Abstract
This study aimed to examine the presence of Autism Spectrum Disorder (ASD) in a sample of female adolescents with Anorexia Nervosa (AN) during the acute phase of illness. We also compare the level of autistic traits, social perception skills and obsessive–compulsive symptoms in four groups: AN, ASD, and two gender- and age-matched control groups. Of the 30 AN participants, only three scored above the conventional ADOS-2 threshold for ASD. The AN participants were similar to their controls on autistic trait measures, and to the ASD group on obsessive–compulsive measures, and on theory of mind ability and affect recognition measures. Further longitudinal studies are needed in order to determine the association between these conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anorexia Nervosa (AN) is an eating disorder characterized by a significantly low body weight [expressed as Body Mass Index (BMI) in adults, and BMI-for-age percentile for children and adolescents] due to restricted food intake, an intense fear of gaining weight, and a disturbance in self-perceived body image (American Psychiatric Association 2013). The onset of AN is usually during adolescence and the lifetime course is highly variable. AN is more common in females [female-to-male (F:M) ratio of 10:1] and the majority of affected individuals have above average intelligence (Anderluh et al. 2009; Lopez et al. 2010). By contrast, Autism Spectrum Disorder (ASD) is a lifelong condition with an early onset characterized by persistent deficits in social communication, as well as restricted and repetitive patterns of behavior (APA 2013). This condition is more prevalent in males (M:F ratio of 4:1), and intellectual functioning ranges from well below average to above average (CDC 2014; De Bildt et al. 2004; Miller et al. 2012). Despite these distinct clinical features, recent studies have focused on the overlap between these conditions with the hypothesis that ASD could be a risk factor for women to develop anorexia. One of the argument supporting this idea is that ASD may present differently in females than in males. For example, females with ASD have been found to have better social skills than males, and being less impaired than males with regard to stereotyped behaviors or restricted interests (Head et al. 2014; Szatmari et al. 2012; Van Wijngaarden-Cremers et al. 2013). Thus, it is possible that females either go undiagnosed or are mislabeled as having other disorders and the presence of undetected ASD symptoms leaves females susceptible to the development of eating disorders (Holtmann et al. 2007; Mondschein et al. 2000; Postorino et al. 2015; Russell et al. 2011; Westwood et al. 2015). In line with this hypothesis, studies on adult samples have reported similarities in cognitive profiles of AN and ASD, including weak central coherence and difficulties in set-shifting (Roberts et al. 2007; Westwood et al. 2015). However, findings on children and adolescent samples are mixed (Lang et al. 2014a, b). Similarly, studies examining theory of mind in patients with AN showed contrasting results (Calderoni et al. 2013; Oldershaw et al. 2010; Russell et al. 2009; Tchanturia et al. 2004). Rigid thinking and obsessive–compulsive features are also observed in both conditions (Solomon et al. 2008; Tchanturia et al. 2011, 2012). Overall, it has to be noticed that distinguish between AN and ASD has proved challenging. In fact, starvation can have profound impacts on brain functioning and may amplify social withdrawal, rigidity and repetitive behaviors in patients with AN (Westwood et al. 2015). Moreover, comorbid psychopathology, such as obsessive–compulsive disorder, anxiety or depression, can raise social and flexibility difficulties that could be mistaken for symptoms of autism (Oldershaw et al. 2010; Mandy and Tchanturia 2015; Mazzone et al. 2012, 2013).
In a recent systematic review, Huke et al. (2013) reported an estimated ASD prevalence in eating disorder populations ranging between 8 and 37% (Huke et al. 2013). However, is worth noting that six out of eight of the studies included in this review were based on the same Swedish community sample, and investigated retrospectively the presence of an ASD (Anckarsäter et al. 2012; Gillberg et al. 1995; Råstam 1992; Råstam et al. 2003; Wentz Nilsson et al. 1998; Wentz Nilsson et al. 2005). Moreover, participants included in these studies were at different stages of recovery, thus it is not possible to exclude that ASD symptoms were artefacts of starvation.
