Abstract
This study investigated the changes in lipid and starch contents, lipid fraction, and lipid profile in the nitrogen-starved Scenedesmus obtusus XJ-15 at different temperatures (17, 25, and 33 °C). The optimal temperature for both growth and lipid accumulation under nitrogen-sufficient condition was found to be 25 °C. However, under nitrogen deprivation, the total and neutral lipids increased with increasing temperature, and achieved the highest lipid content of 47.60 % of dry cell weight and the highest TAG content of 79.66 % of total lipid at 33 °C. In the meantime, the stored cellular starch content decreased with the increasing temperature. Thus, high temperature induced carbon flux from starch toward TAG accumulation in microalgae during nitrogen starvation. In addition, the decreased polar lipids may also serve for TAG synthesis under high temperature, and high temperature further reduced the degree of the fatty acid unsaturation and favored a better biodiesel production. These results suggested that high-temperature stress can be a good strategy for enhancing biofuel production in oleaginous microalgae during nitrogen deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid progress in microalgae-derived biofuels has been made on all levels, from identifying high-yield strains of microalgae, to developing new culture systems, and to improving harvest and extraction techniques (Chen et al. 2011). However, bulk production of single-cell microalgae for biofuels is still expensive. The largest constraint is the acquisition of microalgal biomass with high lipid content. Thus, a number of recent studies have focused on maximizing lipid content to reduce costs (Mandal and Mallick 2009; Rodolfi et al. 2009; Delrue et al. 2013; Gardner et al. 2013; Mus et al. 2013; Münkel et al. 2013).
The production of microalgal lipids is often triggered by environmental stress such as nitrogen limitation, high light intensity or extreme temperature (Hu et al. 2008; Li et al. 2008, 2011a; Ho et al. 2012). Nitrogen limitation is the most effective and widely used method for lipid enhancement, especially in green algae. The Chlorophyta, among 11 oleaginous species, showed the greatest increase in lipid content (almost doubled or even more) in response to nitrogen limitation (Griffiths et al. 2011). Besides lipids, starch content also can be promoted under nitrogen deficiency in green algae (Dragone et al. 2011; Li et al. 2011b; Yao et al. 2013; Takeshita et al. 2014). Upon nitrogen starvation, both starch and lipid contents increased greatly within cells, but starch synthesis precedes lipid accumulation in Chlorella zofingiensis and, after 2 days of stress condition, starch was possibly degraded to support lipid synthesis (Zhu et al. 2014). Given that starch synthesis shares common precursors with lipid synthesis, it is possible that lipid and starch could be interconvertible. However, the interaction between starch metabolism and lipid synthesis is poorly understood. Nevertheless, redirecting carbon flux from starch into lipid synthesis has been proposed as a promising strategy to increase oil production in green algae (Li et al. 2011b).
Numerous studies have reported that the lipid content in microalgae can accumulatively increase under conditions of combined nitrogen deficiency with high light (Ho et al. 2012; Münkel et al. 2013), or with high salt (Su et al. 2010; Feng et al. 2014), or at high pH (Gardner et al. 2010; Mus et al. 2013; Xia et al. 2014b). Furthermore, starch degradation is more or less responsible for inducing lipid accumulation under above combined conditions. However, there is little information on lipid production performance under conditions of combined temperature stress and nitrogen deficiency. Temperature has been identified as a major factor affecting the lipid fraction and lipid profile in microalgae (Li et al. 2011a; James et al. 2013; Venkata Subhash et al. 2014). In many microalgae species, a general trend toward a higher degree of fatty acid unsaturation at lower temperatures is observed (Hu et al. 2008). In addition, a high temperature can induce total and neutral lipids accumulation in microalgae (James et al. 2013; Venkata Subhash et al. 2014). Among different oil-rich microalgae, Scenedesmus has been characterized as an important thermo-tolerant genus (Xia et al. 2013). Scenedesmus spp. can grow at temperatures ranging from 4 to 35 °C (Li et al. 2011a; Chen et al. 2012). However, there are few reports focusing on lipid and starch production in Scenedesmus under high temperatures, let alone in combination with nitrogen deprivation.
The main objectives of this study were to determine the general patterns of starch and lipid synthesis in the oleaginous green alga Scenedesmus obtusus XJ-15 and to further elucidate how photosynthetic carbon partitioning into starch and lipid altered by temperature shift in nitrogen-deprived cells. Lipid fraction and profile were also investigated to emphasize the importance of temperature alteration on lipid modification.
