 h. Dry matter was determined by exposing samples to 105 °C temperature in a drying oven overnight.
Evaluation of growth performance
The parameters were calculated as shown as follows:
where Id is feed intake in dry matter; Nd, Nf, and Ni represent protein contents in diet, final, and initial fish body, respectively.
Measurement of enzymatic activities
The activities of pepsin and lipase in the stomach; trypsin, amylase, and lipase activities in the liver and anterior intestine; LPL activity in abdominal adipose tissue; the Na+/K+-ATPase, alkaline phosphatase (ALP), γ-glutamyl transferase (γ-GT), and creatine kinase (CK) activities in the anterior intestine, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) activities in the liver and serum; total protein (TP) and total cholesterol (TC) levels in the serum; and liver glycogen level were determined with a spectrophotometry. The test kits (pepsin, trypsin, amylase, lipase, LPL, Na+/K+-ATPase, γ-glutamyl transferase, creatine kinase, transaminase, triglyceride, total cholesterol, and glycogen) for these assays were provided by Nanjing Jiancheng Bioengineering Institute.
Determination of apparent digestibility coefficients
The apparent digestibility coefficient (ADC) determination followed the method described by Cho and Kaushik (1990), with chromic oxide (1% dry basis) as the digestibility marker. The ADCs for dry matter, protein, and lipid for the different experimental diets were calculated using the following equations:
Statistical analysis
Results are presented as mean ± SE (n = 3). All data were subjected to one-way ANOVA. Where there were significant differences (P < 0.05), the group means were further compared with Tukey’s multiple range test. All statistical analyses were performed using SPSS 20.0 (USA).
Results
Growth performance and feed utilization
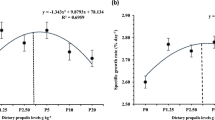
Fish from all groups were fed actively on the experimental diets. The results of different growth parameters are presented in Table 2. The WG, SGR, PER, PR, and the FE of the fish fed with D3 diet improved significantly (P < 0.05) while the poorest growth performance was observed in fish that received D12 diet. FI of the fish on D15 diet was significantly higher than that of the other groups (P < 0.05).
The nutrient digestibility of the experimental diets is presented in Table 3. The ADC of the dry matter and the crude protein of fish from group D3 were significantly higher, while the ADC of the crude lipid of the group D15 was significantly lower than that of the control group (P < 0.05).
Fish body composition
Final body composition of the fish did not show significant variation among the test and control groups, except for that of fish fed with D15 diet, which exhibited significantly lower hepatic lipid content (Table 4), and their HSI lower than that of the control group (P < 0.05) (Table 2).
Digestive and absorptive enzymes activities
The activity of pepsin and lipase in stomach as well as the activities of the pancreatic enzymes including trypsin, lipase, and amylase in the liver and the anterior intestinal amylase activities of the fish were not significantly affected by different levels of dietary G. lemaneiformis supplementation (P > 0.05) (Table 5). The activity of lipase in anterior intestine decreased when the supplemented level of G. lemaneiformis was over 12% (P < 0.05) (Table 5). The activities of Na+/K+-ATPase, ALP, and γ-GT in anterior intestine of D3 group were significantly higher than that of D15 group (P < 0.05); however, the activity of CK in the anterior intestine of the fish was not significantly affected by the G. lemaneiformis experimental diets (P > 0.05) (Fig. 1).
The activities of absorptive enzyme (Na+/K+-ATPase sodium–potassium adenosine triphosphatase, ALP alkaline phosphatase, CK creatine kinase, γ-GT γ-glutamyl transferase) in anterior intestine of juvenile red sea bream fed with the experimental diets for 8 weeks. Different lowercase letters on the similar bars indicate significance limits (P < 0.05). Values represent mean ± SE (n = 3)
Nutrient metabolism indices
The activity of LPL in the abdominal adipose tissue of fish in D3 group, AST activity in the liver, and the serum total cholesterol of fish that received diets D3 and D6 were greatly higher (P < 0.05) than those fed with control diets. The ALT activity in the liver and the TC and TG levels in serum of fish in the D3 group were greatly higher than that of D15 group (P < 0.05). Compared to the control group, the hepatic glycogen of fish in groups D3 and D6 was greatly increased (Table 6).
