Abstract
There has been a good deal of interest in the potential of marine vegetation as a sink for anthropogenic C emissions (“Blue Carbon”). Marine primary producers contribute at least 50% of the world’s carbon fixation and may account for as much as 71% of all carbon storage. In this paper, we analyse the current rate of harvesting of both commercially grown and wild-grown macroalgae, as well as their capacity for photosynthetically driven CO2 assimilation and growth. We suggest that CO2 acquisition by marine macroalgae can represent a considerable sink for anthropogenic CO2 emissions and that harvesting and appropriate use of macroalgal primary production could play a significant role in C sequestration and amelioration of greenhouse gas emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global environment is going through a period of rapid change, the pace of which is unprecedented in our geological history, and life on the planet is being threatened by elevated temperatures and ocean acidification associated with the release of greenhouse gases. While CO2 levels and global temperatures have both been higher, sometimes much higher, in the geological past than they are at present, it is the current rate of change that will pose problems for biota. It is thus critical for the future of our planet that significant changes are made to our emissions of greenhouse gases, of which CO2 is the greatest contributor at present.
Various solutions to the problem of excess emissions have been proposed, and many countries are making good progress in stabilising or even reducing their CO2 outputs. However, rapid economic growth in developing countries has seen their yearly CO2 emissions continue to rise, and the latest IPCC report suggests that unless major steps are taken, CO2 concentrations in the atmosphere will continue to increase exponentially well into the future (Meehl et al. 2007). It is therefore of paramount importance that all possible steps are taken to reduce our atmospheric CO2 load to sustainable levels if severe damage to ecological function, including food chains and ecosystem services, is to be avoided.
Recently, there has been a good deal of interest in the potential of marine vegetation as a sink for anthropogenic C emissions (“Blue Carbon”—Nellemann et al. 2009). Nellemann et al. (2009) point out that marine primary producers contribute at least 50% of the world’s carbon fixation and may account for as much as 71% of all carbon storage in oceanic sediments. Clearly then, the algae and higher marine plants such as mangroves and seagrasses that comprise the vast majority of oceanic primary producers have the potential to make a real contribution to CO2 removal and carbon storage. In this paper, we have analysed the current rate of harvesting of both commercially grown and wild-grown macroalgae, as well as their capacity for photosynthetically driven CO2 assimilation and growth. We suggest that CO2 acquisition by marine macroalgae can represent a considerable sink for anthropogenic CO2 emissions and that harvesting and appropriate use of macroalgal primary production could play a significant role in C sequestration and amelioration of greenhouse gas emissions.
The nature of the problem: current rates of CO2 emission
We live in an era where atmospheric CO2 levels are rising at a rate unprecedented in geological history. Some 7.2 ± 0.3 Pg C (1 Pg = 1015 g or 1 Gigatonne) are released annually from fossil fuel combustion and cement production, while land-use changes and deforestation release a further 1.6 ± 1 Pg C year−1 (Denman et al. 2007). The oceans have played a role as a major sink for anthropogenic CO2 emissions, accounting for 48% of emissions since the Industrial Revolution (Sabine et al. 2004). Behrenfeld et al. (2002) estimate the annual oceanic sink for CO2 as 2 ± 0.8 Pg C with an additional missing sink element of 1.8 Pg C involving both terrestrial and oceanic elements of the biosphere. Despite the drawdown from these biotic and abiotic activities of the oceans, the atmospheric CO2 pool is currently increasing by ∼4.1 ± 0.1 Pg C year−1 (Denman et al. 2007). This rapid increase in atmospheric CO2 has occurred over the last ∼200 years, from a value of 280 ppm (28 Pa) in 1800 to ∼385 ppm (38.5 Pa) at present. Most of this increase has occurred over the last 100 years (Denman et al. 2007). Forecasts are quite variable, depending on the values used for growth in CO2 emissions in the models used, but the most likely scenario is for a two- to threefold increase in atmospheric CO2 concentration over the next 100 years (Meehl et al. 2007).