Separate from the issue of ASD diagnosis is the matter of autistic traits. Several studies have explored the presence of autistic traits in AN samples (Anckarsäter et al. 2012; Baron-Cohen et al. 2013; Calderoni et al. 2015; Coombs et al. 2011; Courty et al. 2013; Hambrook et al. 2008; Huke et al. 2013; Lang et al. 2015; Pooni et al. 2012; Rhind et al. 2014; Tchanturia et al. 2013; Westwood et al. 2015). The majority of these studies have used the Autism-Spectrum Quotient (AQ), a self-report measure assessing five different areas: social skill, attention switching, attention to detail, communication and imagination. For example, in a sample of 66 adolescent females with AN, Baron-Cohen et al. (2013) observed elevated autistic traits compared to healthy female adolescents (Baron-Cohen et al. 2013). However, these authors have not reported much detail about diagnosis (e.g., duration of anorexia), thus it is unclear to what extent these are manifestations of ASD or whether they are the manifestations of the acute phase of illness. In addition to the uncertainty about the phase of illness, to our knowledge only one study used the Autism Diagnostic Observation Schedule (ADOS), to confirm the diagnosis of ASD in AN samples (Mandy and Tchanturia 2015).
The purpose of the present study is to examine the presence of ASD in a sample of female adolescents with AN during the acute phase of illness. We also compare the level of autistic traits, obsessive–compulsive symptoms, and performance on a social perception task across four groups: AN, ASD, and two gender- and age-matched control groups (AN-C, ASD-C).
Participants and Methods
Design
This study design included four groups: the AN and ASD samples, and two control groups. The decision to include typically developing female control group was intended to control for the effects of gender on ASD traits. The inclusion of a male ASD control group permitted exploratory analyses of eating behaviors in the ASD group compared to the AN group.
Participants
Thirty females with AN (27 restrictive and 3 binge/purging; age range 10–17 years; mean ± SD = 14.19 ± 1.56 years), and 27 males with ASD (age range 10–16 years; mean ± SD = 12.22 ± 1.78 years) were enrolled in this study. All participants included in the AN and ASD groups were recruited throughout the outpatient service of the Child and Adolescent Neuropsychiatry Unit of the Children’s Hospital Bambino Gesù of Rome (Italy). Participants were selected from all consecutive patients admitted to the outpatient unit for a first assessment from March 2014 to July 2015. To be included in the AN group, participants had to meet diagnostic criteria for AN according to the Diagnostic and Statistical Manual of Mental Disorders-fifth edition (DSM-5) and to be in the acute phase of AN (see procedure for details) (APA 2013).
Participants were included in the ASD group if they met diagnostic criteria for ASD according to the DSM-5. Exclusion criteria for all participants included in the AN and ASD groups were the presence of specific genetic disorders, other medical disorders and epilepsy. Participants with a less than average intellectual ability [Intelligence Quotient (IQ) > 85 or > 25th percentile] as measured by Raven’s Colored Progressive Matrices (CPM) or Standard Progressive Matrices (SPM) were also excluded (Raven 2008a, b).
The ASD group and the AN group were matched on gender and age to create two typically developing control (C) groups. Participants of the control groups were recruited from a group of students of two different middle schools and one high school. For each participant in the control groups, parents provided a history of normal development and no history of a clinical diagnosis or need for special education services. The AN-C group included 35 females (age range 10–16 years; mean ± SD = 13.60 ± 1.61 years). The ASD-C group included 30 males (age range 10–16 years; mean ± SD = 12.65 ± 1.60 years).
Procedure
Assessment for the AN group and the ASD group was conducted in three separate sessions and data of the present study were collected during these visits by two multidisciplinary teams specialized in eating disorders or ASD. Each team had a pediatric neuropsychiatrist, a child psychologist, a pediatrician and a speech therapist. Parents provided a written informed consent prior to data collection. All participants underwent an in-depth assessment that included medical and developmental histories as well as a complete diagnostic evaluation (see below). After this assessment each case was discussed by these two teams to determine diagnosis and treatment plan. In order to determine the phase of illness of the AN participants a detailed physical examination was conducted. A particular attention was given to: vital signs; physical status (including height, weight, and BMI); heart rate and rhythm; heart sounds; salivary gland enlargement; scarring on the dorsum of the hands (Russell’s sign); evidence of self-injurious behavior such as ecchymoses, linear scars, and cigarette burns; muscular weakness; indications of muscular irritability due to hypocalcaem (Birmingham and Beumont 2004; Mehler and Andersen 1999; Miller et al. 2005; Chakraborty and Basu 2010). Measurement of weight and height confirmed that all participants included in the AN group had a dramatically low BMI-for-age percentile (range < 5th–22th). Moreover, all participants reported a rapid or persistent decline in oral intake, and denial and resistance to participate in their own care in less supervised settings. For these reasons, after this first assessment, all AN participants included in this study were referred to an inpatient service.