Materials and methods
Organism and culture
The freshwater green alga Scenedesmus obtusus XJ-15 used in this study was provided by Prof. Xudong Xu of the Institute of Hydrobiology in China (Xia et al. 2013, 2014a). Stock culture was grown in modified BG-11 medium containing 900 mg NaNO3, 30 mg K2HPO4, 36 mg CaCl2·2H2O, 6 mg ammonium citrate monohydrate, 6 mg ammonium ferric citrate, 1 mg EDTA, 2.86 μg H3BO3, 1.81 μg MnCl2·4H2O, 0.222 μg ZnSO4·7H2O, 0.39 μg NaMoO4·5H2O, 0.079 μg CuSO4·5H2O, and 0.050 μg CoCl2·6H2O in 1 L sterile distilled water. The cultures were maintained at 20 °C with an illumination of 40 μmol photons m−2 s−1.
Before inoculation, the cells were activated by inoculating them into fresh medium. The cultures were carried out at 25 °C under 100 μmol photons m−2 s−1 illumination and were continuously aerated with sterile filtered air. After three times of inoculation, the log phase cells were harvested by centrifugation and washed twice with fresh N-free BG-11 medium. Batch cultures were started by inoculating 0.2 g L−1 washed cells into 500-mL Erlenmeyer flasks filled with 300 mL of BG-11 medium with or without 0.9 g L−1 sodium nitrate and cultured under 17, 25, and 33 °C, and 100 μmol photons m−2 s−1 in temperature-controlled incubators with continuous aeration.
Analytical methods
The dry weight of algal biomass was determined gravimetrically, and growth was expressed in terms of dry weight according to Rai et al. (1991). Namely, 20 mL of culture were harvested by centrifugation. The pellets were then washed twice with distilled water, freeze dried (Christ ALPHA 1–2 LD plus, Germany), and weighed. The supernatant was used for measurement of nitrate concentration according to the Chinese state standard testing methods (Monitoring Methods for Water and Wastewater 2002). The cultures lasted for 10 days to just before the nitrate was depleted in the N-replete treatment at 25 °C. The starch and lipid contents were analyzed at the end of the experiments.
For starch determination, 2 mL sample of cells was harvested and first extracted three times with ethanol at 68 °C for 20 min until the extract remained colorless, in order to remove interfering pigments. Then, the starch was extracted by a modified version of the Klein and Betz method (1978). Namely, the pellet was suspended in 0.1 M pH 4.4 acetate buffer and autoclaved at 110 °C for 15 min to solubilize the starch. Next, 1.5 units of amyloglucosidase (Sigma-Aldrich, USA) was added, and the solution was maintained in a water bath at 55 °C for 1 h to hydrolyze starch to glucose. Glucose was determined by a glucose oxidase-peroxide enzyme system (containing 0.1 g L−1 o-dianisidine dihydrochloride, 1000 U L−1 glucose oxidase and 0.1 g L−1 peroxidase) by measuring the absorption at 522 nm (Tang 1999). The glucose content was determined, and the starch was calculated as glucose (mg) × 0.9 (Goñi et al. 1997).
Total lipid content was determined gravimetrically by the modified Bligh and Dyer method using 1:2 chloroform:methanol (Bligh and Dyer 1959). The crude lipids were separated into three fractions: neutral lipid (NL), glycolipids (GL), and phospholipids (PL) by solid-phase extraction (Bellou and Aggelis 2012). In brief, the extracted lipids were dissolved in 1 mL chloroform and fractionated using a glass column (20 mm × 200 mm) containing 4 g silicic acid, activated by heating overnight at 80 °C. The bed height of silicic acid was 40 mm. Successive applications of chloroform (six times the volume), acetone (four times the volume), and methanol (four times the volume) produced fractions containing NL, GL, and PL, respectively. After evaporation of the respective solvent, lipid fractions were weighted and determined as percent of total lipids.
