Abstract
The Norwegian seaweed industry is expanding and there is a need for accurate estimates of protein content of seaweed species from Norwegian waters. A solid method to calculate protein content is through the sum of the proteomic amino acids; however, it can be expensive and beyond the capacities of many laboratories. The most commonly used method to quantify protein is based on the assessment of crude protein from overall nitrogen content, using the traditional nitrogen-to-protein conversion factor of 6.25. However, this approach can be inaccurate when applied to seaweeds, often resulting in an overestimation of their protein content. Specific nitrogen-to-protein conversion factors, calculated from amino acid composition and total nitrogen, give a more reliable protein quantification in seaweeds. However, no such factors are available for species from Norwegian waters. This study was designed to characterize the amino acid composition of 21 seaweed species from Norwegian waters and use the amino acid data to estimate protein contents of the seaweeds. Crude protein analysis (nitrogen × 6.25) was performed and resulted in overestimation (18–44 %) of the protein content compared to the sum of proteomic amino acids. Specific nitrogen-to-protein conversion factors, calculated for each species, ranged from 3.53 ± 0.1 to 5.13 ± 0.1. This study provides nutritional data on Norwegian seaweeds, covering a relatively wide range of species. Moreover, it is the first study to assess nitrogen-to-protein conversion factors on such species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research on seaweeds has increased in Norway over the last few years. The main reason of this interest is the high potential of the seaweed biomass for a wide range of applications (Skjermo et al. 2014). Seaweeds in Norway are investigated as a possible resource for food and feed due to their beneficial nutrient composition (Mahre et al. 2014). Moreover, Norway has a long and productive coastline that promotes the utilization of seaweeds both wild harvested and cultivated, at an industrial scale (Skjermo et al. 2014). Whether the seaweeds are used as food or feed, accurate knowledge of protein quantity and quality is important. However, nutritional data on seaweeds from Norwegian waters are very limited.
Protein quantification in seaweeds has been approached using different methods. A direct way for quantifying protein is based on the sum of the amino acid residues after hydrolysis, often referred to as “true protein” (Heidelbaugh et al. 1975; Aitken et al. 1991; Diniz et al. 2011). The amino acid residues correspond to the actual molecular fraction of the amino acids after the loss of one molecule of H2O (as when copolymerized in polypeptide chains), thus representing the proteomic amino acids (Mossé 1990). The estimation of total protein based on this method, although generally recommended, requires sophisticated equipment and specialized laboratories. Thus, determination of protein concentration is usually based on other, more available, techniques such as: (1) protein extraction and its quantification by colorimetric assays (Lowry et al. 1951, Bradford 1976), or (2) elemental analysis of nitrogen and indirect estimation of crude protein (Kjeldahl 1883). These methods, although relatively fast and inexpensive, can be inaccurate when applied to seaweeds (Lourenço et al. 2002, Barbarino and Lourenço 2005). Protein extraction procedures are not optimal for seaweeds, and chemical features of the algal material often interfere with both protein extraction and the colorimetric assays, leading to a biased and inaccurate quantification of the protein content (Fleurence 1999a, Barbarino and Lourenço 2005). The elemental analysis of total nitrogen does not require extraction of any material, and it is relatively fast and inexpensive. For estimation of protein content via this method, a nitrogen-to-protein (N-Prot) conversion factor of 6.25 (Kjeldahl 1883) is often used as a default factor to deduce protein concentration from total nitrogen content. The use of 6.25 as N-Prot conversion factor is based on two assumptions: (1) the nitrogen content of total protein is 16 %, and (2) protein is the only source of nitrogen in the analyzed tissue and the amount of non-protein nitrogen (NPN) is negligible. Seaweeds, however, contain considerable amounts of NPN; thus, quantification of crude protein, using a N-Prot conversion factor of 6.25, results in an overestimation of the actual protein content of seaweeds (Lourenço et al. 2002, Diniz et al. 2011, Shuuluka et al. 2013). Specific N-Prot factors (other than 6.25) are recommended for a more accurate estimation of protein, when total nitrogen is a proxy for quantification (Sosulski and Imafidon 1990, Salo-väänänen and Koivistoinen 1996, Sriperm et al. 2011), and must be used for correct protein determination of materials with high NPN (Diniz et al. 2011, Shuuluka et al. 2013, Templeton and Laurens 2015). However, only few studies established specific N-Prot conversion factors for seaweeds (Aitken et al. 1991, Lourenço et al. 2002, Diniz et al. 2011, Shuuluka et al. 2013) and, to our knowledge, no such data exist for seaweed species from Norwegian waters. In a recent meta-analysis, Angell et al. (2015) suggested a universal seaweed N-Prot factor of 5 has to be used when calculation of specific N-Prot factors is not available.
