Abstract
Hydrothermal gasification is a process which uses any biomass or carbon-containing source as substrate to generate biogas of regenerative energy production. We used microalgae as biomass source and evaluated the potential of using the residual water of the conversion process as recycled nutrient source for cultivation of microalgae. Nutrient recycling was tested by monitoring growth of Acutodesmus obliquus and Chlorella vulgaris on residual water from hydrothermal gasification of A. obliquus. Four different gasification set ups were tested. After the procedure, all obtained liquid nutrient phases contained, beside nutrients, growth-inhibiting substances affecting photosynthetic activity and biomass yield of the two algal species. At least 28 potential toxic substances were found within one of the batches. Phytotoxicity on cellular structure was verified by electron microscopy. The cell form remained intact but cell compartments vanished. C. vulgaris was not able to recover to a vital growing organism during cultivation, whereas A. obliquus was able to restore cell compartments, photosynthetic activity and growth after 3 days of cultivation. A 355-fold dilution, UV treatment for 4 h and activated carbon filtration of the residual water from gasification finally enabled the discharge to support microalgal growth. UV treatment eliminated 23 substances but generated 4 new substances that were not detected before treatment. Activated carbon filtration eliminated 26 substances. Growth of microalgae obtained in the treated residual water was comparable with that in control medium. This study demonstrated the possibility to recover nutrients after the hydrothermal gasification process when the discharge got remediated to restart the value adding chain of microalgae and lower additional nutrient supply for microalgal cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae cultivation has an over 50-year long history (Burlew 1953). In recent years, research about microalgae composition, upscaling, and downstream processing of mass cultures has been conducted (Pulz and Gross 2004). Many companies and research institutions are focusing on the production of algae and their valuable compounds. Algal biotechnology does not only focus on the valuable compounds but tries to implement the algae into bioremediation of wastewaters as well (Mallick 2002; Munoz and Guieysse 2006). The industrialization of use of algae has a high potential since microalgae are fast-growing phototrophic organisms and their important cellular compounds can be manipulated depending on the growth conditions (Liang et al. 2009; Converti et al. 2009) for the purpose of the user. However, most of the algal pilot study cultivation systems lack a proper life cycle analysis (Scott et al. 2010). To compete on the global market, products derived from algae such as pharmaceutical products, cosmetics, food products, biofuels, and many more (Spolaore et al. 2006; Mata et al. 2010) need to be produced by high performance and specialized algal species as well as highly efficient and cost effective cultivation systems (Rodolfi et al. 2009; Li et al. 2011).
As algae are discussed as an energy source, either as biodiesel, biogas (Wiley et al. 2011), or directly used as hydrogen factories (Melis and Happe 2001), mass cultivation of algae is necessary to generate the feedstock. The mass cultivation of algae demands for high amounts of nutrients for growth. This demand can be satisfied by nutrients commonly used for agriculture or by nutrient recycling within the energy developing systems itself. Nutrient recycling would also lower the production costs and contribute to shift towards an economic cultivation of algae. The reduction of added nutrients to the cultivation medium is imperative. There is no doubt that nutrient availability becomes scarce within the next generations if no sustainable changes in agriculture take place. For many farmers in sub-Saharan regions, a profitable access to phosphorus as fertilizer is already limited (Ashley et al. 2011). Not only phosphorus deposits will be limited in the near future but also fertilizing the soils with nitrogen for agricultural needs raises several problems, some being pollution of the air, increase of the potent greenhouse gas N2O in the atmosphere, acidification of soils and water bodies and negative effects on biodiversity (Vitousek et al. 1997). These problems derive from a higher demand of mainly nitrogen and phosphorus for agriculture and industry and the inefficiencies in its use (Galloway et al. 2008). This fact implies a recycling of the nutrients not only because of ecological point of view but also, as mentioned above, because of simple economics.
This study investigates the potential of hydrothermal gasification of microalgae to generate energy-rich gaseous molecules as well as the possibility of reutilizing the recovered nutrients of this process for new microalgae cultivation to enable a cyclic nutrient flow. Low demand for additional fertilizers would be the consequence. Three different processes of hydrothermal conversion can be distinguished, as follows: carbonization, liquefaction, and gasification. These processes differ mainly depending on reactor temperatures and pressures from 170 to 250 °C and 10 to 20 bar up to 600 to 700 °C and 250 to 300 bar. The here used hydrothermal gasification involves the highest temperatures and pressures of the conversion processes. Wet biomass is converted into energetically rich gaseous molecules (Boukis et al. 2005). The macro- and micronutrients can be recovered from the process. Microalgae and other kinds of biomass have already been applied for all conversion processes (Chakinala et al. 2009; Brown et al. 2010; Heilmann et al. 2011). Chlorella vulgaris has been successfully cultivated by Du et al. (2012) in the recycled aqueous phase (AP) from a hydrothermal carbonization process. Similar cultivation success was demonstrated for Desmodesmus sp. cultivated on the AP from hydrothermal liquefaction of the same microalgae species by Garcia Alba et al. (2013). Jena et al. (2011) and Biller et al. (2012) cultivated microalgae on the AP of hydrothermal liquefaction as well, but they point out the need of dilution of the AP because of negative effects on microalgae growth, whereas Garcia Alba et al. (2013) stated that rather micronutrient limitation was the main cause for growth reduction.
