Abstract
A great deal of effort has gone into developing capacity in the sphere of human research protection programmes in South Africa and Africa over the last decade or more, by several international organisations. However the promotion of the broader agenda of research integrity or ‘RCR’ (Responsible Conduct of Research) has lagged behind. From a global perspective South Africa and other African countries are actively involved in research endeavours and collaborations across a very broad spectrum of scientific fields. For this research to fulfil its potential social value it must be reliable and trustworthy and hence it is essential that research institutions and universities take the promotion of research integrity seriously. The purpose of this paper is to consider the role of an institutional office of research integrity within the context of academic research particularly in South Africa but also in Africa. I will reflect on my own experience over a period of five years as a research integrity officer at a South African academic institution to highlight concerns in five domains; the promotion of an ethic of responsibility in opposition to compliance and bureaucracy, collaboration ethics and collegiality especially in the context of North-South collaborations, authorship and publication ethics, the problem of plagiarism and the utility of policy and procedure. I will suggest that the establishment of such an office can be of great value in the promotion of a broad culture of research ethics and responsible research conduct. The possible role and scope of function of an institutional office of research integrity will be briefly outlined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Universities and other institutions in Africa that fund, manage, develop or implement research studies are increasingly becoming aware of the need to promote the responsible conduct of research and to ensure that they have systems in place to detect and effectively manage instances of breach of research ethics or scientific integrity. This is particularly important in a world of globalised science (Rossouw et al. 2014). This gathering interest was evident in the small contingent of Africans who attended the 4th World Conference of Research Integrity (4th WCRI Rio de Janiero). While a great deal of effort has gone into developing capacity in the sphere of human research protection programmes over the last decade or more, by organisations such as the Fogarty International Center (FIC) and European and Developing Counties Clinical Trials Partnership (EDCTP), arguably the promotion of the broader agenda of research integrity or ‘RCR’ (Responsible Conduct of Research) has lagged behind. This prompted a speaker to state quite openly, while presenting a plenary presentation at the 4thWCRI that ‘research integrity in Africa doesn’t exist’; meaning I believe, that formalised institutional approaches to research integrity are not well developed in an African context, especially from the perspective of Northern hemisphere funders (Kombe 2015).
From a global perspective South Africa and other African countries overall contribution to research outputs may well be relatively small. However many African Universities are actively engaging with international role players like the National Institutes of Health (NIH) and (EDCTP) to increase their research foot prints. In South Africa several universities, including the Universities of Stellenbosch, Cape Town, Witwatersrand and Kwa-Zulu Natal view themselves as research-focused institutions and collaborate extensively over many scientific fields, with other researchers and institutions globally. Of particular mention is the broad field of health-related research, due in part to the prevalence of those social determinants that contribute to burden of disease (WHO Commission on Social Determinants of Health and World Health Organization 2008) where much collaborative, internationally funded research does occur in Africa and South Africa. Funders include both government bodies and research foundations such as the USA government agencies of the National Institutes of Health (NIH), Center for Disease Control (CDC), and US Agency for International Development (USAID), and foundations like the Wellcome Trust and Bill and Melinda Gates Foundation, to name just a few.
Much has been achieved over the last two decades in developing capacity in research ethics in Africa and in South Africa. The term ‘research ethics’ is commonly used and widely understood to refer to the wide constellation of ethical issues involved with doing research that involves both human and animal participants. Capacity development programmes funded by institutions such as EDCTP, FIC and others, have successfully built a cadre of competent research ethicists that are active throughout Africa; for example two recently published articles reflect on the successes of the FIC research ethics training programmes, and an EDCTP funded book, Research Ethics in Africa, involved research ethicists from all over Africa as authors, many of who had in fact been trained by one of the FIC programmes.Footnote 1(Kass et al. 2016; Ndebele et al. 2014, 22–40; Kruger et al. 2014). Many of these trainees are also actively doing research on research ethics and publishing in this field; the SARETI programme alone lists more than 50 student publications on its webpage (South African Research Ethics Training Initiative (SARETI) 2016). Although exact figures for total research ethics publications by South African and African scholars are difficult to obtain, it is not unreasonable to estimate that there are at least several hundred peer reviewed publications in existence.
In addition to extensive capacity building efforts, South Africa also has a well established legal framework for the protection of animal and human research participants in a health research context. The National Health Act No.61 of 2003. (NHA) devoted an entire chapter (chapter 9) to the regulation of health research and established the National Health Research Ethics Council (NHREC) to oversee matters pertaining to health research ethics in South Africa, including registering and auditing all RECs that review and approve healthFootnote 2 related research. The NHREC released the second edition of its research ethics guideline document in 2015, Ethics in Health Research: Principles, Processes and Structures and is also in the process of revising the 2006 South African Clinical Trials Guidelines (National Health Research Ethics Council, South Africa 2015; Department of Health. South Africa 2006).