Developmental History
Developmental history assessment was completed by a child psychologist and a pediatric neuropsychiatrist specialized in ASD with all participants and their parents. Information gathered from this assessment included: onset of symptoms; if parents ever noticed that something was not quite right in language, relationships, or behavior; age when parents first noticed problems in these areas; motor milestones; toilet training; acquisition and loss of language or other skills; overall level of language; use of other’s body to communicate; ability in reciprocal conversation; social development and play; initiation of appropriate activities; interest in children; response to approaches of other children; friendships; unusual preoccupations; circumscribed interests; repetitive use of objects or interest in parts of objects; unusual sensory interests; hand and finger mannerisms; other complex mannerisms or stereotyped body movements.
Evaluation of ASD Symptoms and Autistic Traits
The AN group and the ASD group were evaluated with the Autism Diagnostic Observation Schedule-second edition (ADOS-2) performed by a trained clinician (Lord et al. 2012a, b). The ADOS-2 is a reliable and valid semi-structured, diagnostic assessment designed to elicit social responses in a naturalistic setting. Each module is aimed at a specific level of expressive language ability (ranging from pre-verbal to fluent speech). The choice of modules is based on the subject’s age and expressive language level. The use of different modules reduces possible biasing effects of differences in language skills. Each ADOS-2 module is scored with a diagnostic algorithm comprised of social affective and repetitive behavior items that best discriminate children with ASD from typically developing children and non-ASD children with intellectual disability. Each algorithm provides threshold criteria for autism as well as the less severe classification of ASD based on the combined scores for Social Affect (SA) and Restricted/Repetitive Behaviors or Interests (RRB). Because diagnostic thresholds vary across the ADOS-2 modules, the ADOS-2 Toddler Module and the Modules 1–3 algorithms have been revised to generate calibrated severity scores (CSS), ranging from 1 to 10, which are comparable across modules. Recently, also the ADOS-2 Module 4 algorithm has been revised to be more comparable to currently used algorithms for ADOS-2 Modules 1–3 and to provide a CSS that can be used to quantify and compare the severity of core symptoms in adults with ASD (Hus and Lord 2014). In the present study, 21 participants in the AN group and all participants in the ASD group performed Module 3, and 9 participants of the AN group performed Module 4. Therefore, in order to evaluate ASD severity, the CSS has been used for all analysis in the present study.
In order to explore the presence of autistic traits, participants of all groups and their parents (for participants <16 years) completed the Autism Spectrum Quotient (AQ) (Auyeung et al. 2008; Baron-Cohen et al. 2001, 2006; Ruta et al. 2012). The AQ is a widely used measure in both clinical and research practice that quantifies the number of autistic traits reported by an individual across five domains: social skill, attention switching, attention to detail, communication and imagination. It consists of three versions divided by age: a parent-report measure for children (aged 4–11 years) and adolescents (aged 12–15 years) and a self-report measure for adults (aged >16 years). The parent-report version for adolescents and the self-report version for adults of the AQ have the same range (0–50) with comparable means and standard deviations. Thus, data from both versions can be analyzed together (Baron-Cohen et al. 2001, 2006, 2013). By contrast, the parent-report version for children has a different range score (0–150), thus analysis had to be performed separately. The AQ showed good test–retest reliability and high internal consistency.
Assessment of Obsessive–Compulsive Symptoms
The Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) was used to measure the current severity of obsessive thoughts, rituals and repetitive behaviors (Scahill et al. 1997).
The CY-BOCS consists of a clinician-rated symptom inventory and severity scales. Symptoms are classified in eight categories for Obsessions (Contamination, Aggressive, Sexual, Saving/Hoarding, Superstition, Somatic, Religious, Miscellaneous) and nine categories for Compulsions (Cleaning, Checking, Repeating, Counting, Ordering, Hoarding, Magical, Other person, Miscellaneous). The CY-BOCS includes five severity scales for Obsessions and five severity scales for Compulsions. The severity scales, which are the same for Obsessions and Compulsions, consider time spent, interference, distress, resistance and control with each scale rated 0–4. Thus, the CY-BOCS yields a total obsession score (0–20), a total compulsion score (0–20), and a combined total score (0–40). The CY-BOCS showed good reliability and validity (Scahill et al. 1997; Storch et al. 2006).
