Abstract
Microalgae are unicellular and photosynthetic organisms which have great potential for providing various products of interest in the food, chemicals, pharmaceuticals, and cosmetics industry. The green alga Ankistrodesmus braunii has been cited as capable of producing large amounts of lipids. In this sense, this work aims to study A. braunii growth in tubular photobioreactor using different amounts of nitrogen and under different cultivation processes. In batch cultures, the maximum biomass concentration (Xm) was 1588 ± 11 mg L−1 using 20 mM of NaNO3. A fed-batch process with the addition of 20 mM NaNO3 each 48 h from the first to the sixth cultivation day reached Xm = 2753 ± 7 mg L−1. The semi-continuous process was effective to eliminate the lag phase, allowing to obtain Xm = 2399 ± 5 mg L−1. In this condition, the protein and lipid levels in the biomass were 33.1 ± 0.2% and 38.6 ± 0.2%, respectively. The maximum specific growth rate (μm) reached a maximum value of 0.998 day−1 in the semi-continuous process using 20 mM NaNO3. The results of this study show the potential for the production in a large scale of A. braunii as a source of protein and lipids for commercial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are found in marine and fresh water and are considered responsible for at least 60% of the Earth’s primary production (Chisti 2004). The exact number of algae species is still unknown but it is reported that there may be between 300,000 to several million representatives (Guiry 2012). This diversity is also reflected in the biochemical composition, and on this basis, microalgae are a source of a large number of bioproducts (Norton et al. 1996; Borowitzka 2013). Many species show high growth rates, which favors high biomass production at short time intervals (Campos et al. 2010; Formighieri 2015). Therefore, some of the microalgae have several potential applications such as the production of compounds of interest in the food, pharmaceutical, and cosmetics industry like nutritional supplements for humans and animals, microbial biomass for animal feed, and use in aquaculture, as well as pharmaceuticals and cosmetic formulations (Borowitzka 2013).
It is important to remember that single-celled photosynthetic organisms are able to use light energy efficiently and are efficient carbon fixers (Brown and Zeiler 1993). Moreover, some microalgae can be grown under controlled conditions producing cultivated biomass with constant composition (Sassano et al. 2010), which facilitates the manufacturing operation. Carbon dioxide fixation by photosynthetic microorganisms also has been proposed as a method for removal of CO2 released into the atmosphere. Consequently, a lot of these microorganisms could be used to reduce greenhouse gases emission (Sayre 2010; Moheimani 2016). Considering the importance of obtaining energy from renewable raw materials, biodiesel production from microalgae coupled to CO2 from alcoholic fermentation could also contribute to greenhouse gases mitigation in Brazil (Carvalho et al. 2009).
The content of lipids in microalgae biomass varies from 1 to 40% of dry biomass, but can reach 85% in certain growing conditions (Becker 2004). Fatty acids correspond to the higher fraction of lipids in microalgae, and in some species, the polyunsaturated fatty acids may comprise between 25 and 60% of the total lipids (Becker 1988, 2004; Brown 1991).
Ankistrodesmus braunii (Chlorophyceae) has been reported as a high producer of lipids which can reach up to 73% of dry biomass (Williams and McMillan 1961). Lipid fraction contains fatty acid composition similar to vegetable oils, but with a predominance of linolenic over the linoleic acid, which is not usual in vegetable oils. Furthermore, this species contains different pigments on its composition, such as β-carotene, astaxanthin, lutein, and a possible derivative of neoxantin, which have antioxidant properties (D’Alessandro and Filho 2016).
Recently, Ankistrodesmus falcatus was evaluated as a potential strain for biofuel production, being cultivated in 500 mL conical flasks under different photoperiods and reaching 67.2% total lipids (George et al. 2014). However, as far as we know, there is no information about A. braunii cultivation in tubular photobioreactors.
Taking into consideration the lack of results evaluating nitrogen sources on A. braunii growth in tubular photobioreactors and this parameter being a fundamental key on biomass composition of photosynthetic microorganisms, this study aimed to evaluate NaNO3 as nitrogen source at different concentrations and under different cultivation processes for assessing and understanding biomass growth of this microorganism, as well as the main kinetic parameters such as maximum biomass concentration, biomass productivity, nitrogen conversion factor into biomass, and maximum specific growth rate. The knowledge of kinetic parameters related to growth and biomass composition is an important contribution for potential industrial applications of this alga.