In contrast to the above there are currently, to my knowledge, no similar substantial RCR training programmes running at South African universities. The RCR agenda is a broad one and seeks to promote ethical conduct in all spheres of the scientific endeavour; research ethics is thus one aspect of RCR. The Singapore Declaration on Research Integrity succinctly captures in four principles and 14 responsibilities, the essence of RCR (Second World Conference on Research Integrity 2010 22 September 2010). A typical RCR training programme would include such topics as mentorship and supervision, ethical peer review, responsible data collection, storage and sharing, ethical obligations of collaborative science, avoidance and management of conflict of interest and conflict of commitment, ethical scientific writing and the avoidance of plagiarism, and the identification, investigation and management of questionable research practices and research misconduct (Office for Research Integrity, Department of Health and Human Services (HHS), USA 2015a; Steneck 2016; Singh and Remenyi 2016). Furthermore, in comparison to research ethics, only a handful of South African and African academics have written about research integrity. Both a Web of Science and Google Scholar literature search of the topic produced only a few articles: Okonta and Rossouw discuss the prevalence of research misconduct in Nigeria, Kombe et al. reflect on the need to promote research integrity in Africa broadly, whereas Rossouw et al. and Horn discuss the topic particularly from a South African academic perspective. Singh and Remenyi, writing in the South African Journal of Science, focus particularly on the rise of plagiarism and ghost writing (Kombe et al. 2014; Rossouw et al. 2014; Horn 2013; Okonta and Rossouw 2013; Singh and Remenyi 2016).
Also unlike the well established legal framework for the protection of human and animal research participants, there is no central framework or organised structure at a national level for the promotion of research integrity and the investigation of irresponsible research and research misconduct (both terms referred to in Responsibility 12 of the Singapore Statement (Second World Conference on Research Integrity 2010). This context has been highlighted by Rossouw et al. as who argue that the issue of research misconduct has yet to become a focus of academic or public debate in South Africa, even though the country has seen a number of high profile cases. They argue for a more centralised framework, possibly supported by national research bodies that can provide policy and capacity development guidance to institutions, especially those that are under-resourced. (Rossouw et al. 2014).
The purpose of this article is to argue for institutional support for the promotion of responsible research conduct (RCR) in a broader sense at South African and African research institutions. I will draw on my own personal experience working in this field at a South African academic institution for more than ten years (including five years as a Research Integrity Officer) to reflect on some issues particularly relevant to the South African context. I shall suggest that the formal establishment of an Office of Research Integrity at institutional level may be one suitable approach to organise and focus RCR activities. The potential role and responsibilities of such an office will be briefly outlined.
Reflections of a Research Integrity Officer
In some instances, as a first step or even as an alternative step to the creation of such an office, some South African institutions, including my own, have developed the portfolio of ‘Research Integrity Officer’ (RIO). RIOs are commonplace in the USA partly because the US regulatory framework requires that all institutions receiving research funding have formal processes and persons in place to facilitate the investigation of allegations of research misconduct, when they occur (Office of Research Intergrity, Department of Health and Human Services, USA 2015b). It was in fact this compliance requirement that prompted my institution to create the portfolio of RIO primarily as a person who would be available to facilitate the investigation of allegations related to the breach of accepted research norms and standards, when and if they arise. Of note, our process includes the investigation of questionable research practices and is thus not confined to only allegations of data fabrication, falsification or plagiarism (Division for Research Development, Stellenbosch University 2014). Also, although the RIO facilitates the investigation process, a formal investigation is done by an ad-hoc committee especially appointed for each case and not by the RIO; the RIO does not sit as a member of this committee but is involved in identifying suitable persons to serve on the committee and is responsible for providing the committee with an investigation brief. The position of RIO is somewhat negatively focused as the main responsibility is to address complaints and facilitate investigations where needed. Other responsibilities may become attached to the position by default, such as playing a role in policy development or the delivery of RCR training. However the establishment of a formal institutional Office for Research Integrity, as described and motivated for in this paper, is, in my opinion, a preferred option.
I have held the portfolio of RIO at my institution for just over five years now and believe that it may be worthwhile to share some selected insights gained from dealing with a wide variety of incidents and ‘cases’ in a South African context.Footnote 3 This discussion is not comprehensive or fully inclusive and could in fact be viewed as somewhat eclectic. I have chosen these specific topics because they relate directly to the cases that I have been involved with over the last five years, and informed by certain patterns that have emerged; for example I have learnt that both senior and junior researchers often struggle with ethical dilemmas and disputes relating to authorship and that breakdown in collegial relationships can be at the root of research misconduct allegations.
Of importance, a discussion of the promotion of RCR at South African institutions must take cognisance of the current academic milieu, particularly the need to develop and conduct research in a context that acknowledges the injustices of the past and that strives to find novel ways to address these injustices and inequities. A 2010 commissioned paper of the Development Bank of South Africa notes:
In South Africa, social inequalities were embedded and reflected in all spheres of social life, as a product of the systemic exclusion of blacks and women under colonialism and apartheid. The higher education system was no exception. Social, political and economic discrimination and inequalities of a class, race, gender, institutional and spatial nature profoundly shaped, and continue to shape, South African higher education. (Badat 2010) p.2.