The feasibility to use the residual water from hydrothermal gasification of microalgae as nutrient source for algae cultivation was presently studied. Nutrients obtained during different gasification conditions were thereby tested in laboratory on two different algae species, Acutodesmus obliquus and Chlorella vulgaris. The effect of the different batches on the algae was determined by monitoring the changes in growth and photosynthetic activity during cultivation.
Materials and methods
Algal biomass of A. obliquus was generated for the hydrothermal conversion and fed to the reactor in which the hydrothermal gasification took place. During the conversion process, the carbon share of the algae was gasified under supercritical water conditions within the reactor. Gas developed as carbon dioxide (CO2) and energy-rich molecules (CH4/H2), and the nutrients remained in an AP and as salt brine (SB). These nutrients were recovered and fed to the microalgae in a culturing device (Fig. 1). Microalgae cell growth and photosynthetic activity measured as maximum quantum yield (F v/F m) of photosystem II (PSII) were determined over a period of several days to generally asses the possibility of microalgae to grow with AP.
Generation of biomass for the hydrothermal conversion
The strain of the green alga A. obliquus was isolated from open waters in Hamburg (no. 10169, Microalgae and Zygnematophyceae Collection Hamburg). Precultures were cultivated at laboratory scale of 10 L, transferred to a pilot plant in Hamburg-Reitbrook, Germany (Hindersin et al. 2013) to produce higher amounts in flat panel bioreactors outdoor. For further increase of the biomass production, about 100 L of algal suspension was transferred from Reitbrook to the algae house (BIQ) in Hamburg-Wilhelmsburg (Germany) where 129 of flat panel bioreactors are attached to a house façade and cover an area of 200 m2 with a total volume of 4000 L. In certain time intervals, the algae were harvested, frozen and sent to our project partner at the Karlsruhe Institute of Technology (KIT; IKFT, Karlsruhe, Germany) for the hydrothermal gasification.
Hydrothermal gasification
The microalgae cultivated in the BIQ in Hamburg were gasified using four different reactor settings at the IKFT (Table 1). Varying parameters included cell dry weight of the feed (2.5–5 %), flow rate (3.6–4.8 g min−1), duration (8–50 h), and temperature (600–650 °C).
Growth of microalgae with AP
The experiments were conducted using the green algae A. obliquus (no. 10169) and C. vulgaris (no. 10126) from the Microalgae and Zygnematophyceae Collection Hamburg (MZCH-SVCK). In the different APs from the batch experiments at the IKFT and in a control medium, algae were cultivated using the Multi-Cultivator MC 1000-OD (Photon Systems Instruments, Czech Republic). The control was a standard medium at concentrations of 2 g L−1 Flory Basis Fertilizer 1 (Euflor, Germany) and 446 mg N–NH4Cl. The aqueous phase which contained up to 99 % of the total recovered nitrogen as N–NH4 was diluted to the amount of nitrogen as in the standard medium; 2 g L−1 Flory Basis Fertilizer 1 was added for supply of micronutrients. The culturing vessels contained 80 mL of inoculum in a water bath of 30 °C and were irradiated continuously with 25, 50, 75, 100, and 500 μmol photons m−2 s−1 depending on the experimental set up. Cultures were bubbled with CO2-enriched air (4 % (v/v)). The pH was set to 7 and adjusted again to 7 with hydrochloric acid or sodium hydroxide if necessary. The dilution experiments were conducted in 300 mL Erlenmeyer flasks with 100 mL inoculum, cultivated at 20 °C and a light/dark cycle of 16:8 at 100 μmol photons m−2 s−1. Flasks were shaken with 120 rpm. Dilution was achieved by adding standard medium to the AP which was already diluted to get the N amount of the control medium. The experiments concerning the sensitivity of A. obliquus to varying light intensities when incubated with AP, dilution, effects of activated carbon filtration of the AP, chemical analysis of potential toxic compounds, and scanning electron microscopy were conducted using the AP of batch 2. Batch 4 of the conversion process was used to identify the different sensitivity of C. vulgaris and A. obliquus to AP as well as the cellular effect by transmission electron microscopy.