Marine productivity and capacity for C drawdown
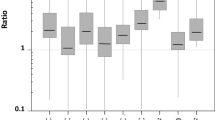
Marine photosynthesis accounts for 50% of the total primary productivity of the planet (54–59 Pg C year−1 from a total of 111–117 Pg C year−1, Beardall and Raven 2004 and references therein). Of this, marine macrophytes (seaweeds and seagrasses) in the coastal regions account for ∼1 Pg C year−1. However, marine macroalgae such as the kelps Macrocystis and Laminaria are capable of very high rates of photosynthesis and productivity of ≥3,000 g C m−2 year−1 (30 × 106 g ha−1 year−1; Jackson 1987; Gao and McKinley 1994; Muraoka 2004). As such, they could potentially make a significant contribution to the annual biological drawdown of CO2 and the global C cycle (Ritschard 1992; Gao and McKinley 1994; Muraoka 2004). Figure 1 shows rates of photosynthesis, on a gram fresh weight basis, for a range of chlorophyte, phaeophyte and rhodophyte algae. A range of other species, not presently cultivated for food and other materials, have high productivities and could also be utilised. Species of genera Sargassum, Ascophyllum and Fucus feature among the highest photosynthetic rates in the phaeophytes, while Porphyra and Palmaria head the rhodophytes with faster photosynthetic rates than the brown algae. In the chlorophytes, simple genera such as Ulva and Enteromorpha also achieve high rates of CO2 assimilation per gram fresh weight.
Rates of primary production rates for selected members of different algal divisions: a Phaeophyta; b Chlorophyta and c Rhodophyta. Only data for species with photosynthetic rates >20 mg CO2 g FW−1 day−1 are shown. Production rates are expressed as mg CO2 gFW−1 day−1. In cases when the original data were not expressed per g FW, ratios from articles in the literature were used to compile a dataset for conversion between FD and DW, and surface area and FW. If data for the appropriate genus were not available, we used the average for the whole group (Chlorophyta, Phaeophyta and Rhodophyta) as a conversion factor. In cases where multiple data for the same species were available, an average of these values was used. References used for calculating conversion factors were: Raven et al. (1989); Beardall and Roberts (1999); Mercado et al. (1998); Johnston et al. (1992) and Raven and Osmond (1992). Sources of data are: 1 Atkinson and Smith (1983), 2 Beardall and Roberts (1999), 3 Brinkhuis (1977), 4 Brown and Tregunna (1967), 5 Dring (1982), 6 Einar and Beer (1993), 7 Fernandez et al. (1990), 8 Fleurence et al. (1994), 9 Gao and Nakahara (1990), 10 Gao and Umezaki (1989a, b, c), 11 Gao et al. (1991), 12 Gao et al. (1993b), 13 Hanisak et al. (1988), 14 Herbert and Waaland (1988), 15 Israel and Hophy (2002), 16 Israel et al. (1999), 17 Johnston et al. (1992), 18 Kremer (1981), 19 Lapointe (1986), 20 Lapointe and Tenore (1981), 21 Levavasseur et al. (1991), 22 Maegawa (1980), 23 Maegawa and Aruga (1983), 24 Middelboe and Hansen (2007), 25 Raven and Osmond (1992), 26 Schaffelke (1999), 27 Titlyanov et al. (2007), 28 Yokohama (1973)
In terms of productivities per unit area substrate, Fig. 2 summarises a range of studies of areal productivity of important phaeophyte and rhodophyte species. A number of species are capable of productivities per unit area substrate in excess of 1,000 g C m−2 year−1. These include Ascophyllum nodosum, Macrocystis integrifolia, Sargassum horneri, Postelsia capillaceae and Ecklonia radiata. Euchema and Gracilaria, among the red algae, are also capable of sustained productivity at this level.
It is informative to compare these rates of productivity with values for terrestrial ecosystems and for crops that have been put forward as possible sources of second generation biofuels. Fig. 3 shows the productivity of selected algae from Fig. 2, expressed as dry wt. ha−1 year−1, compared with estimates for the biomass yield of switchgrass (Panicum virgatus) and Miscanthus (Miscanthus × giganteus), which are frequently cited as potential crops for second generation biofuel, and corn (currently used in a number of countries as a source of bioethanol, Heaton et al. 2008). Clearly the potential annual yields of many of the highly productive algal species are considerably higher than those of the terrestrial plants considered useful candidates for biofuel production, although it should be noted that estimates of areal biomass yield from macroalgae could be influenced by “edge” effects, with the flexibility of macroalgal fronds ascribing a larger surface area than suggested by holdfasts alone. Such effects would be less marked in the more rigid terrestrial species mentioned here.