Social Perception Assessment
The Italian version of the Developmental Neuropsychology Assessment, Second Edition (NEPSY-II) was administered to all participants to evaluate social perception abilities (Korkman et al. 2011; Urgesi et al. 2011). NEPSY–II is a neuropsychological battery designed for children and adolescents, and provides a comprehensive overview of neuropsychological functioning. It evaluates the domains of attention and executive functions, language, memory and learning, sensorimotor processing, social perception, and visuospatial processing. In our study we administered the social perception domains: the Theory of Mind (TOM) and the Affect Recognition (AR). A standard score of ≥8 represented an average or above average performance, a standard score of 6 or 7 slightly below average, and a score of 5 or below significantly below average (Korkman et al. 2007). The NEPSY-II showed good psychometric properties.
Measures of Eating Attitude and Behaviors
All participants completed a battery of psychological tools for a comprehensive evaluation of eating attitude and behaviors.
The Eating Attitude Test-26 (EAT-26) is a reliable measure consisting of 26 items focused on attitudes and behaviors associated with eating disorder. It comprises three subscales: dieting, bulimia, and oral control. Possible total scores range from 0 to 78 with a score over 20 indicating a possible eating problem (Garner et al. 1982).
The Eating Disorder Invenory-3 (EDI-3) is a 91-item self-report questionnaire used to assess the severity of eating disorders and personality traits (Garner 2004; Giannini et al. 2008). It consists of three eating disorder subscales [i.e., drive for thinness (DT), bulimia (B), and body dissatisfaction (BD)], and nine general psychological trait subscales [i.e., low self-esteem (LSE), personal alienation (PA), interpersonal insecurity (II), interpersonal alienation (IA), interoceptive deficits (ID), emotional dysregulation (ED), perfectionism (P), asceticism (AS), and maturity fear (MF)]. The EDI-3 has shown adequate convergent and discriminant validity (Garner 2004; Giannini et al. 2008).
The Body Uneasiness Test (BUT) is a 71-item self-report questionnaire designed to evaluate body image (Cuzzolaro et al. 2000, 2006). It consists of two parts: part A which measures weight phobia, body image concerns, avoidance, compulsive self-monitoring, and feeling detached from one’s own body (depersonalization); part B considers specific worries about particular body parts or functions. The BUT has shown good psychometric properties.
Data Analysis
Data analysis were performed using the Statistical Package for Social Sciences (SPSS 20.0 for Windows) and SAS v.9.4 (Cary, NC). Statistical significance for all analysis was set at 0.05 level. Descriptive statistics were calculated for demographic and clinical variables using means and standard deviations, as appropriate. The study design established two sets of three-group comparisons: the AN group compared to ASD and AN-C groups, and the ASD group compared to AN and ASD-C groups. Analysis of variance (ANOVA) focused on the means of autistic traits measures to determine the extent to which the AN group was similar or different from the ASD group or their controls. In each case, histograms of error residuals were checked for normality and Levene’s tests for common error variance were employed to confirm the assumptions of the ANOVA. In a number of cases, the common error variance assumption was violated, and ANOVAs that accounted for factor-level heterogeneity were utilized by modeling group variances separately and estimating denominator degrees of freedom via the general method proposed by Satterthwaite. Moreover, the means of eating disorder measures were analyzed to determine the extent to which the ASD group was similar or different from the AN group or their controls. All omnibus ANOVAs were followed by Bonferroni-adjusted post-hoc tests (Tables 1, 2). Independent sample t-test were performed to evaluate differences between groups (i.e., the AN and the ASD groups) for ADOS-2 CSS scores.
Results
The AN and AN-C groups, as well as the ASD and ASD-C groups were similar for age and IQ distribution (Age: AN: 14.19 ± 1.56; AN-C: 13.60 ± 1.61; ASD: 12.22 ± 1.78; ASD-C: 12.65 ± 1.60; IQ: AN:105 ± 15; AN-C: 106 ± 13; ASD: 107 ± 5.77; ASD-C:105 ± 6). Comparisons of clinical characteristics are shown in Tables 1 and 2.