Materials and methods
Microorganism and culture maintenance
Ankistrodesmus braunii (UTEX 245) was obtained from The Texas Culture Collection of Algae at the University of Texas at Austin (UTEX) and maintained in glass tubes containing 10 mL solid Bold medium (UTEX 2016) at 25 ± 1 °C.
Medium evaluation
First, with the intention to know which medium could be the best to culture A. braunii, the microorganism was inoculated in three different culture media: Bold (UTEX 2016), Schlösser (Schlösser 1982), and F/2 (Guillard and Rhyter 1962) media. The sodium nitrate concentration added in the formulation of each medium was 80 mM, 20 mM, and 840 mM.
One hundred microliter of each medium was added to a previously sterilized 250 mL Erlenmeyer flask and an inoculating loop with A. braunii inoculum was inoculated aseptically. All broths in duplicate were placed on rotatory shaker (Multitron, INFORTS HT, Switzerland), at 110 rpm, at room temperature of 25 ± 1 °C and exposed to light intensity of 60 μmol photons m−2 s−1 (measured by a model LI-250A, LICOR, USA, meter) for 7 days.
In view of the great importance of the presence of nitrogen for the development and multiplication of this microorganism, it was evaluated which range of NaNO3 concentration dissolved in the medium would be better for biomass growth.
Inoculum preparation
Ankistrodesmus braunii, initially maintained on agar, was collected with the aid of a platinum loop and aseptically inoculated into a test tube containing 10 mL of Bold medium. After 7 days of culture, 10 mL of biomass suspension obtained was divided into 10 parts, each milliliter was added to a 500 mL Erlenmeyer flask with 200 mL Bold medium. All the Erlenmeyers were previously sterilized by autoclaving at 1 atm and 120 °C for 30 min. All Erlenmeyers were placed on a rotatory shaker (model Multitron, INFORTS HT, Switzerland) at 110 rpm, at a temperature of 25 ± 1 °C and exposed to light intensity of 60 μmol photons m−2 s−1. The samples resulting from this process served as the inoculum for feeding the tubular reactors in the following experiments.
Devices for culture and cultivation processes
The tubular photobioreactor was the air-lift type developed in the Laboratory of Microalgal Biotechnology from the University of São Paulo. It is made of transparent tubing with the internal diameter of 1.0 cm which is interconnected and coupled to an air pump (Seven Star, China) to boost the culture flow. The total system volume was 3.5 L and the illuminated volume corresponded to 2.0 L with culture flow of 40 ± 1 L h−1. The initial biomass concentration was 300 mg L−1 and the cultivation was done under aseptic conditions at a temperature of 25 ± 1 °C under light intensity of 60 μmol photons m−2 s−1 supplied by 20 W fluorescent lamps.
The experiments were performed in the photobioreactors by batch, fed-batch, and semi-continuous processes using 2, 5, 10, 20, and 30 mM NaNO3. In batch cultivations, nitrate was added once at the beginning of the process. In fed-batch cultivations in which nitrate was added each 48 h, the first and the last addition was at the beginning and the sixth day of cultivation, respectively. In the semi-continuous process, only when the first peak of maximum biomass concentration was achieved can the first withdrawal of the used medium volume and the restoration of new medium be done, followed by awaiting the second peak of the maximum biomass concentration (first cycle). After the first cycle, the second withdrawal of the used medium was carried out and the restoration of another new medium to reach the third peak of biomass concentration (second cycle). The removed volume corresponded to those which provided to restore the initial biomass concentration of 300 mg L−1.
