Abstract
In this study, we tested the feces of children with ASD and those of healthy children, and the overall changing of the gut fungal community was observed in ASD children compared with controls. However, there were no abundant fungi populations showed significant variations between the ASD and Control group both at phylum and class level. Among the 507 genera identified, Saccharomyces and Aspergillus showed significant differences between ASD (59.07%) and Control (40.36%), indicating that they may be involved in the abnormal gut fungal community structure of ASD. When analyzed at the species level, a decreased abundance in Aspergillus versicolor was observed while Saccharomyces cerevisiae was increased in children with ASD relative to controls. Overall, this study characterized the fungal microbiota profile of children with ASD and identified potential diagnostic species closely related to the immune response in ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders featuring excessively repetitive behaviors, narrow interests, and insistence on sameness (Lord and Bishop 2015). The prevalence of autism in school-age children has climbed dramatically over the past decade in China and the West, at around 1% (Sun et al. 2019). It should be noted that ASD prevalence was significantly higher among males than among females (prevalence ratio: 4.0) (Baio et al. 2018). The exact pathogenesis of ASD is unclear, but it appears to be associated with complicated gene-environment interactions (Kim and Leventhal 2015). Among several comorbidities in ASD, gastrointestinal (GI) dysfunctions are quite common, given its great prevalence and strong correlation with symptom severity (Adams et al. 2011). Indeed, GI symptoms can be related to disturbed gut microbiota and this relationship is spurring an intensive research of the gut microbiota in ASD patients.
The microbiota harbored in our GI tract may influence the brain function and behavior through the so-called “microbiota-gut-brain axis”, a bidirectional network of communication between the gut and the brain (Rhee et al. 2009). Moreover, gut microbiota may regulate the central nervous system (CNS) activities through multiple systems, including neural, immune and endocrine (Collins and Bercik 2009). Thus, alteration in the composition and metabolites of the gut microbiota has been speculated as a possible causative mechanism contributing to ASD pathophysiology. There is enough scientific evidence proved that gut microbiota differs between individuals with ASD and healthy controls (Liu et al. 2019), as well as in mouse models of ASD (Sauer et al. 2019). Specifically, probiotics Bifidobacterium spp and Akkermansia muciniphila (Wang et al. 2011) showed lower counts in ASD children compared to controls while potentially harmful species Desulfovibrio spp (Finegold 2011), Sutterella (Williams et al. 2012) and Alkaliflexus (Finegold et al. 2010)were increased in certain studies. Besides, there are also a few indications that ASD patients have altered fungal components, including reports of the higher incidences of genera Candida (Iovene et al. 2017) and Malassezia, decreased genera Aspergillus and Penicillium compared with controls (Strati et al. 2017). Among Candida spp, Candida albicans is the most represented species. It can shift tryptophan metabolism and toward 5-hydroxytryptophan metabolites (Cheng et al. 2010), an ASD-related neurotransmitter (Ormstad et al. 2018). Genus Candida is two times more abundant in toddlers with ASD than in controls (Strati et al. 2017), and it can release ammonia and other toxin that may cause autistic behavior (Rosenfeld 2015; Reichelt and Knivsberg 2009), suggesting that fungi may play a role in the pathogenesis of autism.
Though a few studies have reported the change of fungal community in children with ASD, the big-sampled well-designed studies have to be conducted, as well as a more in-depth characterization of the fungal flora is strongly needed for ASD children. To elucidate the fecal fungal presence in autistic children and to provide rationale basis for a possible specific therapeutic intervention in ASDs, we performed internal transcribed spacer 2 (ITS2) region sequencing of stool samples from 29 children with ASD and 31 healthy children in China in this study.