The present study aims to characterize amino acid profiles and protein contents of 21 seaweed species from Norwegian waters. Two methods of protein quantification are used: (1) sum of amino acid residues (true protein) and (2) calculation of total nitrogen and indirect estimation of protein content using the default factor of 6.25 (crude protein). We discuss advantages and disadvantages of these methods. We also establish specific N-Prot conversion factors for the species studied, comparing results among species and calculating average group specific N-Prot factors for red, green, and brown algae.
Materials and methods
Sample collection
Seaweeds were collected in October 2014 in the vicinity of Bodø, Northern Norway, between 67.24° and 67.32° N and 14.47° and 14.72° E, in the intertidal or upper subtidal zone. Each sample consisted of pooled material of at least five individuals per species, identified by morphology. The samples were transported moist to the laboratory where they were rinsed in cold freshwater, to remove adhering foreign material such as animals, epiphytes, or debris. The samples were ground to a powder in two steps: first, manually in liquid nitrogen with a mortar and pestle, then, after freeze-drying (FreeZone 18 Liter Console, Labconco, USA), by using a blender (Knife Mill Grindomix GM 100, Retsch, Germany). Powdered samples were stored at −30 °C until further use.
Species identification
Species were identified by DNA sequence comparisons, using gene regions suitable for each group (Table 1). Methods for DNA extraction, amplification, and sequencing followed Heesch et al. (2016). Regions were amplified using published primers: rbcL of Rhodophyta and Pelvetia canaliculata: F8 or F57 and R1150 (Freshwater and Rueness 1994, Mineur et al. 2010), rbcL of Ulva sp.: SHF1 and SHR4 (Heesch et al. 2009), cox1 of Fucales and Chordaria flagelliformis: GazF1 and GazR1 (Saunders 2005), cox1 of Laminariales: GazF2 and GazR2 (Lane et al. 2007), ITS of Fucus spiralis: ITSa and ITSb (Pocock et al. 2004), and LSU of Cladophora rupestris: C'1_mod and D2_rev (Leliaert et al. 2003). New sequences (accession numbers: Table 1) were compared to published sequences by standard nucleotide BLAST (http://www.ncbi.nlm.nih.gov/).
Amino acid analysis
Amino acid analyses were carried out by ultra performance liquid chromatography (UPLC, Waters Acquity UPLC system); freeze-dried powdered samples containing 30–40 mg of protein were hydrolyzed in 6 N HCl at 110 °C for 22 h. Prior to hydrolysis, 3.125 mM Norvaline (Sigma-Aldrich, USA) was added as internal standard, and 0.1 M Dithiothreitol (DTT, Sigma-Aldrich) was added as antioxidant agent to protect methionine from degradation during acid hydrolysis. For a further protective aid, a layer of N2 gas was put into the flasks for 30 s, and the flasks were capped immediately. During acid hydrolysis, tryptophan and cysteine were destroyed. After hydrolysis, the samples were cooled in cold water until room temperature was reached and centrifuged in a vacuum centrifuge to complete dryness. After centrifugation, the residues were diluted in deionized water and filtered through a syringe-driven filter. Prior to the instrumental analysis, a derivatization agent (AccQ.Tag, Waters, USA) was added to each sample. Amino acids were finally analyzed chromatographically in a UPLC System run by Empower chromatography data software. In this study, duplicate samples (n = 2) were submitted to amino acid analysis after considering the standard error (SE%) and the limit of error (L %) estimated from SE%, as described in detail by Cochran and Cox (1953). For example, by using the data provided by Bartolomeo and Maisano (2006), six replicates were estimated, on the basis of an instrumental SE% = 8 and L % = 10, to reach a confidence limit of 95 %. In this study, an instrumental SE% = 3 % (computed from historical data) and L% = 10 % allowed estimating duplicate samples to reach a confidence level of 95 %.