This concern, while not the main focus of this paper, means that those responsible for promoting the responsible conduct of research at their own institutions must keep the big picture constantly in mind. Ideally, as highlighted by both the 2015 South African research ethics guideline and the European Union via its Responsible Research and Innovation (RRI) funding platform, research needs to be responsive to societal values and expectations. It needs to be conducted according to high ethical standards in a context of extended public engagement at all stages in the process and must promote both gender equity and, in a South African context, racial diversity (National Health Research Ethics Council, South Africa 2015; Horizon 2020: The EU Framework Programme for Research and Innovation 2016). RCR programmes must therefore make particular effort to ensure that issues related to researcher capacity development and support amongst previously disadvantaged groups are adequately addressed within the broader RCR agenda.
Some of the selected issues that I explore in this paper include the promotion of an ethic of responsibility in opposition to compliance and bureaucracy, collaboration ethics and collegiality especially in the context of North-South collaborations, authorship and publication ethics, the problem of plagiarism and the utility of policy and procedure.
Academic Production in South Africa: the Tension between Promoting Compliance and Promoting Accountability.
As institutions develop and implement policy for the promotion of responsible research conduct there is a risk that a compliance focused approach, that undermines the value of the underlying principles of ethical research and scientific integrity, but focuses instead on ‘ticking all the boxes’ and complying with rules and policies, will become dominant. Such an approach can lead to a growing burden of research administrative bureaucracy which researchers may resent and view as an obstruction to the research process. The post-modern ‘ethics of responsibility’ articulated by Bauman, places primacy on individual accountability and responsibility (Bauman 1993). Rules, codes and regulations can become structures “to hide behind and almost promote a reduction in individual ethical responsibility for the value-choices we have to make as individual researchers and scientists and for being accountable for those choices” (Horn 2013, 21–24). It is important to note that of the 14 responsibilities identified in the Singapore statement, 12 focus on responsibilities of researchers as individuals; only two of the responsibilities identified are institutional (Second World Conference on Research Integrity 2010). Ultimately, institutions need to aim at promoting a culture that encourages researchers to thoughtfully consider the ethical aspects of their research, including how their own research is situated within the broader societal context and to conduct this research responsibly and sensitively. They should do all of this primarily because it is the right thing to do, rather than in order to simply comply with rules or bureaucratic systems.
Such a culture, I believe, can only be adequately achieved with leadership from the top. When those in senior research leadership positions provide little support, or are openly negative towards issues related to research integrity and research ethics, institutions are left with little choice other than to implement systems which are largely compliant-based and often very bureaucratic. This unfortunately results in a negative feedback loop that can be very difficult to terminate. Once an institution has established a well functioning culture of support for responsible research, built on the foundational Singapore principles of Honesty, Accountability, Professional courtesy and fairness, and Good stewardship (Second World Conference on Research Integrity 2010) it may well be possible to develop trust-based systems that are decentralised and less bureaucratic.
Collaboration and Collegiality in an Emerging Research Context.
A breakdown in research collaboration collegiality can be the starting point from which allegations of research misconduct emerge. In a South African research context, where the availability of funding at a national level is often limited, it is critical that researchers, including those from historically disadvantaged institutions are able to participate in international research collaborations for mutual benefit. They must be able to meet both the ethical and scientific standards required by such collaborations, but also should be able to fully participate without being daunted (and even possibly exploited) by the ‘David and Goliath’ context that such collaborations may represent. In this research context an institutional office of research integrity could potentially play a pivotal role in assisting researchers to effectively navigate the demands of cross-boundary research. The Montreal statement on research collaboration notes that “Research collaborations that cross national, institutional, disciplinary and sector boundaries are important to the advancement of knowledge worldwide. Such collaborations present special challenges for the responsible conduct of research, because they may involve substantial differences in regulatory and legal systems, organizational and funding structures, research cultures, and approaches to training” (3rd World Conference on Research Integrity 2013). This succinct one page statement outlines 20 responsibilities that collaborating partners should agree on, prior to initiating their collaboration. These include general collaborative responsibilities of integrity, trust, purpose and goal setting and more detailed responsibilities involved in managing the collaboration, collaborative relationships and the outcome of the research, including publication. COHRED (Council on Health Research for Development) is in the process of developing a Fairness Index which will provide guidelines for best practice in the development and implementation of research collaborations, particularly those involving Low and Middle Income Countries (LMICs) (Musolino et al. 2015). Another useful guideline for research collaboration is the UNAIDS Good Participatory Practice Guideline for Biomedical HIV Prevention Trials (UNAIDS International Working Group 2011). Although the focus of this document is HIV research, the principles it invokes (respect, mutual understanding, integrity, transparency, accountability and community stakeholder autonomy) and the practical guidelines it provides, are applicable to a wide range of research, not only health research. These tools and documents could also be used by an institutional research office to develop both training (for example a one day workshop) and a memorandum of understanding (MOU) template that researchers could use as a baseline for establishing collaborations. Such pre-emptive action may avoid disputes and unpleasant incidents further along, when pressure to deliver results and funding constraints potentially contribute to tension and disagreement.