Materials and Methods
Subjects
Children with ASD were diagnosed by the Department of Pediatric Neurology at Jinan Central Hospital, which is affiliated with Shandong University, and the diagnosis was reconfirmed at Shandong Provincial Mental Health Center. In the present study, we included 2- to 6-year-old children with ASD diagnosed between 2017 and 2019 based on the following inclusion criteria: (1) age of 2–6 years; (2) diagnosis of autistic disorder defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (Bell 1994) and confirmed using the Autism Diagnostic Interview-Revised (ADI-R); (3) behavioral problems such as irritability, agitation, and/or self-injurious behavior; (4) a Clinical Global Impression Severity of Illness scale (CGI-S) score of ≥ 4 and an Aberrant Behavior Checklist Irritability (ABC-I) subscale score of ≥ 18 at screening and baseline; and (5) mental age of ≥ 24 months.
The exclusion criteria were as follows: (1) Rett's disorder, childhood disintegrative disorder, Asperger's disorder, or pervasive development disorder not otherwise specified according to the DSM-IV-TR; (2) schizophrenia, other psychosis, and mood disorders, including bipolar disorder and major depressive disorder according to the DSM-IV-TR criteria; (3) significant risk of committing suicide based on the subject's medical history or a routine mental status examination; (4) seizure attack in the past year; (5) history of severe head trauma or stroke; (6) history of neuroleptic malignant syndrome; (7) resistance to antipsychotic medication; and (8) presence of a significant comorbid medical illness.
Sample Collection
Stool specimens were collected in the homes of the participants by their parents and transferred to laboratory within three hours. All samples were stored at − 80 °C before DNA extraction. The study was approved by the Medical Ethical Committee of Shanghai Institute of Planned Parenthood Research (NO: PJ2019-17). Written informed consents were obtained from the parents of all participants involved in this study.
Genomic DNA Extraction, PCR Amplification and ITS2 Gene Sequencing
Total genomic DNA was extracted using QIAamp DNA Stool Mini Kit (QIAGEN). Amplifications of ITS2 region were performed with primers ITS3F (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4R (5′-TCCTCCGCTTATTGATATGC-3′) using TransStart Fastpfu DNA Polymerase (TransGen). Cycling conditions were as follows: denaturation at 95 °C for 2 min, 20 cycles of amplification (45 s at 95 °C, 30 s at 55 °C and 30 s at 72 °C), extension 72 °C for 5 min. Three repeat PCR amplifications of each sample were purified with AxyPrep DNA Gel Extraction kit (AXYGEN) and assessed by spectrophotometry (QuantiFluor-ST, Promega). The equivalent pooled ITS2 PCR amplicons were sequenced on an Illumina MiSeq instrument at Chinese National Human Genome Center at Shanghai.
Bioinformatics and Statistical Analysis
Raw paired FASTQ files were processed using QIIME 2 (Bolyen et al. 2019). Sequence merge, denoising and filtering was performed using DADA2 plugin with default parameters. Algorithms VSEARCH was used to assign sequences into OTU at 97% similarity based on de novo clustering method. Community richness and diversity analysis (ACE, Chao, Shannon and Good’s coverage) were performed using QIIME 2 with the same sequence depth. The taxonomic affiliation assignments were based on Ribosomal Database Project at default parameter. Differences between ASD and Control samples were assessed using Analysis of Molecular Variance (AMOVA) in Mothur. The taxonomy features (OTU, genus, family and phylum) abundance differences between ASD and Control groups were analyzed by STAMP (Parks et al. 2014) (p < 0.05).
Results
Fungal Populations in ASD and Control Gut
A total of 60 fecal samples were collected from 29 ASD children (ranging from 2 to 6.5 years old, average 4.5, average BMI = 17.2, 6 females and 23 males) and 31 healthy children (all at 48 months, no overweight or allergy, 16 females and 15 males) (Table 1). All the participants were not on special diets, like taking probiotics or antibiotics. A total of 2,402,928 (18,287 ~ 71,227) high-quality ITS2 sequences from 60 samples were contained by high-throughput DNA sequencing. To normalize data to avoid statistical bias, 18,287 ITS2 sequences of each sample were chosen to calculate richness, evenness, and diversity of the bacterial community at 97% similarity. After 60 samples were classified into two groups (ASD and Control), a total of 1648 OTUs were obtained. The Good’s coverage was over 99.9% for two groups (Table 2), which meant the sequencing depth was sufficient for gut fungi investigation of ASD and healthy children.