Total nitrogen
Total nitrogen (TN) was analyzed according to the Dumas method (Dumas 1831). Briefly, freeze-dried samples were combusted in a CHNS elemental analyzer (Vario Macro Cube, Elementar Analysensysteme GmbH, Germany), using helium as carrier gas. The instrument was calibrated with EDTA (Leco Corporation, Sweden); sulfanilamide (Alfa Aesar GmbH & Co, Germany) and a standard meat reference material (SMRD 2000, LGC Standards AB, Sweden) were used as control samples. Each sample was analyzed in duplicate and mean values were considered.
Protein content
Crude protein content was quantified as follows:
where TN represents the gram N per 100 g of dry weight.
True protein content was determined as sum of amino acid residues. The amino acid residues (E i ) correspond to proteinogenic amino acids, i.e., the actual molecular fraction of the amino acids after the loss of one molecule of H2O (as when copolymerized in polypeptide chains); thus, they were calculated as follows:
where AAi represents the proportion of the single amino acid (g amino acid per 100 g of dry weight).
From the ratio of the nitrogen retrieved in the amino acids after acid hydrolysis (AAN) to TN, we calculated the percentage of protein nitrogen of each sample. Consequently, we estimated the relative percentage of NPN by subtracting the values of protein nitrogen from 100 %.
Calculation of N-Prot conversion factors
In the present study, we determined specific N-Prot conversion factors (k P ) for each seaweed species, as follows (Mossé 1990):
where E i represents the gram of the single amino acid residue per 100 g of dry weight and TN represents the gram of N per 100 g of dry weight.
Results
Amino acid composition
Data from total amino acid analysis are presented in Tables 2, 3, and 4. Glutamic acid and aspartic acid were the two most abundant amino acids in all species studied. The lowest percentage of glutamic acid (10.5 % of total amino acids) was observed in the red alga Porphyra dioica, while the brown alga Alaria esculenta had the highest percentage (25.8 % of total amino acids). The percentage of aspartic acid ranged from 10 % in the red alga Furcellaria lumbricalis to 16 % in the brown alga Ascophyllum nodosum. In all species analyzed, histidine and methionine were the least abundant amino acids, reaching up to 2.1 and 2.4 % of total amino acids, respectively.
Nitrogen
TN in the seaweeds widely varied among species (Tables 5). The lowest value was found in the brown alga A. nodosum (0.7 % of the dry weight), whereas the red alga P. dioica reached the highest value (5 % of the dry weight). NPN in the seaweeds (Table 5) varied from 22 % in the brown alga Fucus serratus to 45.8 % in P. canaliculata with averaged values of 32.5, 33.8, and 35.6 %, in green, red, and brown algae, respectively.
Protein content
The protein content of the seaweeds is presented as crude protein (TN × 6.25) and true protein (sum of amino acid residues) values, both expressed as percentage of dry weight (Table 6). The protein content of the seaweeds widely varied among the species studied. In general, we observed that brown algae had a lower protein content than red and green algae, regardless of the method used. The lowest value was observed in the brown alga A. nodosum (4.5 % crude protein, 3 % true protein), while the red alga P. dioica had the highest (31 % crude protein, 20.6 % true protein). Notably, when comparing crude protein values with true protein values, high differences emerged in all species studied, by up to 43.5 % in the brown alga P. canaliculata (Table 4).
N-Prot conversion factors
The calculated N-Prot conversion factors ranged from 3.53 ± 0.1 in the brown alga P. canaliculata to 5.13 ± 0.1 in the brown alga F. serratus (Table 6). Mean values for each taxonomic group were 3.99 ± 0.39 for red algae (n = 7), 4.24 ± 0.46 for green algae (n = 3), and 4.17 ± 0.44 for brown algae (n = 11). We calculated an overall average N-Prot factor of 4.12 ± 0.42 (n = 21) for all the seaweed species studied.