The fostering of good collegial relationships has been recognised as a key component of successful research and teaching environments (Harrison and Brodeth 1999; Weidman and Stein 2003; Boyle and Boice 1998). Poor communication, poor boundary setting, lingering historical power differentials based on race, language and gender, and lack of a clear understanding regarding expectations and responsibilities may lead to mistrust and suspicion. Large power differentials and an environment conducive to workplace bullying (McKay et al. 2008) may also contribute to a context in which allegations of real or imagined research misconduct surface. Such a context can also lead to disputes over authorship. Researchers, particularly those working in teams need to recognise and acknowledge the importance of maintaining good human relationships. People matter very much and sometimes research teams may need to invest both time and resources into activities that proactively build teams and foster good collegiality. Furthermore it is unwise to ignore signs of disharmony among research teams or those working together on projects, as sooner or later this could lead to suspicion and allegation. Again, an established institutional office for research integrity can play an essential role in assisting researchers to build strong and responsible teams that are able to avoid or proactively manage some of the potential pitfalls mentioned.
Authorship and Publication Ethics.
The academic research context is a very pressured one and both career advancement and the ability to attract funding is determined at least in part by one’s publication record. The Retraction Watch website is one window into this particular issue and some of the consequences that can arise from questionable authorship practices (although not all retractions are related to poor authorship ethics; some are due to genuine error) (Oransky and Marcus 2016). Problematic authorship and publication behaviour can include inappropriate text recycling or self-plagiarism, redundant or duplicate publication, salami slicing and inappropriate allocation of authorship (Roig 2011). As RIO, I have experienced that problems related to the ethics of authorship and publication and the interpretation and implementation of authorship guidelines occur frequently, and it is within this particular context that I find myself most often engaged in attempting to resolve disputes or contentious issues.
South African Universities receive government funding based in part on the their publication unit outputs. This funding amounts to a significant proportion of institutional funding and is used to support research infrastructure including library facillities and Information Technology systems. However, many institutions do distribute some of this funding in a proportional manner back to faculties, departments and in some cases individual authors get a proportion of this funding paid into research funds, or as direct remuneration. This practice could potentially be a driver for unethical authorship such as salami slicing and redundant publication (Horn 2016).Footnote 4 Research done by Franzoni et al. looking at submission trends to the journal Science, note that cash incentives increase submission rates but do not appear to influence acceptance rates, indicating that such incentives may encourage submission of research irrespective of quality (Franzoni et al. 2011). Furthermore in South Africa researchers can apply to be rated by the National Research Foundation (NRF). A favourable rating is directly linked to research funding (National Research Foundation 2015). As Roig (Roig 2011) states “[globally] the current academic reward system is thought to produce a tremendous amount of pressure to generate as many publications as possible. Unfortunately, some of the most serious negative outcomes of the current system are the problems of duplicate and redundant publications”. p.17.
Reusing one’s own work is generally acceptable when the work is clearly referenced. Redundant or duplicate publication is the publication of essentially the same article in separate journals without alerting readers to the fact that the work has been published elsewhere. Salami- slicing is the division of results from one study into separate parcels that are then published as separate articles usually in different journals (Roig 2011). p.16 This is also often referred to as publication of the ‘smallest publishable unit.’ Text recycling is the reuse of ones own text in different places. This can sometimes be appropriate, if adequately refernced. Duplicate publication involves publication of virtually identical data and text in separate journals but with changes made to the title and perhaps order of authors. Retraction guidelines issued by the Committee on Publication Ethics (COPE) recommend that journal editors retract such articles if there is not “proper cross referencing, permission or justification” (Wagner et al. 2009). p.3.
The practice of segmented publication or ‘salami-slicing’ is also problematic. This form of redundant publication involves the splitting of the results of one study into several articles. Often this will be done with the justification that the same data set has been used to answer different research questions. Thus the methodology and data reported are usually the same, whereas the text or discussion is usually different. One of the problems with this practice is that, particularly in the scientific and medical fields, it may distort the body of knowledge and even, in a biomedical context, influence clinical practice unjustifiably. Finally this practice may waste the time of editors, reviewers and readers and also waste sort after journal space (Smolčìć 2013, 237–241).
