Abstract
Relative polyunsaturated fatty acid content and unsaturation index are very important composition variables in the use of microalgae both for animal and human nutrition and biofuel production. A readily available technique to rapidly and inexpensively estimate relative fatty acid composition is very important for mass screening of new strains for the production of different types of oil. This study demonstrates the validity of Nile Red staining and flow cytometry for quick estimation of unsaturation index and relative fatty acid content in microalgae. Nile Red staining allows polar and neutral lipid contents to be estimated, and in this study a significant correlation was observed between polar/neutral ratio and fatty acid content in the species studied, corresponding to higher polyunsaturated fatty acid content in the polar lipid fraction of microalgae. This technique enables quick estimation of relative polyunsaturated fatty acid content and interspecific variation, as well as variations caused by culture conditions. In the species studied, most variability in fatty acid composition was due to variation in monounsaturated and polyunsaturated fatty acids, with less variation observed in saturated fatty acid content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent interest in the production of microalgae is due to several factors, most of which are associated with the possibility of using microalgae as a source of fatty acids for aquaculture, human nutrition or biofuel production.
Microalgae play a decisive role in aquaculture. It is estimated that 18% of aquaculture species worldwide depend on microalgae at some stage of their development (Muller-Feuga 2000), as fatty acids are essential in the productivity of aquaculture facilities (Wikfors and Ohno 2001). However, the implications of microalgae go beyond the field of aquaculture. They are now regarded as a prime alternative to fish oils in human nutrition as a source of polyunsaturated fatty acids (PUFAs; Patil et al. 2007). Microalgae are not only rich in PUFAs but also have greater lipid stability due to their high content of antioxidant compounds and bioencapsulation of lipids in membrane structures. Moreover, declining captures in the world’s principal fishing grounds and the risk of pollutants have led to a decrease in the use of fish oils in human nutrition, particularly in certain applications subject to high levels of control such as infant formulae preparations (Ward and Singh 2005). This has led to the search for alternatives, in which microalgae have emerged as one of the best options.
Microalgae have optimum content of fatty acids which are highly important in human nutrition, such as γ-linoleic acid, arachidonic acid, docosahexaenoic acid (DHA) and eicosapentanoic acid, whose benefits to human health are widely known. However, the reason why production of oils from microalgae has come to be regarded as a highly strategic activity is found in the numerous studies highlighting microalgae as a promising alternative to traditional biofuel sources, especially biodiesel (Chisti 2007; Li et al. 2008; Hu et al. 2008). Apart from providing a source of high oil content biomass (Gouveia et al. 2009), microalgae culture does not compete with other crops either in terms of fertile land or availability of quality water (unlike traditional energy crops) and is a biomass with very limited use in human nutrition. All of these factors make microalgae an ideal energy crop.
In spite of this, microalgae production is not only still at a very early stage but also makes use of a very limited number of species. Just two microalgae species constitute the base of the PUFA production industry for applications in nutraceuticals: Schizochytrium and Crypthecodinium (Ward and Singh 2005). Little more than 30 species are currently in production in the aquaculture sector (Wikfors and Ohno 2001; Pulz and Cross 2004). This limited number of species in use is also due to the low number of screening studies on new strains with major potential for the production of fatty acids (Griffith and Harrison 2009), despite the high interspecific and intraspecific variability observed in microalgae composition (Patil et al. 2007; Mendoza et al. 2009). Difficulties encountered in screening techniques are in part to blame. The precision required and the number of samples that can be processed are key aspects in screening. In the case of fatty acid composition in microalgae, analysis techniques hinder screening as they are relatively expensive and complicated and require large amounts of biomass to be effective.