Discussion
In this study, we evaluate the amino acid composition of 21 seaweed species common in Norwegian waters. The species show similar amino acid profiles, which are comparable to earlier data on seaweeds collected worldwide (Ramos et al. 2000, Wong and Cheung 2000, Lourenço et al. 2002, Diniz et al. 2011, Mahre et al. 2014). From the amino acid composition of the seaweeds, we calculated true protein contents (as sum of amino acid residues), generally considered to be an accurate method for protein evaluation (Heidelbaugh et al. 1975; Aitken et al. 1991, Salo-väänänen and Koivistoinen 1996; Diniz et al. 2011). Calculating protein content based on elemental nitrogen and a N-Prot factor of 6.25 results in an overestimation of the protein content in seaweeds. From the data on amino acid composition and total nitrogen, we calculate specific N-Prot conversion factors for each sample.
Amino acid composition
All the seaweeds in this study are rich in glutamic and aspartic acids. These acidic amino acids, responsible for the typical umami taste of the seaweeds, reach together up to 23 % of total amino acids in red algae, 28 % in green algae, and 30 % in brown algae. Previous studies have also described higher levels of glutamic and aspartic acids in brown algae than in the other two taxonomic groups (Lourenço et al. 2002, Diniz et al. 2011, Mahre et al. 2014). Average values for individual amino acids are similar among the taxonomic groups, although some differences exist. For example, lysine and arginine are higher in red algae than in green and brown algae, in accordance with previous studies (Ramos et al. 2000; Lourenço et al. 2002; Mahre et al. 2014). A balanced essential amino acid (EEA) profile defines (in part) the quality of a protein. Compared to common protein sources like soybean meal (SBM) and fishmeal (FM), the EAA profiles of the seaweeds in this study show lower levels of histidine and methionine, while levels of arginine and lysine in red algae and leucine, phenylalanine, threonine, tyrosine, and valine in all seaweeds are higher compared to SBM and FM.
Protein content
Protein data on seaweeds from Norwegian waters are scarce and only cover a few species. In the present study, we estimate protein contents of the seaweeds using the sum of the amino acid residues after hydrolysis (true protein). Some amino acids are destroyed during acid hydrolysis (22 h HCl), e.g., the acid-labile amino acids tryptophan (Trp) and cysteine (Cys); however, levels of cysteine in seaweeds appears to be very low (Aitken et al. 1991, Mahre et al. 2014). True protein values vary widely between the species studied, ranging from 3 % in the brown alga A. nodosum to 21 % of the dry weight in the red alga P. dioica. True protein levels found by Mahre et al. (2014) were mostly in line with our data; however, some differences are observed. For example, Mahre et al. (2014) reported four and two times higher protein contents in the green algae C. rupestris (L.) Kützing and Ulva lactuca L, respectively, compared to our study. These differences may be explained by different harvesting periods and locations of the seaweeds between the two studies. Seasonal and geographical variations of the protein content in seaweeds are indeed very common (Aitken et al. 1991; Fleurence 1999a; Galland-Irmouli et al. 1999; Rødde et al. 2004; Khairy and El-Shafay 2013). The average true protein value found in this study (9 % of the dry weight) is similar to the value reported for polar seaweed species (8 % of the dry weight) by Angell et al. (2015). When comparing the protein content among taxonomic groups, the brown algae generally have a lower protein content than both red and green algae. This pattern is well established in the literature on seaweeds worldwide (Angell et al. 2015).
Protein evaluation in seaweeds is often based on analysis of total nitrogen and indirect estimation of crude protein (TN × 6.25). In this study, crude protein values of the seaweeds are much higher than protein contents estimated from the amino acid composition. This overestimation of protein content ranges from 18 to 44 %. These results are in agreement with previous studies in which the protein content of seaweeds has been assessed by using both crude and true protein methods (Aitken et al. 1991, Lourenço et al. 2002, Diniz et al. 2011, Shuuluka et al. 2013). For example, Aitken et al. (1991) found up to 31 % higher crude protein than true protein values in two species of red algae, Pyrophyllon subtumens (J. Agardh ex Laing) W.A.Nelson (as Porphyra subtumens) and Pyropia columbina (Montagne) W.A.Nelson (as Porphyra columbina). The overestimation of the protein content by crude protein found in this study confirms that 6.25 is an inappropriate factor for protein estimation for seaweeds.