Cell growth
Cell growth was monitored by measuring the optical density (OD) at 750 nm (OD750) and cell dry weight (CDW; g L−1). CDW was determined using increase of weight of pre-dried filters by filtering of 5 mL of culture and subsequent drying. By calculation of an OD-CDW correlation curve (data not shown), growth was displayed for the days of cultivation.
Measurement of the quantum yield (ΦPSII)
This method enables to detect positive or negative effects of the medium composition on photosynthetic activity of the microalgae. Maximum quantum yield (Genty et al. 1989) of the samples was determined every 24 h by fluorescence measurements with a pulse amplitude modulation (PAM) fluorometer (Imaging PAM, Heinz Walz, Germany) using the saturation pulse method and measuring light at high frequency of 400 kHz for detection of the minimum and maximum fluorescence (F o, F m). The ratio of variable to maximal fluorescence F v/F m = (F m − F o)/F m enables the estimation of the photosynthetic efficiency in vivo (Hanelt and Nultsch 1995). Low F v/F m values indicate photoinhibitory stress of the organism (Ögren 1988). Quantum yield of the pre-culture was determined as comparison parameter and subsequent F v/F m determinations were conducted for the samples irradiated with continuous light (all treatments except dilution experiment) every 24 h. The samples of the dilution experiment with a light/dark cycle of 16:8 were measured every 24 h as well after a 8-h light period. A maximum saturation pulse of 8000 μmol photons m−2 s−1 for 600 ms was applied after a dark adaptation period of 5 min. A longer dark adaptation of the samples did not affect the quantum yield as evaluated by prior laboratory experiments.
Scanning electron microscopy
To observe possible morphological changes while treating the algae with AP, scanning electron microscopy (SEM) was conducted. Samples were fixed in 1 % paraformaldehyde and 0.25 % glutaraldehyde, dehydrated with alcohol and dried at the critical point with Balzers CPD 030 Critical Point Dryer (Bal-Tec, Germany). After coating samples with gold using the SCD 050 sputter coater (Bal-Tec), scanning electron micrographs were taken with a Leo 525 SEM (Zeiss, Germany).
Transmission electron microscopy
Structural changes to the inner cell organization were observed via transmission electron microscopy (TEM). The algae were fixed with 2 % glutaraldehyde and postfixed with 1 % osmium tetroxide. The samples were dehydrated through a series of graded acetone concentrations and finally embedded in plastic. Ultrathin sections were stained with uranyl acetate followed by lead citrate. Sections were viewed with a Leo 906 E TEM (Zeiss, Germany).
Total nitrogen and total organic carbon
Total nitrogen (TN) and total organic carbon (TOC) of the UV-degradation and activated carbon filtration experiment was measured via combustion with a total organic carbon analyzer (TOC-Vcpn) as well as a total nitrogen measuring unit (TNM-1) (Shimadzu, Japan,) to determine the loss of amount of carbon and nitrogen after the treatment.
Chemical oxygen demand
Analyzing the chemical oxygen demand (COD) is a tool to analyze the water quality of a sample. This method evaluates the contamination of water by measuring the total concentration of substances that can be oxidized to inorganic final products. In the COD method, the results obtained are recalculated to the oxygen concentration necessary for total oxidation (Pisarevsky et al. 2005). Photometric cuvette tests (LCK 314 and 514) by Hach Lange (Germany) were used.
Phenol index
Phenols are common groundwater contaminants and are known to be in effluents of industrial waste, but the identification of every single phenol is difficult (Licha et al. 2001). The sum parameter phenol was analyzed using the photometric cuvette test LCK345 from Hach Lange (Germany).
Posttreatment of the AP
Remediation of the aqueous phase to minimize the load of harmful substance was done by photo degradation and activated carbon filtration. Photodegradation was achieved using strong UV radiation by the Sunshine Simulator described by Hanelt et al. (2006) (Isitec, Germany).
Five hundred milliliter of the AP was irradiated for 4 h. Evaporated water was replaced with ddH2O. Activated carbon filtration was conducted using a 4–7-μm pore size filter with 50 g of activated carbon. Five hundred milliliter of the AP was filtered four times.
Qualitative assessment of organic compounds
The original AP, photo-degraded and activated carbon filtrated AP were analyzed regarding the organic compounds to identify potential toxic substances. Solid phase extraction (SPE) was conducted using CHROMABON HR-P polypropylene columns (Macherey-Nagel, Germany), for aromatic compounds, phenols from water, notroaromatics from water, pesticides from water, and polycyclic aromatic hydrocarbons from oil. The samples were subjected to analysis by GC. The GC analysis was performed with an Agilent 6890 plus system equipped with a DB-1MS column and an Agilent 5973 detector.