Discussion
An enhancement of growth performance in terms of WG and SGR was observed with 3% G. lemaneiformis supplementation in red sea bream P. major. The current results are comparable to those previously reported where diets of red sea bream (P. major) were supplemented with 3% Porphyra yezoensis or 5% Ascophyllum nodosum and Undaria pinnatifida (Mustafa et al. 1995; Mustafa and Nakagawa 1995). Feed utilization is generally considered one of the major factors that affect fish growth (Valente et al. 2006). In fact, fish fed with the lower G. lemaneiformis supplemented diets, especially at 3%, exhibited significantly higher FE and ADC of dry matter and crude protein than those of the control group, which implies inclusion of 3% G. lemaneiformis to fish diets might significantly improve efficiency in absorption and assimilation of dietary nutrients. At the same time, it is reasonable to assume that the presence of bioactive substances in G. lemaneiformis, in addition to macronutrients, such as proteins and lipids, might be responsible for stimulating the growth of the fish. For example, non-starch polysaccharides (NSPs) may serve as potential prebiotics (Jia et al. 2009; Xie et al. 2018) while phycobiliprotein has potential antioxidant activity (Zhang et al. 2005; Sfriso et al. 2018).
It has been reported that inclusion of different seaweeds, i.e. Cystoseira barbata (Azaza et al. 2008), Ulva lactuca (Güroy et al. 2007), Ulva rigida (Valente et al. 2006), and Gracilaria cornea (Wassef et al. 2005) decreased growth performance and feed utilization in fish at supplement level of 10%. In the present study, when the supplemented level of G. lemaneiformis was up to 12%, the growth performance was reduced slightly than that of the control group, as indicated by the WG index. The decrease in lipid digestibility and activity of the absorptive-related enzymes might be responsible for the decreased growth performance of the fish in D15 group, although the decrease in growth performance was not significant compared to the control group. This observation might be attributed to the fact that the red sea bream, which itself is capable of ingesting algae, hence, it has a good adaptability to G. lemaneiformis (Nakagawa 1997).
Lipid digestibility can be strongly affected by anti-nutrients (Samarakoon and Jeon 2012). In the present study, a reverse relationship was found between dietary G. lemaneiformis content and apparent digestibility of crude fat when the level of supplemented G. lemaneiformis was above 9%. The main reasons for the anti-nutritive effects might be that the soluble NSPs of G. lemaneiformis, which have gelatinous properties, form a physical barrier that hinders the diffusion of dietary lipids into the intestinal mucosa cells thereby decreasing the rate of triglyceride hydrolysis (Pasquier et al. 1996). The negative effect of G. lemaneiformis on the apparent digestibility of crude fat by Acanthopagrus schlegelii fed with diet containing 20% G. lemaneiformis was demonstrated by Xuan et al. (2013).
Mustafa et al. (1995) found that feeding red algae Porphyra yezoensis increased lipid reserves, especially for muscle and intraperitoneal fat body ratio of red sea bream. In this study, the body composition of fish among the treatment groups was not greatly influenced by the G. lemaneiformis diets; however, the hepatic lipid content and the HSI of fish in the D15 group were significantly decreased, with little effect found on VSI and IFR. This observation is similar to the results reported by Xuan et al. (2013), who found that the effect of G. lemaneiformis diets on the lipid content of fish was apparently related more to the lipid content of the liver than to the adipose tissue around the intestine. It is believed that the effects of dietary algae on lipid metabolism might vary according to the algae species and the growth stage of the particular fish (Mustafa et al. 1995; Morshedi et al. 2018); thus, the reduced hepatic lipid content may partially mirror the decrease in lipid digestibility, which was mentioned above.