N-Prot conversion factors
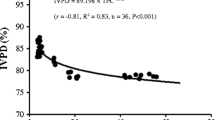
In the present study, we calculate specific N-Prot conversion factors for each seaweed species. In the literature, N-Prot conversion factors have been calculated for different materials using two main methods, based on Mossé (1990). In the first method, a N-Prot conversion factor, referred to as k A, is calculated from the ratio of total amino acid residues to the overall nitrogen content in the amino acid pool after acid hydrolysis (Mossé 1990). This method does not take into account the NPN content of the matrix analyzed. In the second method, a factor referred to as k P is determined from the ratio of amino acid residues to total nitrogen of the sample, each quantified by independent chemical methods (Mossé 1990). Considering that seaweeds typically contain high amounts of NPN, k P is considered to be a better method to calculate specific N-Prot conversion factors for algal samples than k A (Lourenço et al. 2002, Diniz et al. 2011, Templeton and Laurens 2015). In this study, an average of 34 % of total nitrogen is NPN, making the use of k P factors the most suitable for our purpose.
To the best of our knowledge, this is the first study calculating N-Prot conversion factors in Norwegian seaweeds. The k P factors calculated in this study, between 3 and 5, are slightly lower than previous N-Prot factors described for tropical and temperate seaweeds, which ranged between 3.94 and 5.96 (Aitken et al. 1991, Lourenço et al. 2002, Diniz et al. 2011, Shuuluka et al. 2013). Angell et al. (2015), in the framework of a meta-analysis, calculated noticeably lower N-Prot factors in polar seaweeds (mean 3.04), compared to species from temperate and tropical regions. Mean k P factors for red, green, and brown algae were 3.99 ± 0.39, 4.24 ± 0.46, and 4.17 ± 0.44, with an overall mean k P conversion of 4.12 ± 0.42. The k P factors calculated in this work could be applied retrospectively to previous protein data from these species in Norwegian waters. Both tissue nitrogen and amino acid composition of seaweeds can be influenced by the nitrogen content of the environment (Hanisak 1979; Angell et al. 2014), resulting in high individual variations in k P factors (Aitken et al. 1991). For future research, we therefore suggest that sample-specific N-Prot factors are calculated when possible, for a more accurate estimate of protein contents of the seaweeds, especially if the seaweeds are harvested in a different location and/or period of the year.
References
Aitken K, Melton L, Brown M (1991) Seasonal protein variation in the New Zealand seaweeds Porphyra columbina Mont. & Porphyra subtumens J. Ag. (Rhodophyceae). Jap J Phycol 39:307–317
Angell AR, Mata L, de Nys R, Paul NA (2014) Variation in amino acid content and its relationship to nitrogen content and growth rate in Ulva ohnoi (Chlorophyta). J Phycol 50:216–226
Angell AR, Mata L, de Nys R, Paul NA (2015) The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. J Appl Phycol 28:511–524
Barbarino E, Lourenço SO (2005) An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J Appl Phycol 17:477–460
Bartolomeo MP, Maisano F (2006) Validation of a reversed-phase HPLC method for quantitative amino acid analysis. J Biomol Tech 17:131–137
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Cochran WG, Cox GM (1953) Experimental designs. John Wiley & Sons, London
Diniz GS, Barbarino E, Oiano-Neto J, Pacheco S, Lourenço SO (2011) Gross chemical profile and calculation of specific nitrogen-to-protein conversion factors for five tropical seaweeds. Am J Plant Sci 2:287–296
Dumas JP (1831) Lettre de M. Dumas a M. Gay-Lussac, sur le procedes de l’analyse organique. Ann Chim Phys 2:198–215
Fleurence J (1999a) The enzymatic degradation of algal cell walls: a useful approach for improving protein accessibility? J Appl Phycol 11:313–314
Freshwater DW, Rueness J (1994) Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia 33:187–194
Galland-Irmouli AV, Fleurence J, Lamghari R, Luçon M, Rouxel C, Barbaroux O, Bronowicki JP, Vuillaume C, Gueant JL (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10:353–359
Hanisak MD (1979) Nitrogen limitation of Codium fragile ssp. tomentosoides as determined by tissue analysis. Mar Biol 50:333–337