Results
Effects of different APs on growth of A. obliquus
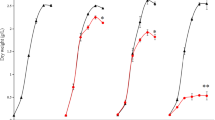
By optimization of the reactor settings at the KIT (Karlsruhe, Germany) (Table 1) the gasification efficiency resulted in maximal conversion efficiency up to 90–98 % (Table 2). Growth of A. obliquus, cultivated with the APs obtained from the different conditions of the hydrothermal conversion, was always inhibited at least for 4 days of cultivation (Fig. 2). However, after this delay algae started to grow while the control had already entered linear growth after 1 day of incubation. Growth measurements were in congruence with measurements of the photosynthetic activity of the microalgae. The precultures were vital and had a photosynthetic efficiency of 0.7. A. obliquus cultivated with AP from batch 1 and 3 displayed no photosynthetic efficiency at days 2 and 3 of cultivation, algae grown in AP of batch 2 had no photosynthetic activity at day 3 and A. obliquus cultivated within AP of batch 4 only at the first day of cultivation. Afterwards, the algae were able to recover from the strong inhibition. At the end, the F v/F m reached almost the values of the control. The photosynthetic efficiency of the control remained more or less constant within a range of 0.55 to 0.7. Cellular growth within the different APs started when the F v/F m passed a threshold of approximately 0.3.
Species-specific sensitivity to different APs
Comparing the reaction of A. obliquus to C. vulgaris, major differences in the species-specific sensitivity to the APs were detected (Fig. 3). A. obliquus was able to grow in the AP batch 3, but start of growth was delayed compared with the control. C. vulgaris behaved differently with respect to growth and photosynthetic efficiency. No growth was observed even after 7 days of cultivation in AP. Despite no visible growth, the photosynthetic efficiency of C. vulgaris fluctuated at low values but was never zero. In the standard medium, cell density of C. vulgaris increased and as well as the F v/F m ratio. The CDW of A. obliquus in AP exceeds that of C. vulgaris after 6 days of cultivation in the standard medium.
Effects of AP on microalgal cell structure
SEM micrographs and thus the outer cell wall of A. obliquus showed no differences of the morphology when exposed to AP compared with that of the control (Fig. 4). However, TEM analysis revealed critical changes of the cellular structures during the growth experiments due to AP compared with the control of the two tested algae (Figs. 5 and 6). The chloroplast membranes are degenerated, and the protoplast is detached from the cell wall. Most of the inner structure vanished and only blurred boundaries between different compartments occurred. In addition, optical inspections by light microscopy revealed that algae turned from green to yellowish-white color while exposed to AP. All changes observed in the fine structure of A. obliquus after exposure to AP have fully recovered until the end of experiments. This was not the case for C. vulgaris.
Transmission electron micrographs of A. obliquus. a Longitudinal section of four intact cells cultivated in standard medium. b The magnified thylakoid membrane of as indicator for a functioning photosynthetic apparatus. c A. obliquus after 1 day of incubation with AP. The sections show still an intact cell wall but no regular inner structures and no thylakoid membranes. d A. obliquus after 7 days of incubation with AP. The sections of A. obliquus show recovered cells with regular cell structure. e The magnified thylakoid membrane as indicator for a functional photosynthetic apparatus
Transmission electron micrographs of C. vulgaris. a Section of an intact cell cultivated in standard medium. b The magnified thylakoid membrane as indicator for a functional photosynthetic apparatus. c C. vulgaris after 6 days of incubation with AP. The section shows still an intact cell wall but no regular inner cellular structure and no intact thylakoid membranes
Effects of light intensity on the growth of A. obliquus in AP
The CDW of the control groups, irradiated with equal light intensities as in the AP experiments correlated to the irradiance (Fig. 7). At the end, CDW is highest with the highest photon flux. The photosynthetic activity showed the same pattern for all four treatments with a final F v/F m of about 0.7. Experiments under dark conditions revealed that light was the main driver for the occurrence of photobleaching of algae incubated with AP (batch 1). When kept the whole time in the dark, the algae remained green. Only during illumination the algae turned yellowish-white (visual observation). Growth of A. obliquus cells in AP at different light intensities occurred as long as the photosynthetic efficiency did not drop below 0.2. For the lowest light intensity (25 μmol photons m-2 s−1) however, the F v/F m declines to 0.3 but remains relatively constant until the end of the experiment, whereas under 50, 75, and 100 μmol photons m−2 s−1, the typical curvature with declining and later on recovering photosynthetic activity values occurred when algae were inoculated with the AP.