ASD Symptoms in the AN Group
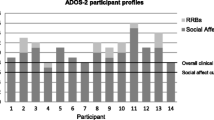
The ASD group scored significantly higher on the CSS ADOS-2 score compared to the AN group (6.78 ± 1.805 versus 1.77 ± 1.305, t: 12.101, p < 0.001, for ASD and AN groups, respectively). Figure 1 presents values for ADOS-2 sub-scores of the AN group. Of the 30 participants of the AN group, 27 were below the established threshold for ASD. One participant was classified on the ADOS-2 algorithm as having autism (ADOS-2: SA = 9, RRB = 0, OT = 9, CSS = 6); two scored in the autism spectrum range (ADOS-2: SA = 7, RRB = 0, OT = 7, CSS = 4 for both participants). Despite exceeding the threshold for ASD, the diagnosis of ASD was not supported by the detailed clinical interviews on developmental history with these participants and their parents. Based on a review by an expert case panel with two multidisciplinary teams specialized in eating disorders or ASD (see methods for details), none of these participants was given a diagnosis of ASD.
Autistic Traits in the AN Group
Table 1 presents ANOVAs for the AN, AN-C, and ASD. When the omnibus test was significant, we examine the post-hoc pairwise comparisons. The AN participants were similar to their controls on autistic trait measures. For example, there were no differences in the AQ total (either child or adolescent and adult versions) or on any of AQ sub-scores (Table 1). By contrast, the AN group had significantly lower scores than the ASD group on the AQ total (either child or adolescent and adult versions) and on the majority of AQ sub-scores. For example, AN participants showed lower mean scores in the AQ child Total score (p < 0.001), and in the AQ adolescent and adult Total score (p < 0.001) as compared to the ASD group.
Participants’ Obsessive–Compulsive Symptoms and Social Perception Skills Differences
As expected, the AN participants resulted similar to the ASD group on obsessive–compulsive measures (Table 1). For example, there were no differences in the CY-BOCS combined scores. By contrast, the AN group had significantly higher scores than their controls on the CY-BOCS combined scores (p < 0.001).
Moreover, the AN participants were similar to the ASD group on theory of mind ability and affect recognition measures (Table 1). Particularly, the AN group reported lower performances on theory of mind ability (p = 0.025) and affect recognition (p = 0.049) measures compared to the AN-C participants.
Participants’ Eating Behaviors Differences
Table 2 presents ANOVA for the ASD, AN, and ASD-C. As expected, the ASD participants were similar to their controls on the eating behavior measures (Table 2). For example, there were no differences on the majority of eating behavior measures. Moreover, the ASD participants had significantly lower scores then the AN group in most of the eating behavior measures.
Discussion
This study examined the presence of ASD in 30 adolescent females with AN during the acute phase of illness using the ADOS-2. Only 10% (n = 3) of participants in the AN group scored above the conventional ADOS-2 threshold for ASD. Although none of these three participants was rated as having RRB, it has to be noted that girls with ASD report less RRB than boys, and may exhibit a different pattern of RRB as that presented by males (Szatmari et al. 2012; Van Wijngaarden-Cremers et al. 2013). Therefore, it is possible that RRB of females may be more difficult to detect. Nonetheless, an in-depth assessment of developmental history with these three participants and their parents did not identify symptoms of ASD during childhood. Basing on review of all information gathered from the assessment by two multidisciplinary teams specialized in eating disorders or ASD (see methods for details), none of these participants was given a diagnosis of ASD. Thus, the current social impairments detected in these participants may be attributed to the acute phase of anorexia. These results are in line with the study of Pooni et al. (2012) investigating whether young people (8–16 years) with an eating disorder have a higher prevalence of ASD. In this study only one out of 22 participants with an early onset eating disorder met criteria for ASD (Pooni et al. 2012). By contrast, our results are not consistent with a case series of ten women with AN and suspected ASD. In that study, seven of ten women exceeded the threshold for ASD on the ADOS (Mandy and Tchanturia 2015). Based on personal history, these authors ruled out the effect of starvation as the cause of the observed social difficulties and inflexibility because these patients revealed the presence of ASD symptoms in childhood. However, the high rate of women with autism in this case series could have been biased by the inclusion criterion of suspected ASD.
As suggested by Pooni et al. (2012), a possible interpretation of the lower rates of autism found in adolescent samples with AN can be related to the fact that the lifetime course of this condition is highly variable (Pooni et al. 2012). In more details, the recovery rate for adolescent AN is relatively high (Mandy et al. 2011). Therefore, adult samples with AN consist of unrecovered adolescent patients, thereby being more chronic. The presence of ASD may be a predictor for treatment non-responders, which might be consequently overrepresented in adult samples. However, further longitudinal studies are needed in order to test this hypothesis.