a Maximum biomass concentration (Xm) as a function of the concentration of sodium nitrate added in the medium in batch, fed-batch, and semi-continuous processes. b Productivity (Px) as a function of the concentration of sodium nitrate added in the medium in batch, fed-batch, and semi-continuous processes
a Nitrogen conversion factor into biomass (YX/N) as a function of the concentration of sodium nitrate added in the medium in batch, fed-batch, and semi-continuous processes. b Maximum specific growth rate (μm) as a function of the concentration of sodium nitrate added in the medium in batch, fed-batch, and semi-continuous processes. The error bars represent the standard deviation
Analytical techniques
Biomass concentration
Biomass concentration (X) was measured by spectrophotometry with samples of medium containing biomass and analyzed for absorbance in a spectrophotometer model 700 PLμS (FEMTO, Brazil) at a wavelength of 550 nm and expressed as a concentration (dry weight per liter) by means of a calibration curve. Calibration curve to determine the biomass concentration was obtained by the initial use of 25 mL of concentrated biomass suspensions in an exponential growth phase, which was subsequently filtered and washed with distilled water in a pre-dried cellulose acetate membrane and then dried at 70 °C for 12 h. The samples were placed in an oven at 100 °C until constant weight. It was possible to obtain the biomass in the filtrate volume of biomass suspension using the difference between the mass of the dried membrane with the biomass with the membrane. From different dilutions of the same suspension, it was possible to obtain an equation that expresses the relationship between biomass concentration (X), expressed in dry weight per liter of suspension, and the sample absorbance at 550 nm (α). The equation obtained from the calibration curve was X = 417.26α – 9.2476 (R2 = 0.997), where X is the biomass concentration and α represents the absorbance value at 550 nm.
Kinetic parameter calculation
All cultivations were evaluated according to the maximum biomass concentration (Xm) parameter obtained in each experimental condition. In addition, biomass productivity (Px), nitrogen-biomass conversion factor (YX/N), and specific growth rate (μ) were calculated. The equation used to calculate biomass productivity was:
where Px corresponds to the biomass productivity (mg L−1 day−1), Xm represents the maximum biomass concentration obtained (mg L−1), Xi is the initial biomass concentration (mg L−1), and tc is the time corresponding to Xm (days).
The equation used to calculate the nitrogen-biomass conversion factor was:
where YX/N is the nitrogen-biomass conversion factor, Xm corresponds to the maximum biomass concentration obtained (mg L−1), Xi represents the initial biomass concentration (mg L−1), V is the volume of the medium (L), and Nt is the total amount of nitrogen added (mg).
The method of Leduy and Zajic (1973) was used to calculate the specific growth rate.
Determination and monitoring of pH
The pH was measured with a potentiometer model M300 (Mettler Toledo, USA) by immersing the electrode in the cultures and was kept constant at values of 7.25 ± 0.25 with a carbon dioxide injection in the medium with the aid of solenoid valve.
Biomass composition
At the end of the cultivation in the photobioreactors, the total amount withdrawn of biomass and culture medium was centrifuged at 5660×g and 10 °C for 20 min and the concentrated biomass was placed in ceramic plates and dried in an oven with air circulation at 55 °C for 12 h. After drying, the dried biomass was ground, frozen, and stored in cleaned dry bottles until used in subsequent analysis. The lipid content was determined by solvent extraction, according to Olguín et al. (2001). Dried biomass sample was ground, with mortar and pestle, and then transferred to a continuous Soxhlet extractor with a solvent reflux using chloroform and methanol in the ratio 2:1, until the sample did not exhibit any color. The solvent was removed with a rotary evaporator and the sample was weighed. The determination of the protein concentration was performed using the micro-Kjeldahl method (AOAC 2007). Dried and degreased samples were digested with sulfuric acid with the addition of catalyst in the digester block at 350 °C. Digested samples were distilled, adding NaOH (60%) to the recovery of nitrogen in ammoniacal form in a saturated boric acid solution. The resulting sample was submitted to titration with 0.02 M hydrochloric acid, to determine the concentration of free nitrogen in the sample. The total protein concentration was determined by multiplying the nitrogen value with the conversion factor 5.95 (López et al. 2010).
Statistical analysis
In order to compare the types of cultivation processes and the quantities of NaNO3 in each process, the parameters analyzed were the maximum biomass concentration (Xm), biomass productivity (Px), and nitrogen-biomass conversion factor (YX/N), as well as biomass composition parameters. The results were compared by two-way analysis of variance—ANOVA—with a significance level of 0.05, performed in MINITAB 17 software.
Results
Medium evaluation
After 7 days of cultivation in a shaker, only the biomass in Bold medium (UTEX 2016) had grown, while in the other media, no biomass production was achieved.
Cultivation in batch process
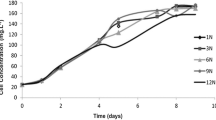
Within these conditions, the maximum biomass concentration (Xm) obtained was 1588 ± 11 mg L−1, which corresponds to the cultivation in the reactor using 20 mM NaNO3 (Figs. 1 and 4a). The batch cultivation with 30 mM was not effective, and the maximum biomass concentration obtained was 784 ± 6 mg L−1 (Figs. 1 and 4a).