Remediation of AP
Different strategies were applied to eliminate the inhibitory effects of AP on microalgal growth: dilution, filtration by activated carbon, and UV treatment. A. obliquus was able to grow on the diluted AP (Fig. 8). Growth occurred at all dilution steps, from 17- to 1778-fold, but differed in final CDW. Obvious differences in final CDW were reached within the 17- and 35-fold dilution (compared with the higher dilutions). Growth stopped after 4 days of cultivation whereas in the other treatments the cell density continued to increase. Growth was in concordance with the measurements of photosynthetic efficiency. F v/F m declined constantly to zero at day 7 for the 17-fold dilution and at day 8 for the 35-fold dilution. The photosynthetic efficiency of A. obliquus in the 177-fold dilution declined as well over time but not as much as in the higher concentrated samples. Algae grown in the higher dilution as well as the control showed a constant F v/F m, but at the 236-fold dilution had a slightly lower F v/F m.
When AP was treated with activated carbon filtration and UV, changes in pH, phenol index and organic matter were observed (Table 3). The pH remained relatively constant at pH 10 during the filtration steps, but decreased to 8.5 after UV treatment. The phenol index decreased with filtration below 2 % of the untreated AP and to 42 % during the UV treatment. Likewise, the total carbon content showed a comparable decrease for the carbon filtrate and UV treatment (82 and 85 %, respectively). Total nitrogen however, had a 13 % decrease for the filtrate and a 80 % decrease for the UV treatment. The chemical oxygen demand was lowered by about 67 % for the filtrated sample and 53 % for the UV-treated sample. Twenty-eight organic compounds were found in the original AP (Table 4). The qualitative assessment of the compounds revealed that 23 of the 28 potential toxic substances could be eliminated with UV light and 26 by activated carbon filtration. Phenol was not removed by UV light nor filtration. Four new substances were generated during UV treatment.
Toxicity of AP was significantly reduced by both treatment methods, as the biological growth test showed and growth started after exposure without any delay (Fig. 9). Yet again, A. obliquus showed no growth in the untreated AP sample and a declining photosynthetic activity. Cell density of the microalgae increased in the two treated samples similar to the control sample. Photosynthetic efficiency of the algae in these three samples was almost identical and increased slightly from a starting F v/F m of 0.59 over the time course of the experiment.
Discussion
In this study, the aqueous phase after hydrothermal gasification of A. obliquus was tested for use as a nutrient source for microalgal growth to reduce use of fertilizers. This would lower the production costs of microalgal mass culture and also minimize environmental exploitation.
Four different aqueous phases were studied obtained from different gasification temperatures varying between 600–650 °C and a pressure of 280 bar. These process conditions were similar to those generally given for gasification in literature (Peterson et al. 2008) and the aqueous phases tested herein can thus be regarded as representative to those generally obtained during gasification of organic matter. However, gasification efficiencies of up to 98 % were reached when material was gasified at a temperature of 600 °C and a dry matter content of 5 % was much higher than the 82 % achieved by Chakinala et al. (2009) at a 7.3 % feed and 700 °C for C. vulgaris. The application as a nutrient source is unfortunately limited to N, because the aqueous phases under study contained high nitrogen concentrations in a range of 2170 to 3200 mg L−1 but only 1.1 to 3.0 mg L−1 of phosphorus. The low concentrations of P made it necessary to supply additional nutrients to the APs for cultivation. For a more efficient reuse of the nutrients from gasification the remaining salt brine which contains higher concentrations of P has to be used in addition to the aqueous phase.
The aqueous phases from hydrothermal gasification always produced an immediate effect on inner algal cell structure, cell growth and the physiological status of photosystem II in both illuminated algal species. The cell structure degenerated resulting in destroyed chloroplasts and hence lack of (A. obliquus) or a decreased (C. vulgaris) photosynthetic activity after incubation with AP. After damaging the chloroplasts, the algae lost their ability for photoautotrophic growth. These effects were independent of reaction parameters of the hydrothermal gasification.
Qualitative assessment of potentially toxic compounds by solid phase extraction of the AP revealed 28 organic compounds which may be the cause for the inhibiting/damaging effects. However, there might be many other toxic compounds which were not detected by us (Garcia Alba et al. 2013), because we focused on aromatic compounds, phenols, nitroaromatics, and pesticides from water. Identification of the one toxic compound or the toxic combination of the 28 organic compounds would need a complex analysis which was beyond the scope of this project. Damaging effects of these compounds to the algal cells occurred only during illumination which was observed macro- and microscopically as bleaching of the culture and cells, respectively. Cultures kept in the dark still remained green. Cells were not starved of nutrients but were damaged by light-induced chlorophyll-mediated electron transport, because the toxic substances unfolded their cell damaging properties only during illumination and thus when photosynthetic electron transport has occurred. Two primary sites of herbicide actions in photosynthetic electron transport are known: inhibition of photosystem II electron transport and diversion of electron flow through photosystem I (Fuerst and Norman 1991). Both sites of action eventually lead to peroxidation of membrane lipids which destroys membrane integrity, leading to cellular disorganization and phytotoxicity which was observed for both algae by TEM. Cell structures clearly vanished when incubated with AP. Chloroplasts may produce reactive oxygen species (ROS) and are susceptible to oxidative stress especially under stressful conditions such as exposure to xenobiotics or pollutants and high PAR (Lesser 2006). Photobleaching is accompanied by ROS (He et al. 1997; Sharma et al. 2012). Already toxicants at low light conditions of 25 μmol photons m−2 s−1 affected photosystem II negatively as demonstrated in this study. At this illumination state, the maximum quantum yield of PSII (F v/F m) was lowered from 0.7 to about 0.3.