Fatty acids were determined as fatty acid methyl esters (FAMEs) after acidic transesterification of lipids. Namely, about 100 mg of lipid sample was suspended in 1 M H2SO4-methanol (2 mL) in a vial. The vial was flushed with nitrogen to ensure an inert atmosphere before sealing and heated at 100 °C for 1 h in a water bath. After methylation, purified water (0.20 mL) and n-hexane (0.60 mL) were added, the mixture centrifuged, and the top hexane layer containing the FAMEs was collected. FAMEs were analyzed by gas chromatograph mass spectrometry (GC-MS) (Thermo Scientific ITQ 700, USA) equipped with a flame ionization detector (FID) and a fused silica capillary column (60 m × 0.25 mm × 0.25 μm; Agilent Technologies, USA). The injector and detector temperatures were maintained at 270 and 280 °C, respectively, with an oven temperature gradient of 50 to 170 °C at 40 °C min−1 after a 1 min hold time at 50 °C, then with an oven temperature gradient of 170 to 210 °C at 18 °C min−1 after a 1 min hold. The fatty acids were identified by comparing the retention times with those of standard fatty acids (Supelco 37 component FAME mix, Sigma-Aldrich, USA), and quantified by comparing their peak area with that of the internal standard (C17:0) (Chi et al. 2007).
Statistical analysis
Results are averages of triplicates, and the values in each graph and table are shown with 5 % error bars. ANOVA was performed using SPSS 18.0 package (SPSS, USA), with values of 0.05 selected for significance.
Results
Effect of temperature on growth and carbon fixation under N-replete conditions
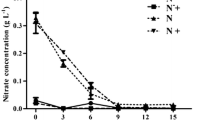
To determine the effect of temperature on growth as well as lipid and starch accumulation in S. obtusus XJ-15, batch cultures were conducted at 17, 25, and 33 °C with an initial nitrate concentration of 0.9 g L−1. S. obtusus XJ-15 grew logarithmically at all temperatures and the maximum growth rate occurred at 25 °C (Fig. 1a). After 10 days cultivation, the biomass concentration increased to 1.45 g L−1 at 25 °C (Fig. 1a, Table 1), followed by 17 and 33 °C which showed no significant differences (p > 0.05). Figure 1b and Table 1 show the lipid and starch contents from 10-day cultures at different temperatures. The highest lipid content was found at 25 °C (33.27 %), followed by 17 °C (27.02 %), and 33 °C (25.49 %). However, the starch content (∼4 % dry cell weight) did not change significantly with temperature (p > 0.05).
Effect of temperature on growth and carbon fixation under N-deficient conditions
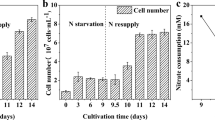
The effect of temperature on the growth and carbon fixation in S. obtusus XJ-15 under nitrogen deprivation was assessed (Fig. 2, Table 1). In N-replete conditions, the N-deficient cultures grew slower than the N-replete cultures (Fig. 2a), with the highest biomass concentration at 25 °C, as expected (Fig. 2a, p < 0.05). However, the lipid content at day 10 was significantly higher under N-deficient than that under N-replete conditions at all temperatures (Table 1, p < 0.05). Notably, the lipid content significantly increased with increasing temperature from 17 to 33 °C (Fig. 2b, p < 0.05), the highest lipid content of 47.60 % was obtained at 33 °C.
The starch content showed a similar pattern as the lipid content, increasing at all temperatures in N-deprived cultures, and was 3.5-fold higher in N-deficient culture than that in N-replete culture at 17 °C, 2.3-fold at 25 °C, and 1.4-fold at 33 °C (Table 1). However, it should be noted that the starch content decreased as the temperature increased from 17 to 33 °C (Fig. 2b) with the lowest starch content of 6.50 % observed at 33 °C (p < 0.05).
Effect of temperature on lipid fraction and profile under N-replete and N-deficient conditions
After 10 days of culture, the highest triacylglycerol (TAG) content was observed at 25 °C under N-replete conditions (Fig. 3a, Table 2). By contrast, TAG content significantly increased with temperature rise in the N-deficient cultures and the highest TAG content of 79.66 % was observed at 33 °C (Fig. 3b, Table 2, p < 0.05). In addition, polar lipids accumulation was also markedly influenced by the temperature shift, both under N-replete and N-deficient conditions. At 17 °C, GL was largely accumulated in the cells under N-deficient conditions and GL content significantly decreased with increasing temperature (Fig. 3, Table 2, p < 0.05). On the other hand, PL content was significantly lower in N-deficient cultures than in N-replete cultures at all temperatures (Table 2, p < 0.05). Furthermore, the TAG concentration was significantly higher in N-deficient medium than in N-sufficient medium at all temperatures (p < 0.05): in N-deficient medium (0.19 g L−1) was 1.7 times higher than in the N-sufficient medium (0.11 g L−1) at 33 °C.