Since its development, flow cytometry has been considered the best option for screening studies of single-cell organisms (Davey and Kell 1996). This technique and the equipment it uses not only allow a large number of cell parameters to be checked at one time but also automate cell selection. Flow cytometry has only recently been applied to obtain quick estimates of lipid cell content and select hyperlipogenic species (de la Jara et al. 2003; Gouveia et al. 2009; Mendoza et al. 2009). This type of analysis is performed using the lipid stain Nile Red (NR), which shows varying fluorescence depending on the polarity of the lipids (Alonzo and Mayzaud 1999) and enables neutral and polar lipid cell content in microalgae to be estimated (Mendoza et al. 2009). This study also demonstrates the feasibility of using Nile Red in conjunction with flow cytometry for quick estimation of relative fatty acid content and unsaturation index in microalgae, assessing both intraspecific differences and differences caused by culture conditions. Ability to estimate these variations would be essential in screening studies to select PUFA-rich species for use in aquaculture and human nutrition and also to identify microalgae for biodiesel production, in which fatty acid composition and unsaturation index are important variables.
Materials and methods
Four microalgae species from different collection were cultured: Isochrysis galbana BNA-40-002 and Chlorella pyrenoidosa BNA-10-013, from the official collection of algae BNA, Tetraselmis suecica 03/0203 from the collection of microalgae of ICMAN-CSIC (Spain) and Dunaliella salina ITC-5.003 from the collection of ITC. The first three were cultured in f/2 medium (Guillard 1975) (C. pyrenoidosa in freshwater). Dunaliella salina was cultured in the hypersaline medium of Semenko and Abdullaev (1980), at a salinity of 1 M NaCl. Cultures were bubbled with 3% CO2 in air (v/v) at continuous light (200 µmol photons m−2 s−1) and grown in 500 mL borosilicate flasks containing 500 mL medium at 25 ± 2°C in a programmable temperature chamber. Stock was maintained by weekly subculturing. In all cases, the stock culture was used as inoculum.
Two test culture conditions were defined, with three replicates in each condition for each species: (1) control (maintenance conditions of stock culture) and (2) nitrogen starvation (−N), where successive washing by centrifugation (5 min, 5,000×g) removed all source of nitrogen. The remaining formula of the culture media was kept constant.
Lipid assessment and chromatography analysis
For the analysis of the lipid composition, samples were taken when they had clearly reached exponential growth in the control and had been exposed to nitrogen starvation in the medium for 48 h. Growth phases were determined from variations in cell density estimated by cytometry analysis. Samples were harvested by centrifugation (5 min, 5,000×g, 5°C).
Gravimetric extraction and lipid composition quantification were conducted by the method described by de la Jara et al. (2003). Total lipid content was obtained from a crude extract of each sample. Frozen cells (aliquots of 50–100 μL) were extracted by adding methanol/chloroform (1:2 v/v). After 2-min centrifugation (5 min, 5,000×g, 5°C), the supernatant was collected in a different tube, and a solution of sodium chloride (0.9% w/v) was added in a proportion of 1:5 mL of lipid extract. The mix was vigorously vortexed for approximately 5 min, after which two phases were observed. The supernatant was discarded, as this phase is rich in nonlipid components. The oily phase was recovered as crude lipid extract. Total lipid content was obtained by evaporating the crude lipid extract and oven-drying it at 85°C for 15 min, after which it was precision-weighed. The polar and neutral lipid fractions were separated by absorption chromatography on silica cartridges as described by Juaneda and Rocquelin (1985), according to the modification made by Yongmanitchai and Ward (1992) for separation of lipids in microalgae.
Fatty acid composition, obtained from fresh samples, was estimated following the method of Mendoza et al. (1999). Biomass aliquots were transmethylated with MeOH acetyl chloride. Gas chromatography analysis was performed in a Varian CP-3800 fitted with an FID detector. Fatty acid methyl esters were identified by comparing retention times with the Supelco standard for FAME Mix C4–C24.
Fatty acid composition was analysed not only for each sample but also for the polar and neutral lipid fractions in the cultures under control conditions. During this analysis, the lipid fractions were concentrated by nitrogen evaporation after separation to avoid degradation of fatty acids.