Current usage of marine macroalgae
The uses of marine macroalgae (seaweeds) are well known. Out of approximately 20,000 known species of seaweed distributed in different parts of the world, only about 221 species are used commercially (Critcheley and Ohno 1998). Many of the species are exploited from their natural habitats as the technology for their cultivation is not yet developed. During the last 50 years, approximately 100 seaweed species have been tested in field farms, but only a dozen are being commercially cultivated (Sahoo and Yarish 2005). Today, around 7.5–8 million tonnes wet weight seaweeds are harvested annually both from wild and cultivated sources. China is the largest producer of seaweeds with 5 million tonnes (wet weight) followed by Korea (800,000 tonnes) and Japan (600,000 tonnes). As detailed by the FAO (2003), although more than a dozen species of macroalgae are cultivated, the bulk of the annual production is attributable to only five genera: Laminaria (4,580,000 tonnes wet weight), Porphyra (1,011,000 metric tonnes wet weight), Undaria (311,105 tonnes wet weight), Eucheuma and Kappaphycus (628,576 tonnes wet weight) and Gracilaria (12,510 tonnes wet weight). While China’s contribution mainly comes from the cultivation of Laminaria japonica, 50% of Korea’s production is contributed by Undaria pinnatifida and 75% of Japan’s harvest is based on the cultivation of Porphyra sp. (Table 1). In addition, countries such as The Philippines, Indonesia, Tanzania and India are involved mainly in the cultivation of Kappaphycus alvarezii and Eucheuma denticulatum (carrageenophytes) as well as Gracilaria species (agarophytes). The growth rate of the various species varies depends on the site of cultivation, the season and the cultivation methodology. For example the daily growth rate of K. alvarezii varies between 3% and 12% and that of Gracilaria spp. between 3.3% and 8.4% depending on various factors.
The above figures give an annual harvest for these species alone of 0.87 × 106 tonnes dry matter. Macroalgae have, on average, 30% carbon so this figure represents 0.26 × 106 tonnes C incorporated into harvested algae annually. Other species such as A. nodosum are also harvested, at rates of ∼5 × 104 tonnes year−1 for alginates and animal fodder (Morand et al. 1991; Moen et al. 1997), so the above figures are a conservative estimate of the potential drawdown of C by macroalgae generally. It should be noted though that these are average figures and achieved yields will be affected by a range of environmental and genetic (strain-specific) factors.
Consideration of the scale of harvesting in the top ten algae-producing countries (Table 2) indicates the extent to which their current harvest might contribute to any offset against their CO2 emissions. These figures exclude calcification, so they do not include for instance the large harvest of maerl in France (Zemke-White and Ohno 1999) which contains a very high level of mineral carbonates. With current levels of harvest photosynthetic incorporation of CO2 into algal biomass represents, for most countries, only a small proportion of C emissions. However, it should be noticed that most countries have a low level of harvest given the extent of their coastlines. If other countries were able to increase their production in line with the values achieved by Korea, then some at least would be in a position where algal utilisation could make significant inroads into their annual C emissions (Table 2). Furthermore, improved production by high producing countries such as China and Korea could enhance drawdown further. Clearly geographic and other constraints will influence the extent to which this can be realised, but it does indicate that there is a significant potential for improved macroalgal based CO2 remediation.
Uses of macroalgae for C sequestration/remediation
Biofuels
Considerable interest and effort has been centred on the possibility of using biofuels (ethanol, biodiesel) as a substitute for fossil fuels. Although the carbon in biofuels is re-released as CO2, it is the anthropogenic release of C from fossil reserves that is largely responsible for the increase in atmospheric CO2 and the ensuing greenhouse effect that we are experiencing. The use of substitutes for fossil fuels thus offers an opportunity to minimise the increase in atmospheric CO2 that has been so evident over the last two centuries or so.
However, although there has been a flurry of activity associated with production of biofuels such as biodiesel from oil bearing plants like oil palm and canola, and bioethanol from sugar cane and corn, it is becoming rapidly apparent that terrestrial production of biofuel has a very significant ecological and social cost and may not compare well with forest restoration as a C mitigation approach (Righelato and Spracklen 2007). Stripping of primary rainforest or savannah and turning arable land over to crops for biofuel can have major impacts on ecosystem health (Tilman et al. 2006; Jordan et al. 2007; Sawyer 2008), biodiversity (Koh 2007) and on the ability of the planet to provide food for humanity (Dalgaard et al. 2006; Fargione et al. 2008). Land clearance changes may also lead to net C release (Searchinger et al. 2008). It must be recognised though that algal harvesting, unless done in a sustainable manner, can also potentially impact on ecosystem functions in coastal areas.