The allocation of inappropriate authorship is also often a contentious issue and authorship disputes occur not infrequently. The allocation of authorship to persons who may not actually deserve authorship credit can be a way of avoiding disputes, but is undesirable. Various international bodies such as the International Committee of Medical Journal Editors (ICJME) provide guidelines for ethical allocation of authorship and for resolving authorship disputes (Albert and Wagner 2003, 32–34; International Committee of Medical Journal Editors 2016). In South Africa some academic institutions have incorporated authorship guidelines into policy documents or have developed their own guideline documents (Office for Research Integrity, University of Cape Town 2010; Senate Research Ethics Committee 2013). Steneck (Steneck 2003) notes that while guidelines are influential, they are not consistently applied across all scientific fields. He argues, in line with the more recent Singapore Declaration of Research Integrity (Second World Conference on Research Integrity 2010), that the responsibility for fair and appropriate allocation of authorship lies with the researchers involved in the project. However, in my own experience, it is not unusual to hear anecdotes from researchers, particularly more junior ones, that research unit or laboratory academic heads expect to have their names on all papers. The reverse is also true; junior academics, including PhD students, sometimes admit to being willing to include a senior well-published colleague who would not ordinarily qualify for authorship on their authorship list, as this may facilitate publication in a higher ranked journal.
At an institutional level the perils and pitfalls of some publication and authorship practices, including publishing in predatory journals, may not necessarily be immediately evident to researchers, particularly those at the start of their career (Beall 2016). Hence creating awareness of these issues within the context of planning a research career and authorship or publication strategy, is very important. Although South Africa is not unique in linking publication outputs directly to institutional funding (Franzoni et al. 2011, 702–703), this practice could, if not carefully implemented and monitored at institutional level, create a fertile ground for encouraging some of the questionable authorship and publication practices mentioned above.
The Problem of Plagiarism
The three big research misdemeanours that count as research misconduct according to the Office for Research Integrity in the USA are plagiarism, data fabrication and data falsification (Office of Research Integrity, Department of Health and Human services, USA 2015b). Hopefully the latter two categories occur infrequently, although the concern remains that in South Africa, in the absence of both public awareness and a central research integrity framework, such events may well be under reported or remain undetected. However plagiarism seems to be a persistent problem world-wide even with the now widespread use of similarity detection software, such as Turnitin. It is likely that most academic institutions will need to deal with a wide variety of allegations of plagiarism ranging from incidents involving undergraduate students and occurring primarily out of ignorance, to more blatant cases. At Stellenbosch University the Post Graduate and International Office offers a very popular course, ‘Avoiding Plagiarism’ (Postgraduate and International Office, Stellenbosch University 2016). I have previously co-presented this workshop on many occasions and each time I have been struck by how complex an issue ‘avoiding plagiarism’ can be for students, especially those who are studying and writing in a second or third language, almost the norm in an African context. Institutions of higher learning need to realise that avoiding plagiarism is not just common sense and that the understanding of graduate students (and even more so undergraduate students) of what constitutes ethical scientific writing is often surprisingly limited and confused. This complexity is echoed in a much cited 1996 article, (worth reading in its entirety) by Alastair Pennycook from the University of Melbourne (Pennycook 1996). In this article he reflects on his experience of teaching English at university level in China, Hong Kong and Japan. He argues that plagiarism is not “a simple black-and-white issue, the prevention of which can be achieved via threats, warnings, and admonitions” but needs to be “understood in terms of complex relationships between text, memory, and learning”. p.201 He also explores in some detail the historical development of current notions of authorship and text ownership. Much of what is written in this article has surfaced at a practical level in our workshops, where students often argue at length and with conviction against many of the illustrative examples presented; examples which to us, as presenters, seem quite ‘black and white’. Penny Cook concludes:
Many of the ways we approach supposed plagiarism are pedagogically unsound and intellectually arrogant. It is not adequate to observe simply on the one hand that students “copy” or that on the other hand they need to learn academic writing practices. Both observations are trivially true but insufficient in terms of an awareness of cultural difference and a self-reflexivity about the practices to which we adhere….. Also needed is an attempt to understand the other side of the coin-our students’ textual and language learning worlds as well as the constraints on their lives and their perceptions of how academic norms operate and may be flouted’. p.227.
The above perspective is a particularly important in academic context where the majority of students and researchers are writing in a language which may be their second or third. While a developmental and remedial approach to plagiarism is appropriate for most students, there will be those who are blatant plagiarists, hoping not to get caught out. Unfortunately, this also applies occasionally to academic staff. In these instances, it is essential that institutions have clear written policy and procedure in place so that such incidents can be effectively managed and appropriate action taken against perpetrators.
Policy and Procedure
Elsewhere (Horn 2013) I have discussed the institutional value of having adequate written policy and procedures in place for all aspects of research and academic integrity. Compared to well established Northern hemisphere institutions, policy development and implementation is still relatively underdeveloped at many institutions in South Africa.Footnote 5 Again, historically disadvantaged institutions in particular often still lack the resources and capacity to undertake the arduous task of drafting effective policy, getting it approved and implemented. Having an institutionally approved robust policy available is particularly important when allegations of research misconduct, including plagiarism occur. Without a good, legally sound written procedure in place; one that fully complies with the tenants of administrative law, mistakes can be made during the investigation that have negative consequences for all involved and can even result in legal action against an individual or institution. In South Africa many academic institutions receive research support from a US federal government agency and must have a written procedure for the investigation of allegations of research misconduct; this procedure must comply with US federal regulations regarding research integrity (Office of Research Integrity, USA 2015) An institutional ORI would be well situated to facilitate the development and implementation of RCR policy, assisting researchers to comply adequately with the demands of international funders, while simultaneously relieving them of the time consuming burden of having to resolve allegations of wrong doing or breaches of accepted ethical and scientific norms and standards.