To the best of our knowledge, few studies have examined the enzymes activities related to the digestive–absorptive function of fish fed with seaweed-based diets (Xuan et al. 2013; Zhu et al. 2017; Wang et al. 2018). Digestive enzymes are mainly synthesized and secreted in the pancreas (Zambonino Infante and Cahu 2001). In the present study, the activities of pepsin and pancreatic enzymes in the liver of fish were not influenced by the G. lemaneiformis diets, which suggest that the secretion of digestive enzymes was not influenced by G. lemaneiformis supplementation. The digestive enzyme activity in the intestine is a comprehensive representation of a dynamic process involving secretion and turnover of enzymes (Schneeman and Gallaher 1980), so the decreased lipase activities in the intestine of the fish in the 12% and 15% groups could be associated with the dietary fiber content of G. lemaneiformis, which may reduce the activity of the digestive enzymes through special characteristics such as ionic interaction, matrix restriction, water and oil holding capacity (Schneeman 1978). However, data on anti-nutritional or anti-physiological factors of G. lemaneiformis are virtually non-existent at present, and the exact reason for the reduced lipase activity needs to be further examined.
The Na+/K+-ATPase which is localized in the basolateral membrane of enterocytes plays an important role in nutrient transport in the small intestine (Gal-Garber et al. 2003). CK catalyzes the transfer of phosphate to creatine in an ATP-dependent manner, and therefore play a role in energy transfer in tissues with high and fluctuating energy demands (Tang et al. 2009). In the present study, the activities of Na+/K+-ATPase were significantly decreased when the G. lemaneiformis supplementation level was above 12%, suggesting that the intestinal uptake of nutrients by active transport across the brush border of the intestine may be reduced, and the physiological mechanism needs to be further studied.
The liver is the main organ that plays an important role in many metabolic processes of nutrients. Aminotransferases of the liver such as AST and ALT, which are important and critical for the Kreb’s cycle, are often considered as an indicator for protein metabolism (Shakoori et al. 1994; Yousef et al. 2002). In this study, the activity of AST in the liver of fish fed with 3 and 6% G. lemaneiformis diets increased greatly. Although the activity of ALT was not significantly affected, the trends shown by the treatment groups were similar to those of AST activity. The higher PER and PR of the 3% group could at least in part be due to effective anabolism of proteins. It is well known that liver glycogen is a readily available source of energy. The hepatic glycogen in fish fed with 3 and 6% G. lemaneiformis diets were greatly increased, which seems to suggest that energy metabolism was stimulated in fish in the D3 and D6 groups. The growth performance of fish fed 3 and 6% G. lemaneiformis diets could partly be attribute to more efficient metabolism of nutrients and energy. Active ingredients such as phycobiliprotein and agar polysaccharide in G. lemaneiformis might be responsible for the effective stimulus of metabolism (Chen et al. 2005b).
It is known that lipid absorption and transportation in fish were similar to that of mammals (Ostos Garrido et al. 1993) and that LPL is a glycoprotein enzyme which hydrolyzes triglycerides, generating free fatty acids that serve as either direct energy source or for storage (Auwerx et al. 1992). In this study, the significantly elevated activity of LPL in the adipose tissue of the fish in D3 group implied an improvement in lipid metabolism. Thus, when taking the great increase in WG into account, it is plausible to conclude that addition of 3% G. lemaneiformis to diets is beneficial, as it promotes lipid metabolism for better growth performance.
In the present study, 3 and 6% G. lemaneiformis supplementation in diets markedly elevated the serum TC levels of fish. The elevated serum TC could be related with endogenous cholesterol biosynthesis and/or better assimilation of cholesterol (Nakagawa et al. 2007; Sathivel et al. 2008). Wong et al. (1999) have reported that some seaweed, such as Ecklonia cava, Colpomenia sinuosa, and Sargassum hemiphyllum elevated serum cholesterol levels of rats due to increase in endogenous synthesis of cholesterol in the liver. In view of the better WG of D3 and D6 groups, the increased plasma cholesterol levels might reflect an increased disease resistance capacity, as proposed by Nakagawa et al. (2007).