Other benefits of algae for biofuels
While the Asia-Pacific region contributes to nearly 80% of the world’s seaweeds production, most of the value addition takes place in developed countries. Of the 221 species harvested currently, 145 species are used for food and 110 species for phycocolloid production (Table 1) (Zemke-White and Ohno 1999). We argue that while the provision of food from macroalgae is of undoubted importance to some nations’ nutrition and/or economy, conversion of algal carbon into biofuel could represent a more important global contribution in terms of CO2 sequestration, analogous to the concept of carbon credits currently being applied in developed, industrialised countries. While the lipid content of macroalgae is considerably less than that of microalgae, and is usually <7% (see e.g. Fleurence et al. 1994), the content of soluble and structural carbohydrate can be much higher (values of >30% soluble carbohydrate are not uncommon for tropical rhodophytes; Renaud and Luong-Van 2006). Lipid can be directly converted to biodiesel, but the other components such as carbohydrate (and protein) can also be chemically converted to useful fuels, including ethanol, and chemical feed-stocks (Petrus and Noordermeer 2006). By converting algal biomass to useful fuels, we decrease our reliance on fossil fuels for both transport and chemical feedstock.
Pulp
Recent development of a red algal pulp could provide an alternative to the use of trees and will thereby minimise further deforestation (Seo et al. 2010). Thus, the use of algal-based fuels and algal pulp would bypass the critical and ecologically damaging conversion of fossil fuel into atmospheric CO2 and could also play a role in conserving terrestrial forest which plays an important role in the global C cycle.
Climate change
In planning future development of algal-based CO2 sequestration programmes, it will be important to take into account the potential impacts of climate change on growth and production of the algae to be used. It is expected that climate change will have an effect on both macroalgal distribution and biodiversity, but also on their physiology and photosynthetic performance. That, in turn, can change their capacity to sequester CO2. Increased CO2 concentration, in some cases, can increase their capacity to photosynthesize and grow. For instance, it was shown by Gao et al. (1991) that high CO2 concentrations increased growth of the macroalga Porphyra yezoensis both in length and width, but did not change the morphology. Other species are essentially CO2 saturated under present day CO2 levels and are not expected to show increased performance in the future (see Beardall et al. 1998). Differences will be expected between inter-tidal species (where photosynthesis is currently not limited by the availability of inorganic carbon (Ci)), and sub-tidal species that show Ci-limited photosynthesis (Beardall et al. 1998). Differences will also exist between calcifying and non-calcifying species, based on performance of calcifying Corallina pilulifera, whose calcification and growth was inhibited by the drop in pH associated with elevated atmospheric CO2 (Gao et al. 1993a; Gao and Zheng 2010).
Temperature shifts may also affect the ability of macroalgae to perform in particular geographic areas (see e.g. Breeman 1990). Long-term data from the Californian coast, where an increase in 2.2°C was recorded over a period of 60 years, showed a dominance of small turfing species, such as Endocladia muricata, Mastocarpus papillatus, Gelidium coulteri, Rhodoglossum affine and Gigartina canaliculata, over larger, non-turfing species, including Pelvetia fastigata and Fucus distichus, which were still common, but not to their previous extent (Barry et al. 1995).
Another long-term study, based on 10 years of observation in waters off the Californian coast, showed that a great majority of species in the nearshore changed abundance, due to an increase in sea surface temperature (Shiel et al. 2004). Macrocystis species extended its distribution down to Mexico and showed a wide tolerance of temperature, while Cryptopleura could not withstand the change in temperature, but was also adversely affected by changes in light climate that arose because of the increased production of Macrocystis. Other species, such as Mazzaella flaccida and E. muricata completely disappeared from the system, due to the increase in temperature (Shiel et al. 2004).
The disappearance of particular species, due to changes in temperature, might pose another threat via increased grazing pressure (due to “newly open” niches for grazers), which might affect subsequent colonisation of these areas by macroalgae (Shiel et al. 2004).
In species where life cycle is controlled by temperature, changes in temperature might desynchronize reproduction, which can be further translated to the food web. Temperature is critical to the reproductive success of many macroalgae, and a shift in temperature distributions is thus likely to impact strongly on the ability of macroalgae to maintain populations in a given area. Breeman and her co-workers studied temperature dependence of growth and the life history characteristics of a range of species found in the North Atlantic (e.g. Breeman 1990; Pakker and Breeman 1996; Pakker et al. 1996). These studies demonstrated that for some algae even a small change of water temperature could bring about major shifts in distribution. Thus, Breeman (1990) predicted significant changes in community structure associated with the northward shift in the southern boundaries of the major canopy forming species Laminaria hyperborea, L. saccharina and L. digitata. The northward migration of warm to tropical species (which are mainly smaller red, green and brown algae) is not likely to have such a major impact (Beardall et al. 1998).