Figure 4b shows that the biomass productivity (Px) reaches a maximum value of 215 ± 2 mg L−1 day−1 in the batch process containing 20 mM NaNO3. The maximum value for nitrogen-biomass conversion factor (YX/N) was 22.5 ± 1.6 mg mg−1 in culture using 2 mM NaNO3 (Fig. 5a).
Cultivation by fed-batch process
The maximum biomass concentration obtained in this process was 2753 ± 7 mg L−1 in the photobioreactor with 20 mM NaNO3 (Figs. 2 and 4a). The biomass productivity (Px) in the process was also increased in proportion to the NaNO3 concentration present in the medium. A peak of 351 ± 1 mg L−1 day−1 using 20 mM of nitrate can be observed (Fig. 4b). On the other hand, nitrogen-biomass conversion factor (YX/N) decreased in cultivations with higher amounts of nitrate, with the highest value obtained of 7.1 ± 0.1 mg mg−1 using 2 mM of nitrate (Fig. 5a).
Cultivation in semi-continuous process
The maximum biomass concentration obtained from the semi-continuous processes was 2399 ± 5 mg L−1 at the end of the second cycle with the addition of 20 mM NaNO3 (Figs. 3 and 4a). Biomass productivity (Px) was an increasing function of NaNO3 concentration and the highest values (Px = 525 ± 1 mg L−1 day−1) were obtained with 20 mM NaNO3 (Fig. 4b). Nitrogen-biomass conversion factor (YX/N) also decreased in cultivations with higher amounts of nitrate, being the highest value obtained at 29.4 ± 0.2 mg mg−1 using 2 mM of nitrate at the end of the first cycle (Fig. 5a).
The maximum specific growth rate (μm) values occurred at the beginning of all experiments performed shortly after the end of the lag phase, more specifically after about 3 days of cultivation, in the exponential phases of cell growth with the highest value (μm = 0.998 day−1) obtained in semi-continuous processes using 20 mM of NaNO3 (Fig. 5b).
Protein and lipid content of the biomass from the semi-continuous process
Biomass from semi-continuous process, the process with the higher growth, was analyzed to determine their lipid and protein concentrations. In cultivation using 2 to 5 mM NaNO3, it was possible to obtain values around 39% of lipids, while in samples cultivated with 10 to 20 mM NaNO3, the values obtained were approximately 38% of lipids (Fig. 6).
While in the run, with 2 mM NaNO3, it was possible to achieve a protein content of 16.5 ± 0.4%; the experiments that used 5 and 10 mM NaNO3 had 48.7 ± 0.1% and 52.7 ± 0.1% protein contents, respectively (Fig. 6).
Discussion
The results of the preliminary tests of evaluation of culture media showed that A. braunii did not grow in the Schösser and F2 media probably because of the high concentrations of dissolved salts in these media, which could inhibit the cells. Therefore, we decided using Bold medium for the main experiments. The results of the experiments in photobioreactors showed significant differences because of both different NaNO3 concentrations and growing processes. Biomass productivity, nitrogen-biomass conversion factor, and proteins and lipid levels were influenced by the different processes used.
It is noteworthy that the maintenance of pH was important in all trials. The pH control between 7.0 and 7.5 was essential for growth in A. braunii cultivation using tubular photobioreactors since pH affects nitrate uptake Hipkin and Syrett (1977).
Cultivation in batch process
The initial cultivation process was batch model by virtue of being a simple process to be carried out, in which the culture medium and inoculum are added only once in the photobioreactor. The low value of biomass concentration obtained in batch cultivation with 30 mM of NaNO3 could be explained by inhibition of biomass growth due to the increase of salinity caused by the NaNO3 added in the cultivation medium.
A lag phase was present in all the batch experiments (Fig. 1) up to 3 days. In fact, the lag phase in cultivations latest up to 3 days and 2 days with 2 mM and 30 mM NaNO3, respectively, is probably due to the limitation of nitrogen in the former and inhibition in the latter. These effects are evidenced by the lowest maximum cell concentrations (Xm) obtained with these nitrate concentrations (Figs. 1 and 4a). On the other hand, in the range of 5–20 mM NaNO3, the biomass production was directly proportional to the amount of nitrate added in the medium, similar to the results obtained by Blair et al. (2014).