An Institutional Office for Research Integrity
The promotion of responsible research conduct can and should happen at many different levels in an academic institution and via multiple interactions. These interaction opportunities are too numerous to mention but involve many role players including students, supervisors, department chairpersons, deans, research directors etcetera. However in recognition of the importance of implementing and upholding internationally accepted standards of scientific integrity, several South African academic institutions have recently established, or are in the process of establishing an institutional Office for Research Integrity. The function of this office may be varied, depending on resources and available human capacity. Such offices are often responsible for developing institutional policy relating to the broad context of the promotion of responsible conduct of research (often referred to as ‘RCR’) and for implementing this policy.
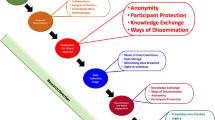
Figure 1 represents a visual overview of how I picture such an office, including the pivotal role it could play with regard to three groups of stakeholders; the institutions own research community; local and international applicable regulatory bodies and government departments, and external research collaborators including funders and reviewers. This illustration represents the interaction flow between the ORI and these three stakeholder groups as bi-directional. This implies that the ORI is not merely there to provide information, set norms and standards and ensure they are adhered to, but that it also needs to listen, learn and adapt to the needs and requirements of its stakeholder population. Also importantly this office would need the capacity to function across all research domains active within the university or institution it serves; that is the available skill-set in the office should be broad and not only competent in one research domain, such as health research.
Conclusion: Prevention is Better Than Cure
This paper focuses on some aspects of the promotion of responsible research conduct within a South African research context and also highlights some of the challenges encountered in this context. Furthermore, it proposes that an established institutional office for the promotion of research integrity represents a critical step in the promotion of a culture of research integrity.
The academic field of research integrity is a new one in South Africa and Africa and as suggested by implication in this article provides much scope for empirical research to be conducted by up-and-coming African researchers with an interest in these matters. Some examples for a reseach agenda include issues related to research collaborations and the challenges they encounter, including the testing of novel interventions; the prevalence of plagiarism and other questionable research practices at African universities; the challenges faced in promoting responsible research conduct in resource limited settings; issues related to effective policy development and implementation; issues related to the understanding and management of conflict of interest and conflict of commitment; issues related to publication and authorship ethics particularly within the context of transformation and research capacity development in Africa. This list is far from exhaustive; the field is currently wide open.
The historical and current social and political context in which South African academic and research institutions exist, must inform the agenda for the promotion of responsible research conduct. The above discussion highlights some of the selective insights I have gained as a Research Integrity Officer, as a window into the complex and complicated arena of research misconduct and the promotion of research integrity. One thing is certain: incidents (either alleged or actual) that involve the breach of accepted norms for ethical and scientifically valid research often have negative effects that can be far-reaching. These negative consequences can adversely effect individual careers and reputations and also damage the reputation of research institutions as they undermine the credibility of research being done at these instutions and negatively impact on public trust. Investigations of such incidents consume significant human resources and have the potential to to disrupt departments and faculties. It is critical that academic institutions take the promotion of responsible research conduct very seriously. Preventing incidents by education, training, mentoring, advocacy, policy, procedure and monitoring, is so much better than trying to resolve the mess created by actual allegations and occurrences. Establishing a well functioning and adequately resourced institutional office for research integrity can be a sound step in the right direction.
Notes
In South Africa the Fogarty International Center has funded three training programmes on a five year, renewable basis. IRENSA (International Research Ethics Network South Africa) was established in 2003 and ran for 10 years. Successful candidates obtained a Diploma in International Research Ethics from the University of Cape Town. SARETI (Southern African Research Ethics Training Initiative) is ongoing and in its third cycle. http://sareti.ukzn.ac.za/Homepage.aspx This Masters degree programme is based at the University of Kwa-Zulu Natal and is planning to expand to include a doctoral programme, if re-funded. ARESA (Advanced Research Ethics training South Africa) is also a research ethics one year diploma programme, based at Stellenbosch University. http://www.sun.ac.za/english/faculty/healthsciences/aresa
The definition of health research used in the South African National Health Act No.61. 2003 is very broad and includes all research that aims at gaining a better understanding of biological, social and psychological processes in human beings.
My portfolio covers all fields of research at my institution (which is a comprehensive university with ten faculties) and not only health research.