Fungi of ASD and Control
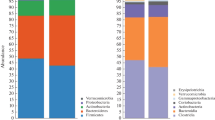
The total gut fungi were revealed through the phylogenetic and taxonomic assessments of the ITS2 region. All fungal populations could be aligned to six phyla, 224 families and 507 genera. At phylum level, Ascomycota (average 89.78%, ± 0.008), Basidiomycota (4.90% in ASD, 9.81% in Control) and Zygomycota (4.28% in ASD, 0.47% in Control), were the three most abundant fungal divisions in gut. At family level, 20 families were major taxa in two groups (> 1% in at least one group, accounting for over 88.91% in each group, Table 3). Among them, Saccharomycetaceae was the most abundant family in each group (58.61% in ASD, 36.93% in Control). In the 507 identified genera, 21 were abundant (> 1% in at least one group, accounting for over 84.81% in each group, Table 4). Among them, Saccharomyces, Akanthomyces, Candida, and Morchella were > 1% both in ASD and Control group.
Gut Mycobiota Changes Between ASD and Control
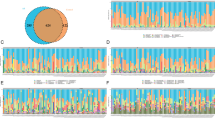
The principal component analysis (PCA) analysis with Bray–Curtis dissimilarity based on genera revealed that the fungi groups show significant differences in similarity tested by AMOVA (P < 0.001) (Fig. 1). According to the evaluation of fungal populations (Table 2), the ASD had lower richness (ACE index and Chao index), lower evenness (Shannon even index) and lower diversity (Shannon diversity). The result interpreted fungi compositions were different between ASD and Control group, and the ASD could change fungi compositions of the gut and have lower biodiversity compared with the Control group.
There were no fungi populations showing significant variations between the ASD and Control group both at phylum and class level. At order level, only Eurotiales were significantly enriched in the control group (1.85% in ASD, 5.89% in Control, FDR = 0.026). Two major families Saccharomycetaceae and Trichocomaceae had significant differences between ASD (60.45%) and Control (42.81%) (Table 3). At genus level (Table 4; Fig. 2), two major genera Saccharomyces and Aspergillus had significant differences between ASD (59.07%) and Control (40.36%). At species level, we found two abundant species had a significant difference between ASD (58.39%) and Control (38.50%) (Table 5; Fig. 3). Saccharomyces cerevisiae was increased in ASD gut fungi populations (58.38% in ASD, 36.72% in Control) while Aspergillus versicolor almost does not exist in ASD group (0.01% in ASD, 1.78% in Control).
Discussion
Recently, extensive evidence suggests that gut microbiota can influence autism behavior by regulating brain chemistry (Gabriele et al. 2014; Zheng et al. 2016) through “microbiota–gut–brain axis” and the intestinal flora became one of the major topics of ASD research interest. However, most gut microbiota studies were focused on bacteria, with few studies reporting the fecal fungi of children with ASD. In fact, fungi are an important part of the human intestinal flora and have an impact on human health, beneficially or harmfully (Andersen et al. 2013). For example, several Candida-related symptoms were found in autistic children (Kidd 2002), for its over-presence, ammonia and toxins are released, and the absorption of carbohydrates and minerals is reduced, which may lead to autistic behavior (Burrus 2012). These studies suggest that we can further investigate the role of fungi in the pathogenesis of autism. Thus, we explored the profile of fungal microbiota in 29 subjects with ASD using ITS2 sequencing and our data showed significant differences in fungal diversity and composition between ASD children and healthy children.