Regarding the presence of autistic traits in patients with AN, a recent systematic review and meta-analysis indicated that this clinical population appears to have significantly greater autistic traits than controls (Westwood et al. 2015). In the current study, however, participants with AN were similar to controls on the AQ total (either child or adolescent and adult versions) and on the AQ sub-scores. Moreover, the AN group scored significantly lower on the AQ total (either child or adolescent and adult versions) and on the majority of AQ sub-scores compared to the ASD group. It has to be noted that sex differences on the developmental stability of autistic traits have been reported among typically developing toddles (Whitehouse et al. 2011). Specifically, Whitehouse et al. (2011) examining the long-term stability of autistic traits in 360 males and 400 females from the general population, found that these traits were stable from childhood to adulthood only in males. Again, in line with the observation that females with ASD may present a different behavioral phenotype, it can be possible that these results have been biased by the fact that self-assessment measures may be not adequate to capture the sub-threshold autistic symptoms in females.
Moreover, studies exploring the presence of autistic traits using the AQ in other clinical populations (e.g., schizophrenia patients), have reported that this measure could be not enough sensitive in making differential diagnoses (e.g., differentiating between individuals with schizophrenia and those with ASD and schizophrenia) (Lugnegard et al. 2015). Although none of the AN participants exceeded the conventional threshold for repetitive behavior on the ADOS-2, obsessive–compulsive symptom severity was mild to moderate in both AN and ASD groups. The mean score on the CY-BOCS was 10.30 ± 10.07 for the AN group which was significantly higher than the 0.46 ± 1.74 in the AN-C group. These results are similar to prior reports (Solomon et al. 2008; Tchanturia et al. 2011, 2012). For example, Rhind et al. (2014) examined obsessive–compulsive symptoms in 150 adolescents with AN or subthreshold AN receiving outpatient treatment (Rhind et al. 2014). In this sample, adolescents with AN had elevated levels of obsessive–compulsive symptoms, and these symptoms did not appear to be familial.
In the current study, the AN and ASD groups showed similar deficits on theory of mind and affect recognition tasks. Indeed, in our sample the AN group performed lower on theory of mind and affect recognition tasks than controls. These findings are similar to previous studies, and may have important treatment implications (Anckarsäter et al. 2012; Baron-Cohen et al. 2013; Carton and Smith 2014; Coombs et al. 2011; Courty et al. 2013; Lopez et al. 2010; Mandy and Tchanturia 2015; Oldershaw et al. 2010; Rhind et al. 2014; Tchanturia et al. 2013). Specifically, to enhance treatment adherence and improve the chance of recovery, patients with AN accompanied by social and affect recognition deficits may benefit from novel interventions that target these deficits, such as Cognitive Remediation and Emotion Skills Training (CREST) (Tchanturia et al. 2014).
In line with prior studies, exploratory analyses of eating behaviors showed that the ASD participants were similar to their controls on these measures (Courty et al. 2013).
This study has several limitations that should to be taken into account. First, this is a cross-sectional study, and although useful for generating hypotheses, these hypotheses need to be confirmed by longitudinal investigations. Second, our sample included a wide age range (from 10 to 17). This required use of the parent-report version of the AQ for children (range = 0–150) versus the adolescent and adult versions (range = 0–50). Thus, analysis for the child version had to be performed separately from the other two versions, and this could have affected the power of our results for this measure. Third, given that the lifetime course of AN is highly variable and this study included only adolescent participants, our results may not generalize to adults with AN. Fourth, given our stringent selection criteria (i.e., age, IQ level, diagnosis and for the AN sample phase of illness) the available participants’ pool was limited, therefore we used consecutive sampling. However, all consecutive patients admitted to the outpatient unit for a first assessment joined the study (100% for the AN sample and 100% for the ASD sample). Therefore, the response rate for the present study was high, and non-response bias was minimal. Finally, the AQ has shown the capacity to distinguish individuals with ASD from the general population. However, as previously discussed, this measure may have missed autistic traits in this sample of young women in the acute phase of AN.
In conclusion, our study adds new insight to the literature on the link between people with AN and ASD. Our findings do not support the presence of autistic traits in individuals with AN. However, in this sample of adolescent females with AN during starvation phase, we observed similar deficits in theory of mind task to ASD patients. The potential association between theory of mind deficits and the starvation phase in AN requires further investigation. For example, longitudinal studies investigating theory of mind in AN patients from illness to recovery could clarify whether these characteristics persist after recovery from AN.