Conclusion
In conclusion, the present study revealed that the incorporation of G. lemaneiformis in diets at the level of 3% is beneficial for red sea bream, as it improved growth performance and physiological state. It is also possible to add up to 15% G. lemaneiformis to diets, as lipid deposition in the liver of fish would be reduced without adverse effects on growth performance in terms of WG. The incorporation of G. lemaneiformis in fish diets will reduce the consumption or reliance on fish meal, given the increasing scale in the culture of red sea bream. Since the dietary fiber of G. lemaneiformis is the main anti-nutritional factors for fish, it remains to be determined if further processing on the G. lemaneiformis such as fermentation could be an alternative way to limit the negative effects of the algal fiber.
References
AOAC (Association of Official Analytical Chemists) (2006) Official methods of analysis of Official Analytical Chemists International, 18th edn. Association of Official Analytical Chemists, International
Araújo M, Rema P, Sousa-Pinto I, Cunha LM, Peixoto MJ, Pires MA, Seixas F, Brotas V, Beltrán C, Valente LMP (2016) Dietary inclusion of IMTA-cultivated Gracilaria vermiculophylla in rainbow trout (Oncorhynchus mykiss) diets: effects on growth, intestinal morphology, tissue pigmentation, and immunological response. J Appl Phycol 28:679–689
Auwerx J, Leroy P, Schoonjans K (1992) Lipoprotein lipase: recent contributions from molecular biology. Crit Rev Cl Lab Sci 29:243–368
Azaza MS, Mensi F, Ksouri J, Dhraief MN, Brini B, Abdelmouleh A, Kraïem MM (2008) Growth of Nile tilapia (Oreochromis niloticus L.) fed with diets containing graded levels of green algae Ulva meal (Ulva rigida) reared in geothermal waters of southern Tunisia. J Appl Ichthyol 24:202–207
Chen MZ, Yu J, Long ZJ, Luo QB (2005a) Studies on antimutagenic and free radical scavenging effect of polysaccharide from Gracilaria lemaneiformis. Food Sci 26:219–222 (in Chinese)
Chen MZ, Yu J, Zhong QL, Chen JL, Ge AS (2005b) Study on the immunity function and anti-oxidation activity of phycobiliproteins from Gracilaria lemaneiformis. Food Sci 26:456–459 (in Chinese)
Cho CY, Kaushik SJ (1990) Nutritional energetics in fish: energy and protein utilisation in rainbow trout (Salmo gairdneri). World Rev Nutr Diet 61:132–172
Cian RE, Bacchetta C, Rossi A, Cazenave J, Drago SR (2018) Red seaweed Pyropia columbina as antioxidant supplement in feed for cultured juvenile Pacú (Piaractus mesopotamicus). J Appl Phycol. https://doi.org/10.1007/s10811-018-1648-2
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S (2015) Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile Red Sea Bream, Pagrus major. Fish Shellfish Immunol 45:33–42
FAO (2016) The State of World Fisheries and Aquaculture 2016 (SOFIA): contributing to food security and nutrition for all. State World Fish Aquacult 4:40–41
Gal-Garber O, Mabjeesh SJ, Sklan D, Uni Z (2003) Nutrient transport in the small intestine: Na+,K+-ATPase expression and activity in the small intestine of the chicken as influenced by dietary sodium. Poult Sci 82:1127–1133
Guerreiro I, Magalhães R, Coutinho F, Couto A, Sousa S, Delerue-Matos C, Domingues VF, Oliva-Teles A, Peres H (2018) Evaluation of the seaweeds Chondrus crispus and Ulva lactuca as functional ingredients in gilthead seabream (Sparus aurata). J Appl Phycol. https://doi.org/10.1007/s10811-018-1708-7