The lower fungal diversity of ASD children presented here (Table 2) might be a consequence of bacterial microbiota imbalance. The bacterial community plays an essential role in the maintenance of intestinal microbial homeostasis and its diversity is inversely related to fungal diversity (Kuhbacher et al. 2006). Several studies have demonstrated that the increased diversity of intestinal bacteria in children with ASD may be due to the over-presence of harmful bacteria (Finegold et al. 2010). Therefore, it can be speculated that harmful bacteria overgrowth in ASD may lead to a lower fungal diversity, compared with controls. Additionally, results revealed that three major fungal phyla were present in autism samples, including Ascomycota, Basidiomycota, and Zygomycota. Interestingly, the relative abundance of Basidiomycota was almost twice as much in Control (9.81%) than in ASD (4.90%), though this difference was not significant (FDR = 0.22). The same is true of Zygomycota (FDR = 0.23), though it is highly enriched in ASD (ASD: 4.28%; Control: 0.47%). A tendency at genus level towards a higher abundance of Saccharomyces and a lower proportion of Aspergillus was revealed in fecal samples of ASD children. Different food groups and different nutrient intake may have distinct effects on the microbiota composition (Berding and Donovan 2018). For example, dairy intake was negatively associated with the abundance of species whereas vegetable intake increased the abundance of certain microbiota (Smith-Brown et al. 2016). However, its effects on certain fungi are less reported, further studies on this point are needed.
The differences of yeast infection among stools from ASDs and healthy controls were investigated in recent years. Kantarcioglu et al. (Kantarcioglu et al. 2016) found Candida species, especially Candida albicans, were prevalent in stool samples of ASD children while our finding demonstrated that Candida albicans were more common in healthy controls (ASD: 2.24%, Control:3.93%).Candida albicans may contribute to the imbalance carbohydrate and mineral absorption and the increased toxin levels, which are believed to cause the autistic behaviors (Borre et al. 2014; Burrus 2012). However, little is known about the exact role of Candida in the intestinal tract of ASD patients and it is impossible to explain the effect of its increased or decreased levels on the condition of ASD children. Contrary to another study report (Kantarcioglu et al. 2016), Candida krusei and Candida glabrata were not detected in our ASD children. We found the increased level of Candida sake and decreased level of Candida parapsilosis in ASD children. Therefore, the correct identification of the species might be a key factor for efficient therapeutic decisions in patients with ASD. Genus Saccharomyces is classified as potential human pathogens (Hittinger 2013), which colonizes the mucous membranes of small intestine (Marra et al. 2007) that potentially results in an endogenous infection (Macfarlane and Dillon 2007). In recent years, an association of autism with endogenous infection was reported and infections have been connected to the incidence of ASD (Meltzer and Van de Water 2017). This reminds us that the overgrowth of Saccharomyces is one of the concerns for the occurrence of infectious symptoms in ASD and therefore may be involved in the formation of ASD indirectly. Among Saccharomyces, the identified Saccharomyces cerevisiae species is at the highest level in ASD children of this study, indicating its essential role. Saccharomyces cerevisiae is known to be a transient component of the normal flora of the gut (Sanata et al. 2014). It was only found in ASD as was reported in another study (Kantarcioglu et al. 2016). Saccharomyces cerevisiae may be involved in ASD pathogenesis through immune factors. It could potentially shape the immune response through a specific way: Saccharomyces cerevisiae cells can enhance TNF-α and IL-6 production upon secondary stimulation with TLR ligands (Rizzetto et al. 2016), and antibodies produced by this way can be used as a marker of intestinal inflammation (Severance et al. 2012). Some immune response can be associated with ASD-related immune dysfunction and may play an essential role in the development of ASD (Ahmad et al. 2019). Additionally, living as a commensal yeast species (Wilson 2017), Saccharomyces cerevisiae and Candida albicans can be imbalanced in the unhealthy human microbiome, and their abundances were previously found elevated in schizophrenia (Severance et al. 2017). Overgrowth of Candida albicans has been noted in ASD in a few studies (Kaluzna-Czaplinska and Blaszczyk 2012; Zimmermann et al. 2012) and their suspected metabolic byproduct, d-arabinitol, was found in ASD children (Noto et al. 2014). These studies seem to suggest that overgrowth of