Relative PUFA content is expressed as the ratio between the percentages of the different fatty acids: saturated (SATs), monounsaturated (MUFAs) and PUFAs, using the formula (PUFA/SAT + MUFA) (Guimarães et al. 1991). The unsaturation index was also estimated in accordance with the formula developed by Thi et al. (1987); the percentage of each fatty acid was multiplied by the number of double bonds presents in the molecule.
Flow cytometry analysis
Cells from the different culture conditions were stained with 15 mM Nile Red (Sigma), as described by de la Jara et al. (2003). Flow cytometry was used to determine yellow and red fluorescence of NR-stained cells, cell size (FSC) and complexity (SSC) and deep red autofluorescence due to chlorophyll, using an EPICS XL flow cytometer (Beckman Coulter Instruments) equipped with an air-cooled 488 nm argon-ion laser. The optical system used in the EPICS XL flow cytometer collects yellow light (575 band pass filter) in the FL2 channel and red light (620 band pass filter) in the FL3 channel. These lengths are similar to the fluorescences of optimum emission described for NR in a neutral lipid fraction (580 nm) and a polar lipid fraction (610 nm), although the excitation length is considerably lower (neutral 530 nm, polar 560 nm; Alonso and Mayzaud 1999). Chlorophyll fluorescence was collected in the FL4 channel (675 band pass filter). Cells were gated according to their chlorophyll fluorescence characteristics to remove nonalgal particles. Approximately 3,000 cells were analysed using a log amplification of the fluorescent signals. Equipment was calibrated daily using Flow-Check™ fluorospheres (Beckman Coulter). Unstained cells were used as autofluorescence control. Data were expressed as fluorescence (arbitrary units). The reference data used were the arithmetic mean of the cytometric data of all parameters for all cells analysed (3,000) for each test condition, species and replicate.
Data analysis
In all cases, data represent the mean of the three replicates. Data were analysed for statistical significance using linear regression analysis. The t test was used to establish statistically significant differences for the culture conditions. The statistical significance values chosen were p < 0.05 for the comparison of test conditions and species and p < 0.01 for correlations.
Results
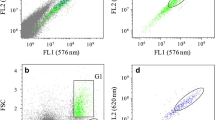
All species responded positively to staining, as there was effective incorporation of Nile Red in the cells (Fig. 1). The coefficient of variation (CV) of FL2 and FL3 signal values in Nile-red-stained cells was low in all species studied (Table 1); in general, the coefficient of variation was not higher on average than 0.097 or 0.060 in FL2 and FL3, respectively (Table 1), and therefore the mean of these parameters was taken as a reference value for all events (3,000 cells analysed) for each cytometric analysis.
A significant correlation was observed (p < 0.01) between variations in the polar/neutral ratio estimated by flow cytometry for Nile-red-stained cells (FL3/FL2) and relative PUFA content and the unsaturation index for all species, culture conditions and replicates (Figs. 2 and 3). The correlation between the polar/neutral ratio and relative fatty acid composition corresponds to the differences observed between the fatty acid content of the polar and neutral lipid fractions in all species, with relative PUFA content and unsaturation index significantly higher in the polar fraction (Fig. 4).
The highest unsaturation index was seen in I. galbana, in both polar and neutral lipids (Fig. 4). Similarly, this specie showed greatest variation in the unsaturation index between the polar and neutral lipid fractions (Fig. 4). The highest relative PUFA content (PUFA/SAT + MUFA) in both lipid fractions was found in C. pyrenoidosa.
In general, in the four species, the main differences between polar and neutral fraction were observed in the PUFA contents whereas the SATs showed less variation between polar and neutral lipids (Table 2); variation in SATs between polar and neutral lipids in D. salina and T. suecica was not detected, while a significant variation in the content of PUFAs and MUFAs in all species was observed. The greatest variations in the fatty acid profile between both fractions of lipids were observed in D. salina and C. pyrenoidosa, with significant differences in the content of most fatty acids. In T. suecica, variation between the two fractions was lower.