Güroy BK, Cirik Ş, Güroy D, Sanver F, Tekinay AA (2007) Effects of Ulva rigida or Cystoseira barbata meals as a feed additive on growth performance, feed utilization, and body composition in Nile tilapia, Oreochromis niloticus. Turk J Vet Anim Sci 31:91–97
Jia W, Slominski BA, Bruce HL, Blank G, Crow G, Jones O (2009) Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during subclinical Clostridium perfringens challenge. Poult Sci 88:132–140
Kader MA, Bulbul M, Koshio S, Ishikawa M, Yokoyama S, Nguyen BT, Komilus CF (2012) Effect of complete replacement of fishmeal by dehulled soybean meal with crude attractants supplementation in diets for red sea bream, Pagrus major. Aquaculture 350:109–116
Lozano I, Wacyk JM, Carrasco J, Cortez-San Martín MA (2016) Red macroalgae Pyropia columbina and Gracilaria chilensis: sustainable feed additive in the Salmo salar diet and the evaluation of potential antiviral activity against infectious salmon anemia virus. J Appl Phycol 28:1343–1351
Morshedi V, Bahabadi MN, Sotoudeh E, Azodi M, Hafezieh M (2018) Nutritional evaluation of Gracilaria pulvinata as partial substitute with fish meal in practical diets of barramundi (Lates calcarifer). J Appl Phycol 30:619–628
Moutinho S, Linares F, Rodríguez JL, Sousa V, Valente LMP (2018) Inclusion of 10% seaweed meal in diets for juvenile and on-growing life stages of Senegalese sole (Solea senegalensis). J Appl Phycol 30:3589–3601
Mustafa M, Nakagawa H (1995) A review: dietary benefits of algae as an additive in fish feed. Aquaculture 47:163–172
Mustafa MG, Wakamatsu S, Takeda T, Umino T, Nakagawa H (1995) Effects of algae meal as feed additive on growth, feed efficiency and body composition in red seabream. Fish Sci 61:25–28
Nakagawa H (1997) Effect of dietary algae on improvement of lipid metabolism in fish. Biomed Pharmacother 51:345–348
Nakagawa H, Sato M, Gatlin DM (2007) Dietary supplements for the health and quality of cultured fish. Cromwell Press, Trowbridge
Ostos Garrido MV, Nunez Torres MV, Abaurrea Equisoain MA (1993) Lipid absorption by enterocytes of the rainbow trout, Oncorhynchus mykiss: diet-induced changes in the endomembranous system. Aquaculture 110:161–171
Pasquier B, Armand M, Castelain C, Guillon F, Borel P, Lafont H, Lairon D (1996) Emulsification and lipolysis of triacylglycerols are altered by viscous soluble dietary fibres in acidic gastric medium in vitro. Biochem J 314:269–275
Peres H, Oliva-Teles A (1999) Effect of dietary lipid level on growth performance and feed utilization by European sea bass juveniles (Dicentrarchus labrax). Aquaculture 179:325–334
Ragaza JA, Koshio S, Mamauag RE, Nguyen BT, Villamor SS, Ishikawa M, Yokoyama S (2012) Effects of Eucheuma denticulatum supplementation on performance and blood chemistry of juvenile red sea bream Pagrus major. Aquacult Sci 60:485–494
Samarakoon K, Jeon YJ (2012) Bio-functionalities of proteins derived from marine algae—a review. Food Res Int 48:948–960
Sathivel A, Raghavendran HRB, Srinivasan P, Devaki T (2008) Anti-peroxidative and anti-hyperlipidemic nature of Ulva lactuca crude polysaccharide on D-galactosamine induced hepatitis in rats. Food Chem Toxicol 46:3262–3267
Schneeman BO (1978) Effect of plant fiber on lipase, trypsin and chymotrypsin activity. J Food Sci 43:634–635