Candida albicans may be influenced by Saccharomyces cerevisiae dysbiosis, which in turn may increase the risk of ASD indirectly. However, using SPSS to calculate the correlation of species, our data showed that these two species were not correlated. Interestingly, although Saccharomyces cerevisiae has been regarded as a potentially harmful fungi for ASD children in this study, its variant, Saccharomyces boulardii, is an effective agent for the prevention and treatment of gastrointestinal complications in autism children (Kobliner et al. 2018). In addition, Aspergillus versicolor only accounted for 0.01% of the fecal samples from ASD children in our study. Aspergillus versicolor has attracted particular attention for its metabolites with anti-inflammatory activities (Chen et al. 2018). We speculate that the decreased abundance of Aspergillus versicolor may reflect a slightly immune dysbiosis in ASD children. It reminds us that Aspergillus versicolor might be taken as a probiotic fungus having beneficial effects on children with ASD. Because the diet is an obvious influence on gut fungal composition (David et al. 2014), some of our results does not necessarily reflect live fungi in the gut. The abundance of Candida was positively correlated with recent carbohydrate intake and negatively correlated with total saturated fatty acid (Hoffmann et al. 2013), almond and pistachio consumption intake (Ukhanova et al. 2014). Recent consumption of short chain fatty acids may also drive down the abundance of Aspergillus (Hoffmann et al. 2013) and higher levels of Aspergillus were detected in the vegetarian than conventional diet samples (Suhr et al. 2016). The significant reduction in Aspergillus levels of ASD children in this study may also be affected by the plant-based diet and the intake of short chain fatty acids. But this is just a hypothesis, and more related studies incorporating detailed dietary information will be valuable in clarifying the effect of diet on the fungus in ASD children.
Taken all together, our results proved that the fungus in intestinal microbiota is most likely involved in the development of ASD. These fungi are opportunistic which may become ASD pathogenic under the influence of general risk factors notably (Kohler et al. 2017), imbalance of gut flora after taking antibiotics (Pais et al. 2016) or deficit of immunity (Stanzani et al. 2005). Although variations on the composition of the fungal flora were described for ASD children compared to controls, their pathogenic mechanism is not clearly defined. Thus, further large-scale study taking into account multiple factors are required for better understanding of their roles in ASD.
The major limitation of this study is that only relative abundance of each fungus was measured. Though the gut fungal richness of ASD children and control can be reflected by ACE and Chao index, the absolute abundance of gut fungal community cannot be determined through ITS sequencing. So the absolute amount of each fungi must be taken into account in further study to discover more pathogenic fungi.
References
Adams, J. B., Johansen, L. J., Powell, L. D., Quig, D., & Rubin, R. A. (2011). Gastrointestinal flora and gastrointestinal status in children with autism - comparisons to typical children and correlation with autism severity. BMC Gastroenterology, 11, 22. https://doi.org/10.1186/1471-230x-11-22.
Ahmad, S. F., Ansari, M. A., Nadeem, A., Bakheet, S. A., Al-Ayadhi, L. Y., Alotaibi, M. R., et al. (2019). Dysregulation of T cell immunoglobulin and mucin domain 3 (TIM-3) signaling in peripheral immune cells is associated with immune dysfunction in autistic children. Molecular Immunology, 106, 77–86. https://doi.org/10.1016/j.molimm.2018.12.020.
Andersen, L. O., Vedel Nielsen, H., & Stensvold, C. R. (2013). Waiting for the human intestinal Eukaryotome. ISME Journal, 7(7), 1253–1255. https://doi.org/10.1038/ismej.2013.21.
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years - Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summary, 67(6), 1–23. https://doi.org/10.15585/mmwr.ss6706a1.
Bell, C. C. (1994). DSM-IV: Diagnostic and statistical manual of mental disorders. Essentials of Pain Medicine, 272(10), 828–829.
Berding, K., & Donovan, S. M. (2018). Diet can impact microbiota composition in children with autism spectrum disorder. Frontiers in Neuroscience, 12, 515. https://doi.org/10.3389/fnins.2018.00515.
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Author correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology, 37(9), 1091. https://doi.org/10.1038/s41587-019-0252-6.
Borre, Y. E., Moloney, R. D., Clarke, G., Dinan, T. G., & Cryan, J. F. (2014). The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential. Advances in Experimental Medicine and Biology, 817, 373–403. https://doi.org/10.1007/978-1-4939-0897-4_17.