Any response to UVB levels in macroalgae is going to be species-specific, depending on the presence of UV-absorbing compounds, and their position within a water column. It appears that red macroalgae have higher levels of these compounds, giving them an ecological advantage over the green and brown algae. Thus, latitudinal distribution will play a major role in their resistance to UVB, with higher tolerance in tropical algae, compared to temperate species, due to their evolution in a naturally high UV environment (Diaz-Pulido et al. 2007).
It has been proposed that global warming will lead to greater temperature gradients between land and oceans which will lead to greater storm activity in coastal environments. Both increased storm events and consequent enhanced runoff from land are likely to have impacts on algal growth (Dayton and Tegner 1984; Nielsen 2003; Diaz-Pulido et al. 2007). These effects might be more pronounced in shallow waters, while species such as turf algae and deep-water species with strong holdfasts might show less susceptibility to physical disturbance by wave action and storm events. On the other hand, stronger wave action might help in dispersal, and thus increase their distribution. However, shallow water species might also be strongly affected by the increased runoff, which can bring both increased nutrients, but also high concentration of herbicides and other toxicants. On one side, enhanced nutrient supply might increase the production of species in the shallow water, but algal growth might further be affected by increased herbicides (Diaz-Pulido et al. 2007). Increased storm activity could also physically disrupt algal farming infrastructure.
Social and economic impacts
Future developments in using marine macroalgae for CO2 sequestration will need to take into account a range of environmental, social and economic aspects if it is to be used effectively. However, we are living in a time where we are seeing dramatic changes to our planet and there is an urgent need for action if we are not to reach a tipping point in our ecosystems, and enhanced macroalgal utilisation offers a possible strategy for amelioration. However, carbon sequestered by macroalgae will only be effective in ameliorating CO2 levels if the algal products are used for biofuels or other products that bypass the use of fossil fuels. Use of seaweeds as foodstuffs has no impact on atmospheric CO2, as the carbon ingested is rapidly respired and re-released into the atmosphere. Algal farming can certainly bring benefits to subsistence level communities (Sievannen et al. 2005). Intensification of macroalgal farming has the potential to cause ecological impacts, though, to our knowledge, there have been few studies directly addressing this possibility. As stated earlier, sustainable running of such ‘algal farms’ will require sensible limits to harvesting to ensure supply of a reasonable number of propagules to “re-seed” cut areas and also to retain sufficient biomass to minimise impacts on the ecosystem functions of macroalgal communities such as providing nursery grounds for animals. In one of the few analyses that have been carried out, Bergman et al. (2001) have shown both increases and decreases in fish diversity associated with algal farming in lagoon systems. One useful approach is to introduce integrated aquaculture, where macroalgal culture is used in parallel with aquaculture of fish or shell fish to ameliorate nutrient release into the environment and improve water quality (Chopin et al. 2001; Neori et al. 2004). Such changes in the use of coastal environments will also undoubtedly have impacts on communities who currently use these resources (Sievannen et al. 2005), and this needs to be taken into consideration in planning future developments.
The present work has not considered the economic costs of algal-based C remediation. Gao and McKinley (1994) suggested that, even over a decade ago, the costs of energy generation from algal biomass were promising. With escalating fossil fuel prices, this is more so today than in the past and only likely to improve the economic plausibility of algal bioremediation of CO2. However, a full economic analysis, including all level of infrastructure and transport requirements such as that carried out for terrestrial biofuel by Davis et al. (2009), is required before hard conclusions can be drawn about the economic potential of marine algae for CO2 remediation.