Table 3 summarizes the lipid composition from 10-day cultures of microalgal cells subjected to N-replete or N-deficient at different temperatures. The monounsaturated fatty acids (MUFA) (mostly C18:1) content was much higher in N-deficient cultures than that in N-replete cultures at all temperatures. By contrast, the polyunsaturated fatty acids (PUFA) (C18:2, C18:3, and C18:4) showed the opposite results. Correspondingly, the saturated fatty acids (SFA) and MUFA contents increased with increasing temperature in both N-replete and N-deficient cultures, especially under nitrogen deficiency.
Discussion
Temperature is an essential factor affecting the algal growth and lipid accumulation (Converti et al. 2009; Li et al. 2011a; Wan et al. 2012). The optimal growth and lipid accumulation temperature for S. obtusus XJ-15 in a nitrogen-sufficient medium were both found at 25 °C. This is consistent with previous studies in Scenedesmus sp. and Chlorella sorokiniana (Li et al. 2011a; Wan et al. 2012). In addition, the total lipid content of 33.27 % at logarithmically growth phase was much higher than most oleaginous strains reported (Mandal and Mallick 2009; Griffiths and Harrison 2009; Nascimento et al. 2013; Pribyl et al. 2012). Besides lipid, starch is the main carbon storage compound in many microalgae and plants. In our strain, S. obtusus XJ-15, the starch content obtained was much lower than in most green microalgae (Dragone et al. 2011; Markou et al. 2012) and was significantly lower than the lipid content (Fig. 1b, Table 1, p < 0.05). Thus, the fixed carbon was largely stored as lipid prior to starch in the nutrient-sufficient cultures of S. obtusus XJ-15.
Under nitrogen deficiency, there was a marked rise in lipid yield compared with nitrogen repletion at the optimal temperate 25 °C (Table 1). High-temperature stress further enhanced the lipid accumulation and the higher the temperature, the higher the lipid content, and the highest lipid content of 47.60 % at the highest temperature 33 °C was much higher than most strains under nitrogen-deprived conditions (Mandal and Mallick 2009; Griffiths et al. 2011, 2014; Pan et al. 2011; Pribyl et al. 2012). Correspondingly, when microalgae are starved of nitrogen, starch is overproduced without it being consumed as a carbon or energy source (Zachleder and Brányiková 2014). The concomitant accumulation of starch and lipids under nitrogen limitation was consistent with that previously reported for Chlamydomonas reinhardtii, Desmodesmus sp. NMX-451 and Neochloris oleoabundans (Wattebled et al. 2003; Hamid Rismani-Yazdi 2012; Xia et al. 2014b). Nevertheless, the starch content was significantly lower than the lipid content in S. obtusus XJ-15 cells (Fig. 2, Table 1, p < 0.05). Moreover, high temperature depressed starch accumulation in nitrate-deficient cells, given that the higher the temperature, the lower the starch content (Table 1). Therefore, high-temperature stress appears to repress starch formation to save energy for lipid accumulation during nitrogen limitation. Using genetic engineering, recent studies have inhibited starch synthesis to overproduce lipid accumulation (Li et al. 2010; James et al. 2013). However, high-temperature stress inducing carbon flux from starch toward lipid accumulation can be a natural and better strategy.
Total lipids are composed of two major groups, polar lipids (mainly phospholipids and glycolipids) and neutral lipids (mainly TAG). Since high temperature shifts carbon flux away from starch synthesis, it is very important to determine how this shift affects carbon partitioning into neutral and polar lipid synthesis (Li et al. 2010) as neutral lipids are the preferred feed stocks for biodiesel production. Neutral lipids showed a significant increase in N-deficient culture compared to N-replete culture, and the high-temperature stress further enhanced TAG content as well as total lipid content. The highest TAG content of 80 % of total lipids, equivalent to 38 % of dry weight, is comparable and even higher than most green algal species subjected to nutrient limitation, such as Chlorella vulgaris, C. zofingiensis, N. oleoabundans, and Scenedesmus obliquus (Breuer et al. 2012, 2013). In addition, our data showed that phospholipid content was much lower in cells under nitrate-sufficient condition than under nitrate-deficient condition at all temperatures (Fig. 3, Table 2), whereas glycolipids content was much higher except at 33 °C. The maintenance of chloroplast-specific glycolipids such as MGDG, may serve for fundamental basal photosynthesis in cells subjected to stress conditions (Guschina and Harwood 2006). Furthermore, under many stress conditions, microalgae undergo rapid degradation of the membrane polar lipids with concomitant accumulation of TAG-enriched lipid bodies (Mus et al. 2013). This may be due to an acyl CoA-independent mechanism for TAG synthesis which uses phospholipids as acyl donors (Hu et al. 2008). Thus, the increase in TAG appears to be more or less from the polar lipids under the combined nitrate deficiency and high-temperature stress condition.