Heesch S, Broom JES, Neill KF, Farr TJ, Dalen JL, Nelson WA (2009) Ulva, Umbraulva and Gemina: genetic survey of New Zealand taxa reveals diversity and introduced species. Eur J Phycol 44:143–154
Heesch S, Pažoutová M, Moniz MBJ, Rindi F (2016) Prasiolales (Trebouxiophyceae, Chlorophyta) of the Svalbard archipelago: diversity, biogeography and description of the new genera Prasionella and Prasionema. Eur J Phycol 51:171–187
Heidelbaugh ND, Huber CS, Bednarczyk JF, Smith MC, Rambaut PC, Wheeler HO (1975) Comparison of the three methods for calculating protein content of foods. J Agr Food Chem 23:611–613
Khairy HM, El-Shafay SM (2013) Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria. Egypt Oceanologia 55:435–452
Kjeldahl J (1883) Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z Anal Chem 22:366–382
Lane CE, Lindstrom S, Saunders GW (2007) A molecular assessment of northeast Pacific Alaria species (Laminariales, Phaeophyceae) with reference to the utility of DNA barcoding. Mol Phylogenet Evol 44:634–648
Leliaert F, Rousseau F, de Reviers B, Coppejans E (2003) Phylogeny of the Cladophorophyceae (Chlorophyta) inferred from partial LSU rRNA gene sequences: is the recognition of a separate order Siphonocladales justified? Eur J Phycol 38:233–246
Lourenço SO, Barbarino E, De-Paula JC, da Pereira LOS, Marquez UML (2002) Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol Res 50:233–241
Lowry OH, Rosebrough NJ, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mahre HK, Malde MK, Eilertsen KE, Elvevoll EO (2014) Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J Sci Food Agric 94:3281–3290
Mineur F, De Clerck O, Le Roux A, Maggs CA, Verlaque M (2010) Polyopes lancifolius (Halymeniales, Rhodophyta), a new component of the Japanese marine flora introduced to Europe. Phycologia 49:86–96
Mossé J (1990) Nitrogen-to-protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and to seed protein content. J Agr Food Chem 38:18–24
Pocock T, Lachance MA, Pröschold T, Priscu JC, Kim SS, Huner NPA (2004) Identification of a psychrophilic green alga from Lake Bonney Antarctica: Chlamydomonas raudensis Ettl. (UWO 241) Chlorophyceae. J Phycol 40:1138–1148
Ramos MV, Monteiro ACO, Moreira RA, Carvalho AFFU (2000) Amino acid composition of some brazilian seaweed species. J Food Biochem 24:33–39
Rødde RSH, Kjell MV, Larsen BA, Sverre MM (2004) Seasonal and geographical variation in the chemical composition of the red alga Palmaria palmata (L.) Kuntze. Bot Mar 47:125–133
Salo-väänänen PP, Koivistoinen PE (1996) Determination of protein in foods: comparison of net protein and crude protein (N × 6.25) values. Food Chem 57:27–31
Saunders GW (2005) Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Phil Trans R Soc B 360:1879–1888
Shuuluka D, Bolton JJ, Anderson RJ (2013) Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. J Appl Phycol 25:677–685
Skjermo J, Aasen IM, Arff J, Broch OJ, Carvajal A, Christie H, Forbord S, Olsen Y, Reitan KI, Rustad T et al (2014) A new Norwegian bioeconomy based on cultivation and processing of seaweeds: opportunities and R&D needs. SINTEF Fisheries and Aquaculture—Report A25981:48
Sosulski FW, Imafidon GI (1990) Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. J Agr Food Chem 38:1351–1356
Sriperm N, Pesti GM, Tillman PB (2011) Evaluation of the fixed nitrogen-to-protein (N:P) conversion factor (6.25) versus ingredient specific N:P conversion factors in feedstuffs. J Sci Food Agric 91:1182–1186
Templeton DW, Laurens LML (2015) Nitrogen-to-protein conversion factors revisited for applications of microalgal biomass conversion to food, feed and fuel. Algal Res 11:359–367
Wong KH, Cheung CK (2000) Nutritional evaluation of some subtropical red and green seaweeds part I: proximate composition, amino acid profiles and some physico-chemical properties. Food Chem 71:475–482
Acknowledgments
This study was supported by the Norwegian Research Council project Aquafly, grant number 238997, and RAFFPINN, grant number 220634. No potential conflict of interest was reported by the author(s).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biancarosa, I., Espe, M., Bruckner, C.G. et al. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J Appl Phycol 29, 1001–1009 (2017). https://doi.org/10.1007/s10811-016-0984-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0984-3