References
Atkinson MJ, Smith SV (1983) C:N:P ratios of benthic marine plants. Limnol Oceanogr 28:568–574
Barry JP, Baxter CH, Sagarin RD, Gilman SE SE (1995) Climate-related, long term faunal changes in a California rocky intertidal community. Science 267:672–675
Beardall J, Raven JA (2004) The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 43:26–40
Beardall J, Roberts S (1999) Inorganic carbon acquisition by two Antarctic macroalgae, Porphyra endiviifolium (Rhodophyta: Bangiales) and Palmaria decipiens (Rhodophyta: Palmariales). Polar Biol 21:310–315
Beardall J, Beer S, Raven JA (1998) Biodiversity of marine plants in an era of climate change: some predictions on the basis of physiological performance. Bot Mar 41:113–123
Behrenfeld MJ, Esaias WE, Turpie KR (2002) Assessment of primary production at the global scale. In: Williams PJ leB, Thomas DN, Reynolds CS (eds) Phytoplankton Productivity. Carbon assimilation in marine and freshwater ecosystems. Blackwell, Oxford, pp 156–186
Bergman KC, Svensson S, Ohman MC (2001) Influence of algal farming on fish assemblages. Mar Pollut Bull 42:1379–1389
Breeman AM (1990) Expected effects of changing seawater temperatures on the geographic distribution of seaweed species. In: Beukema JJ, Wolff WJ, Brouns JJWM (eds) Expected effects of climate change on marine coastal ecosystems. Kluwer, pp 69–76
Brinkhuis BH (1977) Seasonal variations in salt-marsh macroalgae photosynthesis. I. Ascophyllum nodosum ecad scorpioides. Mar Biol 44:165–175
Brown DL, Tregunna EB (1967) Inhibition of respiration during photosynthesis by some algae. Can J Bot 45:1135–1143
Chopin T, Buschmann AH, Halling C, Troell M, Kautsky N, Neori A, Kraemer GP, Zertuche-Gonzalez JA, Yarish C, Neefus C (2001) Integrating seaweeds into marine aquaculture systems: a key toward sustainability. J Phycol 37:975–986
Critcheley AT, Ohno M. (1998) Seaweed resources of the world. Japan International Cooperation Agency. p 431
Dalgaard T, Jørgensen U, Olesen JE, Jensen ES, Kristensen ES, Connor D, Mínguez I, Deluca TH, Koonin SE (2006) Looking at biofuels and bioenergy. Science 312:1743–1744
Davis SC, Anderson-Teixeira KJ, DeLucia EH (2009) Life-cycle analysis and the ecology of biofuels. Trends Plant Sci 14:140–146
Dayton PK, Tegner MJ (1984) Catastrophic storms, El Niño, and patch stability in a Southern California kelp community. Science 224:283–285
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds.) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Diaz-Pulido G, McCook LJ, Larkum AWD, Lotze HK, Raven JA, Schaffelke B, Smith JE, Steneck RS (2007) Vulnerability of macroalgae of the Great Barrier Reef to climate change. In: Marshall P, Johnson J (eds) Climate change and the Great Barrier Reef. Great Barrier Reef Marine Park Authority, Townsville, pp 153–192
Dring MJ (1982) The biology of marine plants. Cambridge University Press
Einar R, Beer S (1993) Photosynthesis in air and in water of Acanthophora najadiformis growing within a narrow zone of the intertidal. Mar Biol 117:133–138
FAO (2003) Guide to the seaweed industry (A). FAO Fisheries Technical Paper No. 441 Rome, p 116
Fargione J, Hill J, Tilman D, Polasky S, Hawthorne P (2008) Land clearing and the biofuel carbon debt. Science 319:1235–1238
Fernandez C, Gutierrez LM, Rico JM (1990) Ecology of Sargassum muticum on the north coast of Spain. Preliminary observations. Bot Mar 33:423–428
Fleurence J, Gutbier G, Mabeau S, Leray C (1994) Fatty acids from 11 marine algae of the French Brittany coast. J Appl Phycol 6:527–532
Gao K, McKinley KR (1994) Use of macroalgae for marine biomass production and CO2 remediation: a review. J Appl Phycol 6:45–60
Gao K, Nakahara H (1990) Effects of nutrients on the photosynthesis of Sargassum thunbergia. Bot Mar 33:375–383
Gao K, Umezaki I (1989a) Comparative studies of photosynthesis in different parts of Sargassum thunbergii. Jpn J Phycol 37:7–16
Gao K, Umezaki I (1989b) Studies on diurnal photosynthetic performance of Sargassum thunbergii I. Changes in photosynthesis under natural sunlight. Jpn J Phycol 37:89–98