Temperature stress also influences the fatty acid composition of algae (Hu et al. 2008; Sharma et al. 2012), and increasing SFA content with increasing temperature has also been observed in many microalgae and cyanobacteria (Sharma et al. 2012; James et al. 2013; Venkata Subhash et al. 2014). However, MUFA and PUFA were much more affected by nitrogen availability than temperature (Table 3). Nevertheless, the highest temperature of 33 °C provided the highest level of MUFA and SFA concomitantly with the lowest levels of PUFA, which is a favorable fatty acid profile for biodiesel production (Knothe 2011). This highlights that high-temperature stress helps to improve fatty acid composition for better biofuel properties and performance in nitrate-deprived cells.
The highest biofuel potential was observed at a high temperature of 33 °C in nitrogen-deprived S. obtusus XJ-15. Depressed starch accumulation and polar lipids accumulation at high temperature may all save energy for TAG synthesis. Therefore, high temperature can be a very good strategy for enhancing lipid production in nitrogen-deprived microalgal cells.
References
Bellou S, Aggelis G (2012) Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J Biotech 164:318–329
Bligh E, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2013) Effect of light intensity, pH, and temperature on triacylglycerol (TAG) accumulation induced by nitrogen starvation in Scenedesmus obliquus. Bioresour Technol 143:1–9
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Chen Z, Gong Y, Fang X, Hu H (2012) Scenedesmus sp. NJ-1 isolated from Antarctica: a suitable renewable lipid source for biodiesel production. World J Microbiol Biotech 28:3219–3225
Chi Z, Pyle D, Wen Z, Frear C, Chen S (2007) A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem 42:1537–1545
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48:1146–1151
Delrue F, Li-Beisson Y, Setier PA, Sahut C, Roubaud A, Froment AK, Peltier G (2013) Comparison of various microalgae liquid biofuel production pathways based on energetic, economic and environmental criteria. Bioresour Technol 136:205–12
Dragone G, Fernandes BD, Abreu AP, Vicente AA, Teixeira JA (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335
Feng P, Deng Z, Hu Z, Wang Z, Fan L (2014) Characterization of Chlorococcum pamirum as a potential biodiesel feedstock. Bioresour Technol 162:115–122
Gardner R, Peters P, Peyton B, Cooksey KE (2010) Medium pH and nitrate concentration effects on accumulation of triacylglycerol in two members of the chlorophyta. J Appl Phycol 23:1005–1016
Gardner RD, Lohman E, Gerlach R, Cooksey KE, Peyton BM (2013) Comparison of CO2 and bicarbonate as inorganic carbon sources for triacylglycerol and starch accumulation in Chlamydomonas reinhardtii. Biotechnol Bioeng 110:87–96
Goñi I, Garcia-Alonso A, Saura-Calixto F (1997) A starch hydrolysis procedure to estimate glycemic index. Nutr Res 17:427–437
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Griffiths MJ, Hille RP, Harrison STL (2011) Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J Appl Phycol 24:989–1001
Griffiths MJ, van Hille RP, Harrison ST (2014) The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl Microbiol Biotechnol 98:2345–2356
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hamid Rismani-Yazdi BZH, Carol Hsin, Jordan Peccia (2012) Transcriptomic analysis of the oleaginous microalga Neochloris oleoabundans reveals metabolic insights into triacylglyceride accumulation. Biotechnol Biofuels 5:74
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–52
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
James GO, Hocart CH, Hillier W, Price GD, Djordjevic MA (2013) Temperature modulation of fatty acid profiles for biofuel production in nitrogen deprived Chlamydomonas reinhardtii. Bioresour Technol 127:441–447
Klein U, Betz A (1978) Fermentative metabolism of hydrogen-evolving Chlamydomonas moewusii. Plant Physiol 61:953–956
Knothe G (2011) A technical evaluation of biodiesel from vegetable oils vs. algae. Will algae-derived biodiesel perform? Green Chem 13:3048–3065
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Li Y, Han D, Hu G, Sommerfeld M, Hu Q (2010) Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol Bioeng 107:258–268
Li X, Hu HY, Zhang YP (2011a) Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour Technol 102:3098–102
Li Y, Han D, Sommerfeld M, Hu Q (2011b) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–9