There is an inversely proportional relationship between YX/N values and NaNO3 concentration added in the culture medium (Fig. 5a), probably due to the fact that the cells also use nitrogen not only for their growth but also to perform the maintenance of their vital functions, in which case, small concentrations of nitrogen promote higher conversion rates (Cruz-Martinez et al. 2015). Figure 4b shows that productivity (Px) increases as the concentration of sodium nitrate in the medium increases as well.
Cultivation by fed-batch process
Despite the batch process being a simple method, it has drawbacks, among which we can describe mainly giving lower yields when compared with other processes (Carvalho et al. 2004). The same nitrogen source was added by a fed-batch process with the purpose of investigating a possible enhancement in growth rates. In order to confirm the trend of the values obtained in the first experiments, NaNO3 concentrations used in the cultures by fed-batch were 2, 5, 10, and 20 mM. Since with 30 mM of NaNO3 growth inhibition was previously observed, this concentration was discarded for this new analysis. The culture medium and inoculum are added to the reactor at the beginning of fed-batch process, with subsequent addition of nutrients at preset time intervals, which could optimize its growth, since this could already be studied by other researchers, where the chemical composition of Arthrospira platensis, for example, could be modified by the manipulation of the growth conditions (Colla et al. 2007). During each fed-batch culture, the respective nitrate concentration was added within 48 h starting the addition on the beginning and ending on the last day of the experiment. A higher biomass concentration was obtained in comparison to the batch experiment (Figs. 2 and 4a), which can be explained due to the addition of higher concentrations of nitrogen in the medium, which are required for the synthesis of the amino acids which make up proteins (Colla et al. 2007).
However, in all the experiments, the prevalence of lag phase was observed (Fig. 2), which is a period where there is biomass adaptation to the new environmental conditions in the reactor (Carvalho et al. 2004). This phase was characterized by a decrease in biomass or inappreciable cell growth in the first 2 days of culture followed by growth from the third day.
Cultivation in semi-continuous process
In an attempt to reduce the lag phase with consequent increase in biomass productivity, cultivation tests were performed by semi-continuous cultivation. In this process, after reaching a steady value of maximum biomass concentration (batch process phase), a quantity of medium containing biomass was removed and renewed by a new medium. This operation allowed returning to the starting biomass concentration (300 mg L−1). A possible advantage of this process is the fact that the use of adapted growing cells left in the reactor may help to reduce or even eliminate the lag phase in the subsequent cultivations. It also avoids the “dead time” in the bioreactor, which is characterized by the time the reactor is not being used for microbial cultivation itself.
Initially, in the batch startup phase, all cultivations showed a lag phase for 2 or 3 days, probably because of the initial adjustment of microalgae. However, after the first medium withdrawal and the reset of cultivation in 300 mg L−1 biomass, no more lag phase was observed. The biomass had adapted to the culture conditions, being able to multiply in a much faster rate and reaching maximum biomass concentration in fewer days then when initially inoculated in the reactor (Fig. 3). Not only the high maximum cell concentration (Xm) but also the shorter time for achieving this value was responsible for this high Px value (Fig. 4b). These values follow the same trend of cultivation of A. platensis by semi-continuous cultivation (Bezerra et al. 2011).
In all cultivations, regardless of the type of process, there was a decrease in YX/N with the increase of NaNO3 added (Fig. 5a). In a fed-batch process, there was a more significant decrease in YX/N values when compared with batch and semi-continuous processes with the same amount of nitrogen. The same trend of decreasing YX/N by increasing the amount of nitrogen added to the culture medium had been observed by Carvalho et al. (2004), cultivating A. platensis with ammonium chloride as nitrogen source in an open photobioreactor.
There was a direct relationship between the maximum specific growth rate (μm) and nitrate addition, as can be seen in Fig. 5b. By adding higher concentrations of nitrate up to 20 mM in the medium, it was possible to obtain higher values of μm, since larger amounts of nitrogen source were consumed by microalgae. However, the amount of nitrate added that allowed higher values of μm was in the 20 mM nitrate, and above this concentration, the μm was low (0.374 day−1), as well as the biomass concentration probably, due to the nitrate excess in the medium. This relationship between the concentration of nitrate added in the culture and the values of μm can be clearly demonstrated by a trend line of the values obtained which generated an R2 = 0.988 which shows that the values of both parameters are directly proportional. Through the results in Tables 1 and 2, it can be seen that the use of the semi-continuous processes as well as the addition of 20 mM nitrate gives better results for the cultivation of A. braunii when compared with other processes and with other nitrate concentrations added.