Gao K, Umezaki I (1989c) Studies on diurnal photosynthetic performance of Sargassum thunbergii II. Explanation of diurnal photosynthesis patterns from examinations in the laboratory. Jpn J Phycol 37:99–104
Gao K, Zheng Y (2010) Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta). Global Change Biology 16:2388–2398
Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M (1991) Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2 concentrations. J Appl Phycol 3:355–362
Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M (1993a) Calcification in the articulated coralline alga Corallina pilulifera, with special reference to the effect of elevated CO2. Mar Biol 117:129–132
Gao K, Aruga Y, Asada K, Kiyohara M (1993b) Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J Appl Phycol 5:563–571
Hanisak MD, Littler MM, Littler DS (1988) Significance of macroalgal polymorphism: intraspecific tests of the functional- form model. Mar Biol 99:157–165
Heaton EA, Dohleman FG, Long SP (2008) Meeting US biofuel goals with less land: the potential of Miscanthus. Global Change Biology 14:2000–2014
Herbert SK, Waaland JR (1988) Photoinhibition of photosynthesis in a sun and a shade species of the red algal genus Porphyra. Mar Biol 97:1–7
Israel A, Hophy M (2002) Growth, photosynthetic properties and Rubisco activities and amounts of marine macro algae grown under current and elevated seawater CO2 concentrations. Global Change Biology 8:831–840
Israel A, Katz S, Dubinsky Z, Merrill JE, Friedlander M (1999) Photosynthetic inorganic carbon utilization and growth of Porphyra linearis (Rhodophyta). J Appl Phycol 11:447–453
Jackson GA (1987) Modelling the growth and harvest yield of the giant kelp Macrocystis pyrifera. Mar Biol 95:611–624
Johnston AM, Maberly SC, Raven JA (1992) The acquisition of inorganic carbon by four red macroalgae from different habitats. Oecologia 92:317–326
Jordan N, Boody G, Broussard W, Glover JD, Keeney D, McCown BH, McIsaac G, Muller M, Murray H, Neal J, Pansing C, Turner RE, Warner K, Wyse D (2007) Sustainable development of the agricultural bio-economy. Science 316:1570–1571
Koh LP (2007) Can palm oil plantations be made more hospitable for forest butterflies and birds? J Appl Ecol 44:703–713
Kremer BP (1981) Carbon metabolism. In: Lobban CS, Wynne MJ (eds) The biology of seaweeds. Botanical Monographs Vol. 17., pp 493–533
Lapointe BE (1986) Phosphorus-limited photosynthesis and growth of Sargassum natans and Sargassum fluitans (Phaeophyceae) in the western North Atlantic. Deep-Sea Res 33:391–399
Lapointe BE, Tenore KR (1981) Experimental outdoor studies with Ulva fasciata Delile. 1. Interaction of light and nitrogen on nutrient uptake, growth, and biochemical composition. J Exp Mar Biol Ecol 53:135–152
Leigh EG, Paine RT, Quinn JF, Suchanek TH (1987) Wave energy and intertidal productivity. Proc Natl Acad Sci USA 84:1314–1318
Levavasseur G, Edwards GE, Osmond CB, Ramus J (1991) Inorganic carbon limitation of photosynthesis in Ulva rotundata (Chlorophyta). J Phycol 27:667–672
Littler MN, Murray SN (1974) The primary productivity of marine macrophytes from a rocky intertidal community. Mar Biol 27:131–135
Maegawa M (1980) Measurements of photosynthesis and productivity of the cultivated Monostroma population. La Mer 18:116–124
Maegawa M, Aruga Y (1983) Photosynthesis and productivity of the cultivated Monostroma latissimum population. La Mer 21:164–172
Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM,. Noda A, Raper SCB, Watterson IG, Weaver AJ, Zhao Z-C (2007) Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB Tignor M, Miller HL (eds.) Climate change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Mercado JM, Javier F, Gordillo L, Figueroa FL, Niell FX (1998) External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. J Exp Mar Biol Ecol 221:209–220
Middelboe AL, Hansen PJ (2007) Direct effects of pH and inorganic carbon on macroalgal photosynthesis and growth. Mar Biol Res 3:134–144
Moen E, Horn S, Østgaard K (1997) Biological degradation of Ascophyllum nodosum. J Appl Phycol 9: 347–357.