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: an overview of the f actors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 96:631–645
Münkel R, Schmid-Staiger U, Werner A, Hirth T (2013) Optimization of outdoor cultivation in flat panel airlift reactors for lipid production by Chlorella vulgaris. Biotechnol Bioeng 110:2882–2893
Mus F, Toussaint JP, Cooksey KE, Fields MW, Gerlach R, Peyton BM, Carlson RP (2013) Physiological and molecular analysis of carbon source supplementation and pH stress-induced lipid accumulation in the marine diatom Phaeodactylum tricornutum. Appl Microbiol Biotechnol 97:3625–42
Nascimento I, Marques S, Cabanelas I, Pereira S, Druzian J, Souza C, Vich D, Carvalho G, Nascimento M (2013) Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res 6:1–13
Pan YY, Wang ST, Chuang LT, Chang YW, Chen CN (2011) Isolation of thermo-tolerant and high lipid content green microalgae: oil accumulation is predominantly controlled by photosystem efficiency during stress treatments in Desmodesmus. Bioresour Technol 102:10510–10517
Pribyl P, Cepak V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561
Rai LC, Mallick N, Singh JB, Kumar HD (1991) Physiological and biochemical characteristics of a copper tolerant and a wild type strain of Anabaena doliolum under copper stress. J Plant Physiol 138:68–74
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–12
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Su CH, Chien LJ, Gomes J, Lin YS, Yu YK, Liou JS, Syu RJ (2010) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Takeshita T, Ota S, Yamazaki T, Hirata A, Zachleder V, Kawano S (2014) Starch and lipid accumulation in eight strains of six Chlorella species under comparatively high light intensity and aeration culture conditions. Bioresour Technol 158:127–134
Tang Z (1999) Experimental handbook of modern plant physiology. Science Press, Beijing, pp 127–128
Venkata Subhash G, Rohit MV, Devi MP, Swamy YV, Venkata Mohan S (2014) Temperature induced stress influence on biodiesel productivity during mixotrophic microalgae cultivation with wastewater. Bioresour Technol 169:789–793
Wan MX, Wang RM, Xia JL, Rosenberg JN, Nie ZY, Kobayashi N, Oyler GA, Betenbaugh MJ (2012) Physiological evaluation of a new Chlorella sorokiniana isolate for its biomass production and lipid accumulation in photoautotrophic and heterotrophic cultures. Biotechnol Bioeng 109:1958–1964
Wattebled F, Ral JP, Dauvillée D, Myers AM, James MG, Schlichting R, Giersch C, Ball SG, D’Hulst C (2003) STA11, a Chlamydomonas reinhardtii locus required for normal starch granule biogenesis, encodes disproportionating enzyme. Further evidence for a function of α-1, 4 glucanotransferases during starch granule biosynthesis in green algae. Plant Physiol 132:137–145
Xia L, Ge H, Zhou X, Zhang D, Hu C (2013) Photoautotrophic outdoor two-stage cultivation for oleaginous microalgae Scenedesmus obtusus XJ-15. Bioresour Technol 144:261–267
Xia L, Song S, He Q, Yang H, Hu C (2014a) Selection of microalgae for biodiesel production in a scalable outdoor photobioreactor in north China. Bioresour Technol 174:274–280
Xia L, Yang H, He Q, Hu C (2014b) Physiological responses of freshwater oleaginous microalgae Desmodesmus sp. NMX451 under nitrogen deficiency and alkaline pH-induced lipid accumulation. J Appl Phycol doi:10.1007/s10811-014-0371-x
Yao CH, Ai JN, Cao XP, Xue S (2013) Characterization of cell growth and starch production in the marine green microalga Tetraselmis subcordiformis under extracellular phosphorus-deprived and sequentially phosphorus-replete conditions. Appl Microbiol Biotechnol 97:6099–6110
Zachleder V, Brányiková I (2014) Starch overproduction by means of algae. In: Bajpai R, Prokop A, Zappi M (eds) Algal biorefineries. Springer, Netherlands, pp 217–240
Zhu S, Huang W, Xu J, Wang Z, Xu J, Yuan Z (2014) Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour Technol 152:292–298
Acknowledgments
This work was financially supported by the National 863 program (2013AA065804), International Partner Program of Innovation Team (Chinese Academy of Sciences), Platform Construction of Oleaginous Microalgae (Institute of Hydrobiology, CAS of China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, L., Song, S. & Hu, C. High temperature enhances lipid accumulation in nitrogen-deprived Scenedesmus obtusus XJ-15. J Appl Phycol 28, 831–837 (2016). https://doi.org/10.1007/s10811-015-0636-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0636-z