16:0 was one of the predominant fatty acids in the composition of all species (Tables 2 and 3), with similar relative content in the neutral and polar lipid fractions; only in C. pyrenoidosa was variation detected in the content of 16:0 (Table 2). In contrast, high variability was observed in the oleic acid content (18:1ω9), in both the cis and trans forms, C. pyrenoidosa was also the exception in this case, presenting similar content of 18:1ω9 in polar and in neutral lipids. This variability was also high in the content of the principal PUFAs between the two lipid fractions of the four species (Table 2).
Higher variability between MUFAs and PUFAs compared to the relative stability of the principal SAT contents also occurred in the variation in the composition of fatty acids in the culture conditions studied. In I. galbana, T. suecica and D. salina, a significant decrease was observed in relative PUFA content and unsaturation index between cultures that had clearly reached exponential growth (control cultures) and cultures exposed to nitrogen starvation (Fig. 5). This variation occurred primarily in relation to variation in MUFA content (which increased in the nitrogen-starved (−N) cultures) and PUFA content, with higher relative content in cultures in the exponential growth phase (control conditions). In contrast, SAT content showed little variation or remained practically stable, particularly in the case of T. suecica, in which a decrease in SAT content was seen in the control, although this was not significant (Fig. 5). By contrast, there were no great differences in the fatty acid composition between the two experimental growth conditions.
Discussion
Earlier studies demonstrated the possibility of using flow cytometry and Nile Red staining for quick estimation of lipid content in microalgae (Cooksey et al. 1987; de la Jara et al. 2003; Mendoza et al. 2009; da Silva et al. 2009). This method makes it possible not only to detect total lipid content but also to quickly determine the ratio of polar/neutral lipids, which earlier studies had associated with relative fatty acid content in microalgae (Roessler 1990; de la Jara et al. 2003). In general, polar lipids, which are associated with membrane structures, have higher PUFA content than other lipids (Roessler 1990), which means that the polar/neutral ratio could be used as an effective indicator of relative PUFA content in microalgae for screening studies, as shown in the present study.
The polar/neutral ratio estimated by cytometry and Nile Red staining has been used to quickly determine variations in relative fatty acid content in microalgae in response to culture conditions (Mendoza et al. 2009). The present study, at least in the case of the species studied, demonstrates the validity of using this indicator to study interspecific differences in fatty acid composition and even to quickly analyse variations in response to culture conditions.
The polar/neutral ratio is closely related to the two basic indicators of relative fatty acid content (unsaturation index and PUFA/MUFA + SAT ratio), which further supports the validity of this ratio as a quick indicator of relative fatty acid content. At present, it is not possible to specifically stain fatty acids to analyse their composition using fluorometry techniques, although analogous fluorescent lipids can be used in studies on metabolism (Bai and Pagano 1997). Although the polar/neutral ratio is an indirect indicator of relative fatty acid composition in microalgae, the ability to quickly estimate this ratio by cytometry on a large number of samples in a short space of time (>3,000 events min−1) and associate analyses with automatic cell sorting makes cytometry and the polar/neutral ratio an optimum method for conducting mass microalgae screening programmes for PUFA production or for other applications, e.g. biodiesel production where parameters such as the unsaturation index are extremely important (Ferrari and Sousa 2009).
In previous works, the inability to use the Nile Red method to quantify cellular polar lipids in microalgae has been indicated because overlap of fluorescent signal of chlorophyll and Nile Red in this lipids exists (Chen et al. 2009). This is true in the case of spectrofluorometry analysis, but in the flow cytometry the overlap of fluorescent is less decisive; since it is possible that an active compensation of the different signals, besides the usual problems of residual fluorescent in the spectrofluorometry analysis, is less important in flow cytometry, the data generated correspond to an aggregate of individual fluorescents signals measured by cells. Another problem associated with the use of Nile Red in quantifying lipid in microalgae is related with the interspecific differences observed in the degree of cell permeation of Nile Red (Chen et al. 2009), but the present results suggest that this does not affect the relative values of fluorescence; the ratio of FL3/FL2 is significantly correlated with the ratio of polar/neutral lipids. However, despite these results, it is necessary to note that the proposed technique is mainly qualitative and that its application is mainly orientated to the work of mass screening of microalgae.