References
American Psychiatric Association. (2013). The diagnostic and statistical manual of mental disorders. (5th ed). Washington, DC: American Psychiatric Association.
Anckarsäter, H., Hofvander, B., Billstedt, E., Gillberg, I. C., Gillberg, C., Wentz, E., et al. (2012). The sociocommunicative deficit subgroup in anorexia nervosa: autism spectrum disorders and neurocognition in a community-based, longitudinal study. Psychological Medicine, 42, 1957–1967.
Anderluh, M., Tchanturia, K., Rabe-Hesketh, S., Collier, D., & Treasure, J. (2009). Lifetime course of eating disorders: Design and validity testing of a new strategy to define the eating disorders phenotype. Psychological Medicine, 39, 105–114.
Auyeung, B., Baron-Cohen, S., Wheelwright, S., & Allison, C. (2008). The autism spectrum quotient: Children’s version (AQ-Child). Journal of Autism and Developmental Disorders, 38, 1230–1240.
Baron-Cohen, S., Hoekstra, R. A., Knickmeyer, R., & Wheelwright, S. (2006). The autism spectrum quotient (AQ)-adolescent version. Journal of Autism and Developmental Disorders, 36, 343–350.
Baron-Cohen, S., Jaffa, T., Davies, S., Auyeung, B., Allison, C., & Wheelwright, S. (2013). Do girls with anorexia nervosa have elevated autistic traits? Molecular Autism, 4, 24.
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The autism spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31, 5–17.
Birmingham, C. L., & Beumont, P. J. V. (2004). A Practical handbook for healthcare professionals. Medical management of eating disorders. Cambridge: Cambridge University Press.
Calderoni, S., Fantozzi, P., Balboni, G., Pagni, V., Franzoni, E., Apicella, F., et al. (2015). The impact of internalizing symptoms on autistic traits in adolescents with restrictive anorexia nervosa. Neuropsychiatric Disease and Treatment, 11, 75–85.
Calderoni, S., Muratori, F., Leggero, C., Narzisi, A., Apicella, F., Balottin, U., et al. (2013). Neuropsychological functioning in children and adolescents with restrictive-type anorexia nervosa: an in-depth investigation with NEPSY-II. Journal of Clinical and Experimental Neuropsychology, 35(2), 167–179.
Carton, A. M., & Smith, A. D. (2014). Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eating and Weight Disorders, 19(3), 285–293.
Chakraborty, K., & Basu, D. (2010). Management of anorexia and bulimia nervosa: an evidence-based review. Indian Journal of Psychiatry, 52(2).
Coombs, E., Brosnan, M., Bryant-Waugh, R., & Skevington, S. M. (2011). An investigation into the relationship between eating disorder psychopathology and autistic symptomatology in a non-clinical sample. British Journal of Clinical Psychology, 50, 326–338.
Courty, A., Maria, A. S., Lalanne, C., Ringuenet, D., Vindreau, C., Chevallier, C., et al. (2013). Levels of autistic traits in anorexia nervosa: a comparative psychometric study. BMC Psychiatry, 13, 222.
Cuzzolaro, M., Vetrone, G., Marano, G., & Battacchi, M. (2000). Body uneasiness test (BUT) In L. Conti (Eds.), Repertorio delle scale di valutazione in psichiatria. Firenze: SEE.
Cuzzolaro, M., Vetrone, G., Marano, G., & Garfinkel, P. E. (2006). The body uneasiness test (BUT): Development and validation of a new body image assessment scale. Eating and Weight Disorders, 11, 1–13.
De Bildt, A., Systema, S., Kraijer, D., & Minderaa, R. (2004). Prevalence of pervasive developmental disorders in children and adolescents with mental retardation. Journal of Child Psychology and Psychiatry, 46, 275–286.
Developmental Disabilities Monitoring Network Surveillance Year (2010). Principal Investigators; Centers for Disease Control and Prevention (CDC). (2014). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report. Surveillance Summaries, 63(2), 1–21.
Garner, D. M. (2004). Eating disorder inventory-3. Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc.
Garner, D. M., Olmsted, M. P., Bohr, Y., & Garfinkel, P. (1982). The eating attitude test: psychometric features and clinical correlates. Psychological Medicine, 12(4), 871–878.