Burrus, C. J. (2012). A biochemical rationale for the interaction between gastrointestinal yeast and autism. Medical Hypotheses, 79(6), 784–785. https://doi.org/10.1016/j.mehy.2012.08.029.
Chen, R., Liu, D., Guo, P., & Lin, W. (2018). Varicuothiols A and B, new fungal metabolites from Aspergillus versicolor with anti-inflammatory activities. Chemistry & Biodiversity, 15(1), e1700445. https://doi.org/10.1002/cbdv.201700445.
Cheng, S. C., van de Veerdonk, F., Smeekens, S., Joosten, L. A., van der Meer, J. W., Kullberg, B. J., et al. (2010). Candida albicans dampens host defense by downregulating IL-17 production. The Journal of Immunology, 185(4), 2450–2457. https://doi.org/10.4049/jimmunol.1000756.
Collins, S. M., & Bercik, P. (2009). The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology, 136(6), 2003–2014. https://doi.org/10.1053/j.gastro.2009.01.075.
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563. https://doi.org/10.1038/nature12820.
Finegold, S. M. (2011). Desulfovibrio species are potentially important in regressive autism. Medical Hypotheses, 77(2), 270–274. https://doi.org/10.1016/j.mehy.2011.04.032.
Finegold, S. M., Dowd, S. E., Gontcharova, V., Liu, C., Henley, K. E., Wolcott, R. D., et al. (2010). Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe, 16(4), 444–453. https://doi.org/10.1016/j.anaerobe.2010.06.008.
Gabriele, S., Sacco, R., & Persico, A. M. (2014). Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. European Neuropsychopharmacology, 24(6), 919–929. https://doi.org/10.1016/j.euroneuro.2014.02.004.
Hittinger, C. T. (2013). Saccharomyces diversity and evolution: A budding model genus. Trends in Genetics, 29(5), 309–317. https://doi.org/10.1016/j.tig.2013.01.002.
Hoffmann, C., Dollive, S., Grunberg, S., Chen, J., Li, H., Wu, G. D., et al. (2013). Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PLoS ONE, 8(6), e66019. https://doi.org/10.1371/journal.pone.0066019.
Iovene, M. R., Bombace, F., Maresca, R., Sapone, A., Iardino, P., Picardi, A., et al. (2017). Intestinal dysbiosis and yeast isolation in stool of subjects with autism spectrum disorders. Mycopathologia, 182(3–4), 349–363. https://doi.org/10.1007/s11046-016-0068-6.
Kaluzna-Czaplinska, J., & Blaszczyk, S. (2012). The level of arabinitol in autistic children after probiotic therapy. Nutrition, 28(2), 124–126. https://doi.org/10.1016/j.nut.2011.08.002.
Kantarcioglu, A. S., Kiraz, N., & Aydin, A. (2016). Microbiota-gut-brain axis: Yeast species isolated from stool samples of children with suspected or diagnosed autism spectrum disorders and in vitro susceptibility against nystatin and fluconazole. Mycopathologia, 181(1–2), 1–7. https://doi.org/10.1007/s11046-015-9949-3.
Kidd, P. M. (2002). Autism, an extreme challenge to integrative medicine. Part 2: Medical management. Alternative Medicine Review, 7(6), 472–499.
Kim, Y. S., & Leventhal, B. L. (2015). Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biological Psychiatry, 77(1), 66–74. https://doi.org/10.1016/j.biopsych.2014.11.001.
Kobliner, V., Mumper, E., & Baker, S. M. (2018). Reduction in obsessive compulsive disorder and self-injurious behavior with Saccharomyces boulardii in a child with autism: A case report. Integrative Medicine (Encinitas), 17(6), 38–41.
Kohler, J. R., Hube, B., Puccia, R., Casadevall, A., & Perfect, J. R. (2017). Fungi that infect humans. Microbiol Spectrum, 5(3), a019273. https://doi.org/10.1128/microbiolspec.FUNK-0014-2016.