The differential response of A. obliquus and C. vulgaris to the toxic aqueous phase is in concordance with Rojíčková and Maršálek (1999) who demonstrated different sensitivities of the algae species to several toxicants explained by the morphology, cytology, physiology, and genetics of the organisms. A. obliquus could cope better with AP than C. vulgaris even though both algae strains showed the same pattern of structural damage to the inner cell structure. The cultures of C. vulgaris were always photosynthetic active when incubated with the AP whereas A. obliquus totally lost its photosynthetic activity after the 1–2 days. Decrease of initial maximum efficiency at which light absorbed by PSII is used for reduction of Q A might be the cause for the decrease of the photosynthetic activity below detection limit in A. obliquus, whereas C. vulgaris in fact declined but still stayed photosynthetically active. At the start of the experiment, a higher primary PSII efficiency of A. obliquus and thus a higher potential electron transport rate than observed for C. vulgaris allows more electrons to enter the electron transport chain until reaching the site of action of the herbicide raising the chances of forming 3Chl, leading to 1O2 formation and protein damage (Rutherford and Krieger-Liszkay 2001). A. obliquus displayed linear growth after recovering its photosynthetic activity. Growth occurred only after the algae recovered there photosynthetic activity above a maximum quantum yield of PSII of 0.3 suggesting this value as a threshold for growth. On the contrary, C. vulgaris has never entered linear growth because its photosynthetic efficiency was below 0.3. A possible explanation for the recovery of A. obliquus is that microalgae are known for their bioremediation activity of aromatic and phenolic compounds (Klekner and Kosaric 1992; Semple and Cain 1996; Semple et al. 1999; Pinto et al. 2002) and thus were able to alter their direct environment until growth was possible. A delay of growth was also observed by Papazi and Kotzabasis (2013) who found that phenols are able to inhibit microalgae growth or decelerate growth due to increased energy need for biodegradation of the growth inhibitors. Since only unialgal and not axenic cultures were used, bacteria can also play a role in metabolism of toxicants. The degradation of toxicants by bacteria is known (Tabak et al. 1964) and possibly remediated the AP for microalgal growth. As previously described, light-induced damage to the chloroplasts affects the reaction center with the heterodimer protein D1 and D2, particularly the D1 protein (Aro et al. 1993) resulting in a loss or decrease of photosynthetic activity. Regaining the photosynthetic activity afterwards occurred because photosynthesis has evolved highly specialized repair mechanism that restores the functional status of PSII which includes degradation of photodamaged D1 and de novo D1 biosynthesis and insertion in the thylakoid membrane for a reconstituted D1/D2 heterodimer and thus a reactivation of electron transport in the chloroplast (Melis 1999).
Pretreatment of the AP was necessary to remove or dilute toxic substances under the toxicity threshold to obtain microalgal growth comparable with growth in standard medium as demonstrated by the dilution and treatment experiments. The inhibitory compounds within the AP and eliminated/diluted toxicity after treatment displayed the good properties of algae as indicators for bioactivity of industrial waste (Walsh and Merrill 1984). In this study, the microalgae were not nutrient limited due to adding of fertilizer to supply for micronutrients, a lack of nutrients within the AP was not the cause for reduced growth but their toxic compounds.
Dilution of the AP to reduce toxicity has been tested successfully in other studies as mentioned above to avoid the effects of growth inhibitors. A 1:355 dilution of the AP was necessary to gain growth and photosynthetic activity comparable with the standard medium. Yet, dilution of the AP seems not applicable in a holistic algal mass culture approach combined with hydrothermal conversion. The amount of water necessary for dilution of the APs derived from any kind of conversion process would exceed the original capacity of the culturing device. UV treatment can remediate the toxic compounds in the AP for the algae as well, which was demonstrated by growth and photosynthetic activity. Twenty-three of the 28 found organic compounds were eliminated. Four new organic compounds were found after photodegradation enabled by UV irradiation. UV radiation is known to alter organic molecules by oxidizing or reducing peripheral carbon atoms creating new molecules (Bernstein et al. 1999). However, the amount of nitrogen also decreased by 79.6 % during the UV treatment resulting in a considerable loss in this macronutrient for microalgal cultivation. This probably occurred because during hydrothermal gasification the organic nitrogen was converted to ammonia which is volatile. To rehabilitate the AP for microalgal growth, we suggest treating the aqueous phase with activated carbon. Activated carbon is a common tool to remediate wastewaters (Sánchez-Polo et al. 2006; Snyder et al. 2007; Macova et al. 2010) and after four filtration steps only 13.2 % of nitrogen is lost. These losses can be explained by possible organic nitrogen compounds within the AP which got adsorbed by the activated carbon. Twenty-six of the 28 organic compounds were adsorbed by the activated carbon. The loss of nutrients during filtration decreases the value of AP for microalgae cultivation.