Morand P, Carpentier B, Charlier RH, Maz’e J, Orlandini M, Plunkett BA, de Wart J (1991) Bioconversion of seaweeds. In: Guiry MD, Blunden G (eds) Seaweed resources of Europe: uses and potential. Wiley, Chichester, pp 95–148
Muraoka D (2004) Seaweed resources as a source of carbon fixation. Bull Fish Res Agen Supplement 1:59–63
Nellemann C, Corcoran E, Duarte CM, Valdés L, De Young C, Fonseca L, Grimsditch G (2009) Blue carbon. A rapid response assessment. United Nations Environment Programme, GRID-Arendal, www.grida.no
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231:361–391
Nielsen KJ (2003) Nutrient loading and consumers: agents of change in open-coast macrophyte assemblages. Proc Natl Acad Sci USA 13:7660–7665
Pakker H, Breeman AM (1996) Temperature responses of tropical to warm temperate seaweeds. II Evidence for ecotypic differentiation in amphi-Atlantic tropical-Mediterranean species. Eur J Phycol 31:133–141
Pakker H, Breeman AM, Prud’homme van Reine WF, van den Hoek C (1996) Temperature responses of tropical to warm temperate seaweeds. I. Absence of ecotypic differentiation in amphi-Atlantic tropical-Canary Islands species. Eur J Phycol 31:123–132
Petrus L, Noordermeer M (2006) Biomass to biofuels, a chemical perspective. Green Chem 8:861–867
Raven JA, Osmond CB (1992) Inorganic carbon assimilation processes and their ecological significance in inter- and sub-tidal macroalgae of North Carolina. Funct Ecol 6:41–47
Raven JA, Beardall J, Roberts S (1989) The ecophysiology of inorganic carbon assimilation by Durvillaea potatorum (Durvillaeales, Phaeophyta). Phycologia 28:429–437
Renaud SM, Luong-Van JT (2006) Seasonal variation in the chemical composition of tropical Australia marine macroalgae. J Appl Phycol 18:381–387
Righelato R, Spracklen DV (2007) Carbon mitigation by biofuels or by saving and restoring forests? Science 317:902
Ritschard RL (1992) Marine algae as a CO2 sink. Water Air Soil Pollut 64:289–303
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DWR, Tilbrook B, Millero FJ, Peng T-H, Kozyr A, Ono T, Rios AF (2004) The oceanic sink for anthropogenic CO2. Science 305:367–371
Sahoo D, Yarish C (2005) Mariculture of seaweeds. In: Anderson RA (ed.) Algal culturing techniques. Elsevier, pp 219–237
Sawyer D (2008) Climate change, biofuels and eco-social impacts in the Brazilian Amazon and Cerrado Phil Trans Royal Soc B 363:1747–1752
Schaffelke B (1999) Short term nutrient pulses as tools to assess responses of coral reef macroalgae to enhanced nutrient availability. Mar Ecol Prog Ser 182:305–310
Searchinger T, Heimlich R, Houghton RA, Dong F, Elobeid A, Fabiosa J, Tokgoz S, Hayes D, Yu T-H (2008) Use of U.S. croplands for biofuels increases greenhouse gases through emissions from land-use change science 319:1238–140
Seo Y-B, Lee Y-W, Lee C-H, You H-C (2010) Red algae and their use in papermaking. Bioresour Technol 101:2549–2553
Shiel DR, Steinbeck JR, Foster MS (2004) Ten years of induced ocean warming causes comprehensive changes in marine benthic communities. Ecology 85:1833–1839
Sievannen L, Crawford B, Pollnac R, Lowe C (2005) Weeding through assumptions of livelihood approaches in ICM: seaweed farming in the Philippines and Indonesia. Ocean Coast Manage 48:297–313
Tilman D, Hill J, Lehman C (2006) Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 314:1598–1600
Titlyanov EA, Yakovleva IM, Titlyanova TV (2007) Interaction between benthic algae (Lyngbya bouillonii, Dictyota dichotoma) and scleractinian coral Porites lutea in direct contact. J Exp Mar Biol Ecol 342:282–291
Yokohama Y (1973) A comparative study on photosynthesis temperature relationships and their seasonal changes in marine benthic algae. Int Rev Gesamten Hydrobiol 58:463–472
Zemke-White L, Ohno M (1999) World seaweed utilization: an end of century summary. J Appl Phycol 11:369–376
Acknowledgments
This review is the first activity of the WG-Asian Network of the Asian Pacific Phycological Association and has been supported by a grant ‘Greenhouse Gas Emissions Reduction Using Seaweeds’ Project funded by the Korean Ministry of Land, Transport and Maritime Affairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, I.K., Beardall, J., Mehta, S. et al. Using marine macroalgae for carbon sequestration: a critical appraisal. J Appl Phycol 23, 877–886 (2011). https://doi.org/10.1007/s10811-010-9604-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9604-9