Kuhbacher, T., Ott, S. J., Helwig, U., Mimura, T., Rizzello, F., Kleessen, B., et al. (2006). Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut, 55(6), 833–841. https://doi.org/10.1136/gut.2005.078303.
Liu, F., Li, J., Wu, F., Zheng, H., Peng, Q., & Zhou, H. (2019). Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Translational psychiatry, 9(1), 43. https://doi.org/10.1038/s41398-019-0389-6.
Lord, C., & Bishop, S. L. (2015). Recent advances in autism research as reflected in DSM-5 criteria for autism spectrum disorder. Annual Review of Clinical Psychology, 11, 53–70. https://doi.org/10.1146/annurev-clinpsy-032814-112745.
Macfarlane, S., & Dillon, J. F. (2007). Microbial biofilms in the human gastrointestinal tract. Journal of Applied Microbiology, 102(5), 1187–1196. https://doi.org/10.1111/j.1365-2672.2007.03287.x.
Marra, A. R., Opilla, M., Edmond, M. B., & Kirby, D. F. (2007). Epidemiology of bloodstream infections in patients receiving long-term total parenteral nutrition. Journal of Clinical Gastroenterology, 41(1), 19–28. https://doi.org/10.1097/01.mcg.0000212606.13348.f7.
Meltzer, A., & Van de Water, J. (2017). The role of the immune system in autism spectrum disorder. Neuropsychopharmacology, 42(1), 284–298. https://doi.org/10.1038/npp.2016.158.
Noto, A., Fanos, V., Barberini, L., Grapov, D., Fattuoni, C., Zaffanello, M., et al. (2014). The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. The Journal of Maternal-Fetal & Neonatal Medicine, 27(Suppl 2), 46–52. https://doi.org/10.3109/14767058.2014.954784.
Ormstad, H., Bryn, V., Verkerk, R., Skjeldal, O. H., Halvorsen, B., Saugstad, O. D., et al. (2018). Serum tryptophan, tryptophan catabolites and brain-derived neurotrophic factor in subgroups of youngsters with autism spectrum disorders. CNS & Neurological Disorders: Drug Targets, 17(8), 626–639. https://doi.org/10.2174/1871527317666180720163221.
Pais, P., Costa, C., Cavalheiro, M., Romao, D., & Teixeira, M. C. (2016). Transcriptional control of drug resistance, virulence and immune system evasion in pathogenic fungi: A cross-species comparison. Frontiers in Cellular and Infection Microbiology, 6, 131. https://doi.org/10.3389/fcimb.2016.00131.
Parks, D. H., Tyson, G. W., Hugenholtz, P., & Beiko, R. G. (2014). STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics, 30(21), 3123–3124. https://doi.org/10.1093/bioinformatics/btu494.
Reichelt, K. L., & Knivsberg, A. M. (2009). The possibility and probability of a gut-to-brain connection in autism. Annals of Clinical Psychiatry, 21(4), 205–211.
Rhee, S. H., Pothoulakis, C., & Mayer, E. A. (2009). Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature Reviews Gastroenterology & Hepatology, 6(5), 306–314. https://doi.org/10.1038/nrgastro.2009.35.
Rizzetto, L., Ifrim, D. C., Moretti, S., Tocci, N., Cheng, S. C., Quintin, J., et al. (2016). Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with Saccharomyces cerevisiae. Journal of Biological Chemistry, 291(15), 7961–7972. https://doi.org/10.1074/jbc.M115.699645.
Rosenfeld, C. S. (2015). Microbiome disturbances and autism spectrum disorders. Drug Metabolism and Disposition, 43(10), 1557–1571. https://doi.org/10.1124/dmd.115.063826.
Sanata, B., Salam, O. A., Ibrahim, S., Adama, Z., Mamoudou, C., Simplice, K. D., et al. (2014). Digestive fungal flora in asymptomatic subjects in Bobo-Dioulasso, Burkina Faso. Asian Pacific Journal of Tropical Biomedicine, 4(8), 659–662. https://doi.org/10.12980/apjtb.4.201414b27.