Protein and lipid content of the biomass from the semi-continuous process
There was no significant difference in the amount of lipids produced in all cultivations (Fig. 6), and the average lipid concentration is very close to the values found by Williams and McMillan (1961) who also studied A. braunii and for Ankistrodesmus falcatus, Jayanta et al. (2012). The protein content was significantly influenced by the nitrogen amount of different experiments (Fig. 6). The amount of NaNO3 added can directly affect the content of proteins produced by biomass in culture because it provides nitrogen essential for the synthesis of amino acids for proteins (Becker 1994). The results obtained are also close to those found by Sipaúba-Tavares and Pereira (2008) for Ankistrodesmus gracilis.
Conclusion
The major results of the maximum biomass concentration (Xm) and biomass productivity (Px) were obtained when the culture was subjected to a concentration of 20 mM of NaNO3 employed in all processes, leading to values of 1588 ± 11, 2753 ± 7, and 2399 ± 5 mg L−1 for Xm and 215 ± 2, 351 ± 1, and 525 ± 1 mg L−1 day−1 for Px, respectively. The tests made in the batch process with 30 mM of NaNO3 concentration led to an inhibition of biomass growth, and concentrations lower than 20 mM of NaNO3 were not efficient for allowing high biomass concentrations and productivities. The batch and fed-batch processes led to the lag phase, slowing the overall process time. However, the semi-continuous process could avoid this phase on growth, decreasing the cultivation time and favoring the cultivation process. The type of culturing process employed led to different values in biomass productivity (Px) and nitrogen-biomass conversion factor (YX/N). The highest Px value was 525 ± 1 mg L−1 day−1 obtained in the semi-continuous process with 20 mM NaNO3. The highest values of YX/N were obtained in batch and semi-continuous processes with 2 mM NaNO3, corresponding to 22.5 ± 1.6 and 29.2 ± 0.1 mg mg−1, respectively. It was observed that independent of the type of process, there was a drop in YX/N values with increasing the addition of NaNO3. The maximum specific growth rate (μm) achieved major results in the exponential phase of growth of all the experiments in which higher nitrate concentrations were used, with the highest value of 0.998 day−1. In the semi-continuous process, the protein content increased with the use of higher concentrations of NaNO3 until the 10 mM limit, a condition in which a protein content of 52.7 ± 0.1% was obtained. The lipid content reached values of approximately 38 to 39%, regardless of the amount of NaNO3 used. Based on these values of the kinetic parameters evaluated, the results show that the optimal concentration of NaNO3 to be added to the culture, as well as the most appropriate type of process, was obtained. Both aspects were very important in this work, because through them, we can define the best growing conditions for A. braunii cultivation in a tubular photobioreactor. In addition, new research could be done in the future to complement this work, such as the nutrient dosage in the processed culture media after the reactor cultures so we could know how much was consumed by the microalgae and allowing greater control of the process.
References
AOAC (2007) Official methods of analysis of AOAC international, 18th edn. Association of Official Analytical Chemists, Washington
Becker EW (1988) Micro-algae for human and animal consumption. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University Press, Cambridge, pp 222–256
Becker EW (1994) Microalgae: biotechnology and microbiology. Cambridge University Press, Cambridge
Becker W (2004) Microalgae in human and animal nutrition. In: Richmond A (ed) Handbook of microalgal culture. Biotechnology and Applied Phycology. Blackwell Science, Oxford, pp 312–351
Bezerra RP, Montoya EYO, Sato S, Carvalho JCM, Converti A (2011) Effects of light intensity and dilution rate on the semi continuous cultivation of Arthrospira (Spirulina) platensis. A kinetic Monod-type approach. Bioresour Technol 102:3215–3219
Blair MF, Kokabian B, Gude VG (2014) Light and growth medium effect on Chlorella vulgaris biomass production. J Environ Chem Eng 2:665–674