Schneeman BO, Gallaher D (1980) Changes in small intestinal digestive enzyme activity and bile acids with dietary cellulose in rats. J Nutr 110:584–590
Sfriso AA, Gallo M, Baldi F (2018) Phycoerythrin productivity and diversity from five red macroalgae. J Appl Phycol 30:2523–2531
Shakoori AR, Butt U, Riffat R, Aziz F (1994) Hematological and biochemical effects of danitol administered for two months on the blood and liver of rabbits. Z Angew Zool 80:165–180
Tang L, Wang GX, Jiang J, Feng L, Yang L, Li SH, Kuang SY, Zhou XQ (2009) Effect of methionine on intestinal enzymes activities, microflora and humoral immune of juvenile Jian carp (Cyprinus carpio var. jian). Aquac Nutr 15:477–483
Tschirner M, Kloas W (2017) Increasing the sustainability of aquaculture systems: insects as alternative protein source for fish diets. GAIA 26:332–340
Valente LMP, Gouveia A, Rema P, Matos J, Gomes EF, Pintoae IS (2006) Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 252:85–91
Wang C, Hu W, Wang L, Qiao H, Wu H, Xu Z (2018) Effects of dietary supplementation with Sargassum horneri meal on growth performance, body composition, and immune response of juvenile turbot. J Appl Phycol 31:771–778
Wassef EA, El-Sayed AFM, Kandeel KM, Sakr E (2005) Evaluation of Pterocla dia (Rhodophyta) and Ulva (Chlorophyta) meals as additives to gilthead seabream Sparus aurata diets. Egypt J Aquacult Res 31:321–332
Wilson RP (1994) Utilization of dietary carbohydrate by fish. Aquaculture 124:67–80
Wong KH, Sam SW, Cheung PCK, Ang PO Jr (1999) Changes in lipid profiles of rats fed with seaweed-based diets. Nutr Res 19:1519–1527
Xie D, Li X, You C, Wang S, Li Y (2018) Supplementation of macroalgae together with non-starch polysaccharide-degrading enzymes in diets enhanced growth performance, innate immune indexes, and disease resistance against Vibrio parahaemolyticus in rabbitfish Siganus canaliculatus. J Appl Phycol. https://doi.org/10.1007/s10811-018-1662-4
Xuan XZ, Wen XB, Li SK, Zhu DS, Li YY (2013) Potential use of macro-algae Gracilaria lemaneiformis in diets for the black sea bream, Acanthopagrus schlegelii, juvenile. Aquaculture 412:167–172
Yousef MI, EI Hendy HA, El-Demerdash FM, Elagamy EI (2002) Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 175:223–234
Zambonino Infante JL, Cahu CL (2001) Ontogeny of the gastrointestinal tract of marine fish. Comp Biochem Physiol C 130:477–487
Zhang YY, Chen MZ, Yu J, Lin YX (2005) Studies on the antimutagenic and antitumour effects of phycobiliproteins from Gracilaria lemaneiformis. Chin J Mar Drugs 24:36–38 (in Chinese)
Zhu D, Wen X, Li S, Xuan X, Li Y (2017) Evaluation of the red alga Gracilaria lemaneiformis and brown alga Sargassum horneri as ingredients in diets for white spotted snapper Lutjanus stellatus Akazaki juveniles. J Appl Phycol 29:3211–3219
Funding
This research was supported by the Science and Technology Development Project of Suzhou (SNG2018054), the Guangdong Provincial Key Laboratory of Marine Biology Open Fund (GPKLMB201802), and the Special Project of Science and Technology in North of Jiangsu (SZ_SQ2017026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xuan, X., Li, W., Zhu, W. et al. Effects of different levels of macroalga Gracilaria lemaneiformis on growth performance and feed utilization on the red sea bream, Pagrosomus major. J Appl Phycol 31, 3213–3222 (2019). https://doi.org/10.1007/s10811-019-01787-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01787-9