Sauer, A. K., Bockmann, J., Steinestel, K., Boeckers, T. M., & Grabrucker, A. M. (2019). Altered intestinal morphology and microbiota composition in the autism spectrum disorders associated SHANK3 mouse model. International Journal of Molecular Sciences, 20(9), 2134. https://doi.org/10.3390/ijms20092134.
Severance, E. G., Alaedini, A., Yang, S., Halling, M., Gressitt, K. L., Stallings, C. R., et al. (2012). Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophrenia Research, 138(1), 48–53. https://doi.org/10.1016/j.schres.2012.02.025.
Severance, E. G., Gressitt, K. L., Stallings, C. R., Katsafanas, E., Schweinfurth, L. A., Savage, C. L. G., et al. (2017). Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain, Behavior, and Immunity, 62, 41–45. https://doi.org/10.1016/j.bbi.2016.11.019.
Smith-Brown, P., Morrison, M., Krause, L., & Davies, P. S. (2016). Dairy and plant based food intakes are associated with altered faecal microbiota in 2 to 3 year old Australian children. Scientific Reports, 6, 32385. https://doi.org/10.1038/srep32385.
Stanzani, M., Orciuolo, E., Lewis, R., Kontoyiannis, D. P., Martins, S. L., St John, L. S., et al. (2005). Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood, 105(6), 2258–2265. https://doi.org/10.1182/blood-2004-09-3421.
Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome, 5(1), 24. https://doi.org/10.1186/s40168-017-0242-1.
Suhr, M. J., Banjara, N., & Hallen-Adams, H. E. (2016). Sequence-based methods for detecting and evaluating the human gut mycobiome. Letters in Applied Microbiology, 62(3), 209–215. https://doi.org/10.1111/lam.12539.
Sun, X., Allison, C., Wei, L., Matthews, F. E., Auyeung, B., Wu, Y. Y., et al. (2019). Autism prevalence in China is comparable to Western prevalence. Molecular Autism, 10, 7. https://doi.org/10.1186/s13229-018-0246-0.
Ukhanova, M., Wang, X., Baer, D. J., Novotny, J. A., Fredborg, M., & Mai, V. (2014). Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. British Journal of Nutrition, 111(12), 2146–2152. https://doi.org/10.1017/s0007114514000385.
Wang, L., Christophersen, C. T., Sorich, M. J., Gerber, J. P., Angley, M. T., & Conlon, M. A. (2011). Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Applied and Environmental Microbiology, 77(18), 6718–6721. https://doi.org/10.1128/aem.05212-11.
Williams, B. L., Hornig, M., Parekh, T., & Lipkin, W. I. (2012). Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio, 3(1), 1–11. https://doi.org/10.1128/mBio.00261-11.
Wilson, D. (2017). A tale of two yeasts: Saccharomyces cerevisiae as a therapeutic against candidiasis. Virulence, 8(1), 15–17. https://doi.org/10.1080/21505594.2016.1230580.
Zheng, Z., Zhu, T., Qu, Y., & Mu, D. (2016). Blood glutamate levels in autism spectrum disorder: A systematic review and meta-analysis. PLoS ONE, 11(7), e0158688. https://doi.org/10.1371/journal.pone.0158688.
Zimmermann, K., Haas, A., & Oxenius, A. (2012). Systemic antibody responses to gut microbes in health and disease. Gut Microbes, 3(1), 42–47. https://doi.org/10.4161/gmic.19344.
Acknowledgements
This work was supported by the Development Fund for Shanghai Talents (Grant Number 201567).
Author information
Authors and Affiliations
Contributions
HZ designed the project. Sample collection was performed by RZ and QZ. DNA extraction and sequencing was performed by MD and MG. Bioinformatics analysis was performed by RZ and YW. The first draft of the manuscript was written by RZ, YW and HZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, R., Wang, Y., Duan, M. et al. Dysbiosis of Gut Fungal Microbiota in Children with Autism Spectrum Disorders. J Autism Dev Disord 51, 267–275 (2021). https://doi.org/10.1007/s10803-020-04543-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-020-04543-y