Borowitzka MA (2013) High-value products from microalgae – their development and commercialization. J Appl Phycol 25:743–756
Brown MR (1991) The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J Exp Mar Biol Ecol 145:79–99
Brown LM, Zeiler KG (1993) Aquatic biomass and carbon dioxide trapping. Energy Convers Manag 34:1005–1013
Campos VB, Barbarino E, Lourenço SO (2010) Growth and chemical composition of ten species of marine microalgae. Ciênc Rural 40:339–347
Carvalho JCM, Francisco FR, Almeida KA, Sato S, Converti A (2004) Cultivation of Spirulina platensis by fed batch process at exponentially-increasing feeding rate of ammonium chloride. J Phycol 40:589–597
Carvalho JCM, Sato S, Converti A, Bezerra RP, Matsudo MC, Vieira DCM, Ferreira LS, Rodrigues MS (2009). Método de aproveitamento de dióxido de carbono e seu uso no cultivo de microrganismos fotossintetizantes. Brazil Patent Application No. 0805123-2, p 82
Chisti Y (2004) Microalgae: our marine forests. Biotechnol Adv 22:565–567
Colla LM, Reinehr CO, Reichert C, Costa JAV (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol 98:1489–1493
Cruz-Martinez LC, Jesus CKC, Matsudo MC, Danesi EDG, Sato S, Carvalho JCM (2015) Growth and composition of Arthrospira (Spirulina) platensis in a tubular photobioreactor using ammonium nitrate as the nitrogen source in a fed-batch process. Braz J Chem Eng 32:347–356
D’Alessandro EB, Filho NRA (2016) Concepts and studies on lipid and pigments of microalgae: a review. Renew Sust Energy Rev 58:832–841
Formighieri C (2015) Solar-to-fuel conversion in algae and cyanobacteria. Springer, Berlin
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus – a potential strain for bio-fuel production. Bioresour Technol 171:367–374
Guillard RR, Rhyter JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Guiry MD (2012) How many species of algae are there? J Phycol 48:1057–1063
Hipkin CR, Syrett PJ (1977) Nitrate reduction by whole cells of Ankistrodesmus braunii and Chlamydomonas reinhardi. New Phytol 79:639–648
Jayanta T, Chandra KM, Chandra GB (2012) Growth, total lipid content and fatty acid profile of a native strain of the freshwater oleaginous microalgae Ankistrodesmus falcatus (Ralf) grown under salt stress condition. Int J Biol Sci 8:27–35
Leduy A, Zajic JE (1973) A geometric approach for differentiation of an experimental function at a point applied to growth and product formation. Biotechnol Bioeng 25:805–810
López CVG, García MCC, Fernández FGA, Bustos CS, Chisti Y, Sevilla JMF (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591
Moheimani NR (2016) Tetraselmis suecica culture for CO2 bioremediation of untreated flue gas from a coal-fired power station. J Appl Phycol 28:2139–2146
Norton TA, Melkonian N, Andersen R (1996) Algal biodiversity. Phycologia 35:308–326
Olguín EJ, Galicia S, Angulo-Guerrero O, Hernández E (2001) The effect of low light flux and nitrogen deficiency on the chemical composition of Spirulina sp. (Arthrospira) grown on digested pig waste. Bioresour Technol 77:19–24
Sassano CEN, Gioielli LA, Ferreira LS, Rodrigues MS, Sato S, Converti A, Carvalho JCM (2010) Evaluation of the composition of continuously-cultivated Arthrospira (Spirulina) platensis biomass using ammonium chloride as nitrogen source. Biomass Bioenergy 34:1732–1738
Sayre R (2010) Microalgae: the potential for carbon capture. Bioscience 60:722–727
Schlösser UG (1982) Sammlung von Algenkulturen. Ber Deut Bot Ges 95:181–276
Sipaúba-Tavares LH, Pereira AML (2008) Large scale laboratory cultures of Ankistrodesmus gracilis (Reisch) Korsikov (Chlorophyta) and Diaphanosoma biergei Korinek, 1981 (Cladocera). Braz J Biol 68:875–883
UTEX (2016) The culture collection of algae at the University of Texas at Austin. http://www.sbs.utexas.edu/utex/. Accessed 13 April 2016
Williams VR, McMillan R (1961) Lipids of Ankistrodesmus braunii. Science 133:459–460
Acknowledgments
The authors acknowledge the support of “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq), São Paulo, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bresaola, M.D., Morocho-Jácome, A.L., Matsudo, M.C. et al. Semi-continuous process as a promising technique in Ankistrodesmus braunii cultivation in photobioreactor. J Appl Phycol 31, 2197–2205 (2019). https://doi.org/10.1007/s10811-019-01774-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01774-0