This issue is currently being investigated as part of a national research project being conducted under the auspices of SciSTIP (DST-NRF Centre of Excellence in Scientometrics and Science, Technology and Innovation Policy) based at Stellenbosch Univeristy. The project
entitled Authorship and reward: An investigation of researchers’ understanding and experience of scientific authorship and publication incentives in South Africa will use both a national online survey and qualitative methodology to explore publication ethics and the role of publication incentives in influencing publication behaviour at institutions across South Africa. It is hoped that results of this study will be presented at the 5th World Conference on Research Integrity in Amsterdam in 2017.
This insight is largely based on personal communication from attendees of research ethics and research integrity workshops that I have presented on behalf of SARIMA (Southern African Research and Innovation Managers Association) http://www.sarima.co.za /
References
3rd World Conference on Research Integrity. (2013). Montreal Statement on Research Integrity in Cross-Boundary Research Collaborations (2013). http://www.researchintegrity.org/Statements/Montreal%20Statement%20English.pdf. Accessed 12 January (2016).
Albert, T., & Wagner, E. (2003). How to Handle Authorship Disputes: A Guide for New Researchers COPE Report (2003). Committee on Publication Ethics. http://publicationethics.org/files/2003pdf12_0.pdf. Accessed 11 October 2016.
Badat, S. (2010). The challenges of transfornation in higher education and training institutuions in South Africa. http://www.dbsa.org/EN/About-Us/Publications/Documents/The%20challenges%20of%20transformation%20in%20higher%20education%20and%20training%20institutions%20in%20South%20Africa%20by%20Saleem%20Badat.pdf. Accessed 25 May 2016.
Bauman, Z. (1993). Postmodern ethics. Oxford: Blackwell.
Beall, J. (2016). Scholarly Open Access: Critical analysis of scholarly open-access publishing. Blog page. 2016. https://scholarlyoa.com/ Accessed 27 October 2016.
Boyle, P., & Boice, B. (1998). Best practices for enculturation: collegiality, mentoring, and structure. New Directions for Higher Education, 101, 87–94.
Department of Health, South Africa (2006). Guidelines for good practice in the conduct of clinical trials with human participants in South Africa. (SA GCP) (2nd ed.). Pretoria: Department of Health, South Africa.
Division for Research Development, Stellenbosch University. (2014). Procedure for the Investigation of Allegations of Breach of Research Norms and Standards. http://www0.sun.ac.za/research/research-integrity-and-ethics/investigation-procedure.html. Accessed 12 Sept 2016.
Franzoni, C., Scellato, G., & Stephan, P. (2011). Science Policy. Changing Incentives to Publish. Science (New York, N.Y.), 333(6043), 702–703.
Harrison, B. T., & Brodeth, E. (1999). Real work through real collegiality: faculty seniors views on the drive to improve learning and research. Journal of Higher Education Policy and Management, 21(2), 203–214.
Horizon 2020: The EU framework programme for research and innovation. Responsible Research and Innovation. https://ec.europa.eu/programmes/horizon2020/en/h2020-section/responsible-research-innovation. Accessed 21 April 2016.
Horn, L. (2013). Promoting responsible research conduct in a developing world academic context. South African Journal of Bioethics and Law, 6(1), 21–24.
Horn, L. (2016). Publication incentives: just reward or misdirection of funds? Proceedings of the 4th World Conference on Research Integrity. Research Integrity and Peer Review 1(Suppl 1):CS08.2: 11–1-56.
International Committee of Medical Journal Editors (2016). International Committee of Medical Journal Editors (ICMJE) Guidelines. http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html. Accessed 11October 2016.
Kass, N., Ali, J., Hallez, K., et al. (2016). Bioethics training programmes for Africa: evaluating professional and bioethics related achievements of African trainees after a decade of Fogarty NIH investment. BMJ Open, 6, e012758.
Kombe, F. (2015) Enhancing research integrity in Africa. Plenary Session. 4th World Conference on Research Integrity. http://wcri2015.org/plenaries.html. Accessed 22 september 2016.
Kombe, F., Anunobi, E., Tshifugula, P., Wassenaar, D., Njadingwe, D., Mwalukore, S., et al. (2014). Promoting research integrity in Africa: an African voice of concern on research misconduct and the way forward. Developing World Bioethics, 14(3), 158–166.
Kruger, M., Ndebele, P., & Horn, L. (Eds.) (2014). Research ethics in Africa: a resource for research ethics committees. South Africa: SUNMeDIA.
McKay, R., Arnold, D. J., Fratzl, J., & Thomas, R. (2008). Workplace bullying in academia: a Canadian study. Employee Responsibilities and Rights Journal, 20(2), 77–100.
Musolino, N., Lazdins, J., Toohey, J., & Ijsselmuiden, C. (2015). COHRED fairness index for international collaborative partnerships. Lancet (London, England), 385(9975), 1293–1294.
National Health Research Ethics Council, South Africa (2015). Ethics in Health Research: principles, structures and processes (2nd ed.). South Africa: Department of Health.
National Research Foundation, South Africa. (2015) National Research Foundation Rating System. http://www.nrf.ac.za/rating. Accessed 13 January 2016.