However, activated carbon filtration is superior to the other tested remediation approaches. Dilution simply decreases amount of toxicants below their toxicity threshold, but it is questionable to use such treated AP in outdoor algae mass cultures (e.g., production of food quality products with contaminated AP) and regarding special safety rules while handling the undiluted AP. UV and AC treatment could be directly connected to the hydrothermal gasification apparatus and thus special safety rules need not to be applied. UV irradiation affects only molecules which absorb the radiation and thus not all toxic compounds get inactivated. In addition, there is always the risk of synthesizing new or simply altering toxic compounds as discussed above. Activated carbon filtration adsorbs and thus removes toxicants and the adsorptive capacity of saturated activated carbon can be reactivated. As shown in this study, the loss of nutrients by activated carbon filtration is also lower than with UV irradiation.
It is possible to recycle the remaining nutrients and achieve growth and photosynthetic activity comparable with the control and thus reduce the costs for adding fertilizer. To further optimize the nutrient balance, the salt brine should also be used. Future research should focus on HTC-reactor optimization to minimize or prevent the generation of toxic compounds which would allow use of the by-products of hydrothermal gasification without any treatment directly for microalgal cultivation. This proof-of-concept demonstrates the possibility of microalgae to grow on the aqueous phase of the hydrothermal gasification of the same algae to recycle nutrients and restart the value adding chain.
References
Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. BBA-Bioenerg 1143:113–134
Ashley K, Cordell D, Mavinic D (2011) A brief history of phosphorus: from the philosopher’s stone to nutrient recovery and reuse. Chemosphere 84:737–746
Bernstein MP, Sandford SA, Allamandola LJ, Gillette JS, Clemett SJ, Zare RN (1999) UV irradiation of polycyclic aromatic hydrocarbons in ices: production of alcohols, quinones, and ethers. Science 283:1135–1138
Biller P, Ross AB, Skill SC, Lea-Langton A, Balasundaram B, Hall C, Riley R, Llewellyn CA (2012) Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res 1:70–76
Boukis N, Galla U, D’Jesus P, Müller H, Dinjus E, (2005) Gasification of wet biomass in supercritical water. Results of pilot plant experiments. In: 14th European Biomass Conference, Paris. pp 964–967
Brown TM, Duan P, Savage PE (2010) Hydrothermal liquefaction and gasification of Nannochloropsis sp. Energ Fuel 24:3639–3646
Burlew JS (ed) (1953) Algal culture. From laboratory to pilot plant. Carnegie Institution, Washington
Chakinala AG, Brilman DW, van Swaaij WP, Kersten SR (2009) Catalytic and non-catalytic supercritical water gasification of microalgae and glycerol. Ind Eng Chem Res 49:1113–1122
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48:1146–1151
Du Z, Hu B, Shi A, Ma X, Cheng Y, Chen P, Liu Y, Lin X, Ruan R (2012) Cultivation of a microalga Chlorella vulgaris using recycled aqueous phase nutrients from hydrothermal carbonization process. Bioresour Technol 126:354–357
Fuerst EP, Norman MA (1991) Interactions of herbicides with photosynthetic electron transport. Weed Sci 39:458–464
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Garcia Alba L, Torri C, Fabbri D, Kersten SR, Brilman DW (2013) Microalgae growth on the aqueous phase from hydrothermal liquefaction of the same microalgae. Chem Eng J 228:214–223
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen Subj 990:87–92
Hanelt D, Nultsch W (1995) Field studies of photoinhibition show non-correlations between oxygen and fluorescence measurements in the arctic red alga Palmaria palmata. J Plant Physiol 145:31–38
Hanelt D, Hawes I, Rae R (2006) Reduction of UV-B radiation causes an enhancement of photoinhibition in high light stressed aquatic plants from New Zealand lakes. J Photochem Photobiol B 84:89–102
He JA, Hu YZ, Jiang LJ (1997) Photodynamic action of phycobiliproteins: in situ generation of reactive oxygen species. BBA-Bioenerg 1320:165–174
Heilmann SM, Jader LR, Harned LA, Sadowsky MJ, Schendel FJ, Lefebvre PA, von Keitz MG, Valentas KJ (2011) Hydrothermal carbonization of microalgae II. Fatty acid, char, and algal nutrient products. Appl Energ 88:3286–3290
Hindersin S, Leupold M, Kerner M, Hanelt D (2013) Irradiance optimization of outdoor microalgal cultures using solar tracked photobioreactors. Bioproc Biosyst Eng 36:345–355