Despite the differences observed in the species studied, most of them follow a similar pattern in the variation of fatty acid content, which agrees with earlier findings for other species. Therefore, in restricted growth conditions such as nitrogen starvation, most species show a decrease in the unsaturation index (Roessler 1990). C. pyrenoidosa was the exception; no significant variation was observed in the content of fatty acids in both experimental conditions; probably, the culture of this species required a more prolonged exposure to the condition of nitrogen starvation to be able to observe significant variations in the fatty acid composition.
Variations in the unsaturation index between the marine and hypersaline species studied occurred primarily in relation to variation in PUFA and MUFA content, whereas SATs showed high stability. This may suggest that PUFA and MUFA mobility in response to variations in culture conditions is more simple or more active, although it is also possible that mobility could be associated with greater impact of nitrogen starvation in the cultures on these fatty acids, which could be linked to the strong effect of nitrogen starvation on the photosynthesis system (Rao and Terry 2000). In this sense, PUFAs are the predominant fatty acids in the composition of structures with chloroplast membrane, and their role in membrane behaviour is decisive (Yokthongwattana et al. 2005; Wu et al. 2009). Earlier studies detected variation patterns in PUFA and MUFA content, particularly in the case of 18:1, in response to nitrogen starvation and activation of photoadaptation strategies (Mendoza et al. 1999).18:1 is the initial fatty acid in −ω3 PUFA synthesis and predominates in the composition of the species studied, in whose synthesis thylakoid membranes play a major role (Mustardy et al. 1996; Andreu et al. 2007). The metabolic relation between MUFAs (primarily oleic acid) and −ω3 PUFAs would explain why the greatest variations in response to nitrogen starvation are detected in the latter. The increase in MUFAs (primarily 18:1) in cultures with reduced nitrogen may correspond to a reduction in the synthesis activity of the PUFAs.
In general, as the results of this study show, fatty acid composition in microalgae has high plasticity. Production can be efficiently altered by modifying culture conditions, to the point that this method can be a decisive factor in producing fatty acids and biodiesel from microalgae (Gouveia et al. 2009). A readily available technique to quickly estimate the lipid composition of microalgae would be a key element in optimising cultures. In this sense, using Nile Red with spectrofluorimetric analysis techniques and relatively simple and inexpensive equipment could constitute an effective alternative to traditional analysis techniques. Moreover, in just a few seconds, cytometry could provide a wide range of information on other essential aspects of culture viability, such as cell integrity, chlorophyll content and the presence of pollutants.
References
Alonzo F, Mayzaud P (1999) Spectrofluorometric quantification of neutral and polar lipids in zooplankton using Nile Red. Mar Chem 67:289–301
Andreu V, Collados R, Testillano PS, Risueño MC, Picorel R, Alfonso M (2007) In situ molecular identification of the pastid ω3 fatty acid desaturase FAD7 from soybean: evidence of thylakoid membrane localization. Plant Physiol 145:1336–1344
Bai J, Pegano RE (1997) Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipids vesicles. Biochemistry 36(29):8840–8848
Chen W, Chengwu Z, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Meth 77:41–47
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Cooksey KE, Guckert JB, Williams GA, Callis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J Microbiol Methods 6:333–346
Da Silva TL, Santos CA, Reis A (2009) Multi-parameter flow cytometry as a tool to monitor heterotrophic microalgal batch fermentations for oil production towards biodiesel. Biotechnol Bioprocess Eng 14(3):330–337
Davey HM, Kell DB (1996) Flow cytometry and cell sorting of heterogeneous microbial populations-the importance of single-cell analyses. Microbioll Rev 60:641–696