Ndebele, P., Wassenaar, D., & Benatar, S. (2014). Research ethics capacity building in sub-Saharan Africa: a review of NIH Fogarty-funded programs 2000–2012. Journal of Empirical Research on Human Research Ethics, 9(2), 22–40.
Office for Research Integrity, University of Cape Town (2010). Authorship practices policy University of Cape Town. Cape Town: University of Cape Town http://www.researchoffice.uct.ac.za/research_integrity/overview/ Accessed 27 October 2016.
Office for Research Integrity, (2015a). Department of Health and Human Services, USA. Responsible Conduct of Research (RCR) General Resources., http://ori.hhs.gov/general-resources-0. Accessed 8 September 2016.
Office of Research Integrity (2015b). Department of Health and Human Services, USA. Definition of Research Misconduct. http://ori.hhs.gov/definition-misconduct. Acessed 14 January 2016.
Office of Research Integrity, USA (2015). Statutes and Regulations. http://ori.hhs.gov/statutes-regulations. Accessed 14 January 2016.
Office of Research Intergrity, Department of Health and Human Services, USA (2015). Research Inegrity Officer Responsibilities. http://ori.hhs.gov/sites/default/files/SamplePolicyandProcedures-AppendixA-5-07.pdf. Accessed 8 January 2016.
Okonta, P., & Rossouw, T. (2013). Prevalence of scientific misconduct among a Group of Researchers in Nigeria. Developing World Bioethics, 13(3), 149–157.
Oransky, I., & Marcus, A. (2016) Retraction watch. Blog page. http://retractionwatch.com/. Accessed 10 October.
Pennycook, A. (1996). Borrowing others’ words: text, ownership, memory, and plagiarism. TESOL Quarterly, 201–230.
Postgraduate and International Office, Stellenbosch University. (2016) Avoiding Plagiarism Workshop Description., http://www0.sun.ac.za/international/current-students/postgraduate-students/postgraduate-skills/workshops-and-courses/browse-by-a-z/avoiding-plagiarism.html. Accessed 14 January.
Roig, M. (2011). Avoiding Plagiarism. Self-Plagiarism and Other Questionable Writing Practices: A Guide to Ethical Writing https://ori.hhs.gov/images/ddblock/plagiarism.pdf. Accessed 13 January 2016.
Rossouw, T., Van Zyl, C., & Pope (2014). Responsible conduct of research: global trends, local opportunities. South African Journal of Science, 110(1–2), 1–6.
Second World Conference on Research Integrity (2010). Singapore Statement on Research Integrity. http://www.singaporestatement.org/. Accessed 27 October 2016.
Senate Research Ethics Committee, Stellenbosch University. (2013). Policy for responsible research conduct at Stellenbosch University. http://www0.sun.ac.za/research/assets/files/Integrity_and_Ethics/SU%20Research%20Ethics%20policy%20approved%20by%20Council_24%20June%202013.pdf Accessed 12 October 2015.
Singh, S., & Remenyi, D. (2016). Plagiarism and ghostwriting: the rise in academic misconduct. South African Journal of Science, 112(5/6), 32–46.
Smolčìć, V. (2013). Salami publications: definitions and examples. Biochemical Medicine, 23(3), 237–241.
South African Research Ethics Training Initiative (SARETI) (2016). South African Research Ethics Training Initiative (SARETI): Student Publications. http://sareti.ukzn.ac.za/StudentPublications.aspx. Accessed 12 October 2016.
Steneck, N. (2003). Introduction to the responsible conduct of research. USA: Office for Research Integrity. Department of Health and Human Services.
Steneck, N. Epigeum (2016). Web page portal for online courses https://epigeum.com/courses/research/research-integrity/. Accessed 8 September 2016.
UNAIDS International Working Group. (2011). Good participatory practice guidelines for biomedical HIV prevention trials 2011 http://www.unaids.org/sites/default/files/media_asset/JC1853_GPP_Guidelines_2011_en_0.pdf ed. Switzerland: UNAIDS (Joint United Nations HIV Programme). Accessed 27 October 2016.
Wagner, E., Barbour, V., Kleinert, S., et al. (2009). Guidance from the committee on publication ethics (COPE). Maturitas, 64, 201–203.
Weidman, J., & Stein, E. (2003). Socialization of doctoral students to academic norms. Research in Higher Education, 44(6), 641–656.
WHO Commission on Social Determinants of Health. (2008). Closing the Gap in a Generation: Health Equity through Action on the Social Determinants of Health: Commission on Social Determinants of Health Final Report World Health Organization.
Acknowledgment
I would like to express my sincere and grateful appreciation to my colleague Robert H McLaughlin, University of Cape Town, for reviewing an earlier draft of this paper and for encouraging me to see the article as one relevant to both an international and local audience.
To my good friend Lise Day for language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horn, L. Promoting Responsible Research Conduct: A South African Perspective. J Acad Ethics 15, 59–72 (2017). https://doi.org/10.1007/s10805-016-9272-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10805-016-9272-8