Jena U, Vaidyanathan N, Chinnasamy S, Das KC (2011) Evaluation of microalgae cultivation using recovered aqueous co-product from thermochemical liquefaction of algal biomass. Bioresour Technol 102:3380–3387
Klekner V, Kosaric N (1992) Degradation of phenols by algae. Environ Technol 13:493–501
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Li Y, Zhou W, Hu B, Min M, Chen P, Ruan RR (2011) Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: strains screening and significance evaluation of environmental factors. Bioresour Technol 102:10861–10867
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Licha T, Herfort M, Sauter M (2001) Phenolindex–ein sinnvoller Parameter für die Altlastenbewertung? Grundwasser 6:8–14
Macova M, Escher BI, Reungoat J, Carswell S, Chue KL, Keller J, Mueller JF (2010) Monitoring the biological activity of micropollutants during advanced wastewater treatment with ozonation and activated carbon filtration. Water Res 44:477–492
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15:377–390
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Melis A (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4:130–135
Melis A, Happe T (2001) Hydrogen production. Green algae as a source of energy. Plant Physiol 127:740–748
Munoz R, Guieysse B (2006) Algal–bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40:2799–2815
Ögren E (1988) Photoinhibition of photosynthesis in willow leaves under field conditions. Planta 175:229–236
Papazi A, Kotzabasis K (2013) “Rational” management of dichlorophenols biodegradation by the microalga Scenedesmus obliquus. PLoS One 8(4):e61682
Peterson AA, Vogel F, Lachance RP, Fröling M, Antal MJ Jr, Tester JW (2008) Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energ Environ Sci 1:32–65
Pinto G, Pollio A, Previtera L, Temussi F (2002) Biodegradation of phenols by microalgae. Biotechnol Lett 24:2047–2051
Pisarevsky AM, Polozova IP, Hockridge PM (2005) Chemical oxygen demand. Russ J Appl Chem 78:101–107
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotetechnol 65:635–648
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low‐cost photobioreactor. Biotechnol Bioeng 102:100–112
Rojíčková R, Maršálek B (1999) Selection and sensitivity comparisons of algal species for toxicity testing. Chemosphere 38:3329–3338
Rutherford AW, Krieger-Liszkay A (2001) Herbicide-induced oxidative stress in photosystem II. Trends Biochem Sci 26:648–653
Sánchez-Polo M, Salhi E, Rivera-Utrilla J, Von Gunten U (2006) Combination of ozone with activated carbon as an alternative to conventional advanced oxidation processes. Ozone-Sci Eng 28:237–245
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286
Semple KT, Cain RB (1996) Biodegradation of phenols by the alga Ochromonas danica. Appl Environ Microbiol 62:1265–1273
Semple KT, Cain RB, Schmidt S (1999) Biodegradation of aromatic compounds by microalgae. FEMS Microbiol Lett 170:291–300
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. doi:10.1155/2012/217037
Snyder SA, Adham S, Redding AM, Cannon FS, DeCarolis J, Oppenheimer J, Wert EC, Yoon Y (2007) Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 202:156–181
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Tabak HH, Chambers CW, Kabler PW (1964) Microbial metabolism of aromatic compounds I. Decomposition of phenolic compounds and aromatic hydrocarbons by phenol-adapted bacteria. J Bacteriol 87:910–919
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Matson PA, Schindler DW, Schleisinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Walsh GE, Merrill RG (1984) Algal bioassays of industrial and energy process effluents. In: Shubert LE (ed) Algae as ecological indicators. Academic Press, London, pp 329–360
Wiley PE, Campbell JE, McKuin B (2011) Production of biodiesel and biogas from algae: a review of process train options. Water Environ Res 83:326–338
Acknowledgments
Special thanks are dedicated to Mr. Florian Mundt for helping taking samples and to Mrs. Michaela Schafberg for her support analyzing the organic compounds. This study was funded by the German Federal Ministry of Food and Agriculture (KF 22403411).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patzelt, D.J., Hindersin, S., Elsayed, S. et al. Hydrothermal gasification of Acutodesmus obliquus for renewable energy production and nutrient recycling of microalgal mass cultures. J Appl Phycol 27, 2239–2250 (2015). https://doi.org/10.1007/s10811-014-0496-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0496-y