de la Jara A, Mendoza H, Martel A, Molina C, Nordströn C, de la Rosa V, Díaz R (2003) Flow cytometric determination of lipid content in marine dinoflagellate Crypthecodinium cohnii. J Appl Phycol 15:433–438
Ferrari RA, de Souza WL (2009) Evaluation of oxidation stability of sunflower oil biodiesel with antioxidants. Quim Nova 32(1):106–111
Gouveia L, Marques AE, da Silva TL, Reis A (2009) Neochloris oleoabundas UTX#1185: a suitable renewable lipid source for biodiesel production. J Ind Microbiol Biotechnol 36:821–826
Griffith MJ, Harrison TL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanle MH (eds) Culture invertebrate animals. Plenum, New York, pp 26–60
Guimarães ARP, Costa Rosa LFBP, Sitnik RH, Curi R (1991) Effect of polyunsaturates (PUFA n-6) and saturated fatty acid-rich diets on macrophage metabolism and function. Biochem Int 23:1739–1751
Hu Q, Sommerfeld M, Jarvis E, Ghirardi E, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Juaneda P, Rocquelin G (1985) Rapid and convenient separation of phospholipids in rat heart using silica cartridges. Lipids 20:40–41
Li Q, Du W, Liu D (2008) Perspective of microbial oils for biodiesel production. Appl Microbiol Biotechnol 80:749–756
Mendoza H, Martel A, Jiménez del Río M, García-Reina G (1999) Oleic acid is the main fatty acid related with carotenogenesis in Dunaliella salina. J Appl Phycol 11:15–19
Mendoza H, de la Jara A, Carmona Duarte L, Freijanes Presmanes K (2009) Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquac Int. doi:10.1007/s10499-008-9235-1
Muller-Feuga A (2000) The role of microalgae in aquaculture: situation and trends. J Appl Phycol 12:527–534
Mustardy L, Los DA, Gombos Z, Murata N (1996) Immunocytochemical localization of acyl-lipid desaturases in cyanobacterial cells: evidence that both thylakoid membranes and cytoplasmic membranes are sites of lipid desaturation. Proc Natl Acad Sci USA 93:10524–10527
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød HR (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac Int 15:1–9
Pulz O, Cross W (2004) Valuable products from biotechnology of microalgae. J Appl Microbiol Biotechnol 65:635–648
Rao IM, Terry N (2000) Photosynthetic adaptation to nutrient stress. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis mechanisms, regulation and adaptation. New York, Taylor & Francis, pp 380–397
Roessler PG (1990) Environmental control of glycerolipids metabolism in microalgae: commercial implications and future research directions. J Phycol 26:393–399
Semenenko VE, Abdullaev AA (1980) Parametric control of β-carotene biosynthesis in Dunaliella salina cells under conditions of intensive cultivation. Sov Plant Physiol 27:22–30
Thi P, Borrel-Flood C, Vieira da Silva J, Justin AM, Mazliak P (1987) Effects on lipid metabolism in cotton leaves. Phytochemistry 24:723–727
Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of productions. Proc Biochem 40:3267–3652
Wikfors GH, Ohno M (2001) Impact of algal research in aquaculture. J Phycol 37:968–974
Wu Q, Liu T, Liu H, Zheng GC (2009) Unsaturated fatty acid: metabolism, synthesis and gene regulation. Afr J Biotechnol 8:1782–1785
Yokthongwattana K, Savchenko T, Polle JEW, Melis A (2005) Isolation and characterization of a xanthophylls-rich fraction from the thylakoid membrane of Dunaliella salina (green algae). Photochem Photobiol Sci 4:1028–1034
Yongmanitchai W, Ward OP (1992) Separation of lipid classes from Phaeodactylum tricornutum using silica cartridges. Phytochemistry 31:3405–3408
Acknowledgments
This study was conducted with the support of the Spanish Ministry of Science and Innovation, through the PSE microalgae project (PSE-440000-2008-1-FEDER).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendoza Guzmán, H., de la Jara Valido, A., Carmona Duarte, L. et al. Analysis of interspecific variation in relative fatty acid composition: use of flow cytometry to estimate unsaturation index and relative polyunsaturated fatty acid content in microalgae. J Appl Phycol 23, 7–15 (2011). https://doi.org/10.1007/s10811-010-9526-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9526-6