Giannini, M., Pannocchia, L., Dalle Grave, R., Muratori, F., & Viglione, V. (2008). Eating Disorder Inventory-3. Firenze: Giunti OS.
Gillberg, C., Råstam, M., & Gillberg, C. (1995). Anorexia nervosa 6 years after onset: Part I. Personality disorders. Comprehensive Psychiatry, 36, 61–69.
Hambrook, D., Tchanturia, K., Schmidt, U., Russell, T., & Treasure, J. (2008). Empathy, systemizing, and autistic traits in anorexia nervosa: A pilot study. British Journal of Clinical Psychology, 47, 335–339.
Head, A. M., McGillivray, J. A., & Stokes, M. A. (2014). Gender differences in emotionality and sociability in children with autism spectrum disorders. Molecular Autism, 5(1), 19.
Holtmann, M., Bolte, S., & Poustka, F. (2007). Autism spectrum disorders: Sex differences in autistic behavior domains and coexisting psychopathology. Developmental Medicine & Child Neurology, 49(5), 361–366.
Huke, V., Turk, J., Saeidi, S., Kent, A., & Morgan, J. F. (2013). Autism spectrum disorders in eating disorder populations: a systematic review. European Journal of Eating Disorders Review, 21(5), 345–351.
Huke, V., Turk, J., Saeidi, S., Kent, A., & Morgan, J. F. (2013). The clinical implications of high levels of autism Spectrum disorder features in anorexia nervosa: A pilot study. European Journal of Eating Disorders Review, 22(2), 116–121.
Hus, V., & Lord, C. (2014). The autism diagnostic observation schedule, Module 4: Revised algorithm and standardized severity scores. Journal of Autism and Developmental Disorders, 44(8), 1996–2012.
Korkman, M., Kirk, U., & Kemp, S. (2011). NEPSY– II. Italian adaptation. (2nd ed). Firenze: Giunti O.S. Organizzazioni Speciali.
Korkman, M., Kirk, U., & Kemp, S. L. (2007). NEPSY-II. Clinical and interpretative manual. San Antonio TX: Psychological Corporation.
Lang, K., Dapelo, M. M., Khondoker, M., Morris, R., Surguladze, S., Treasure, J., & Tchanturia, K. (2015). Exploring emotion recognition in adults and adolescents with anorexia nervosa using a body motion paradigm. European Eating Disorders Review.
Lang, K., Lopez, C., Stahl, D., Tchanturia, K., & Treasure, J. (2014a). Central coherence in eating disorders: An updated systematic review and meta-analysis. The World Journal of Biological Psychiatry, 15(8), 586–598.
Lang, K., Stahl, D., Espie, J., Treasure, J., & Tchanturia, K. (2014b). Set shifting in children and adolescents with anorexia nervosa: An exploratory systematic review and meta-analysis. International Journal of Eating Disorders, 47(4), 394–399.
Lopez, C., Stahl, D., & Tchanturia, K. (2010). Estimated intelligence quotient in anorexia nervosa: A systematic review and meta-analysis of the literature. Annals in General Psychiatry, 9, 40.
Lord, C., Luyster, R., Gotham, K., & Guthrie, W. (2012). Autism diagnostic observation schedule, Second edition (ADOS-2) Manual (Part II): Toddler module. Torrance, CA: Western Psychological Services.
Lord, C., Rutter, M., Di Lavore, P.C., Risi, S., & Gotham, K. (2012). ADOS-2: Autism diagnostic observation schedule. Los Angeles: Western Psychological Service.
Lugnegard, T., Hallerback, M. U., & Gillberg, C. (2015). Asperger syndrome and schizophrenia: Overlap of self-reported autistic traits using the Autism-spectrum Quotient (AQ). Nordic Journal of Psychiatry, 69(4), 268–274.
Mandy, W., Charman, T., Gilmour, J., & Skuse, D. (2011). Towards specifying pervasive developmental disorder unspecified. Autism Research, 4, 121–131.
Mandy, W., & Tchanturia, K. (2015). Do women with eating disorders who have social and flexibility difficulties really have autism? A case series. Molecular Autism, 6, 6.
Mazzone, L., Postorino, V., De Peppo, L., Fatta, L., Lucarelli, V., Reale, L., et al. (2013). Mood symptoms in children and adolescents with autism spectrum disorders. Research in Developmental Disabilities, 34(11), 3699–3708.
Mazzone, L., Ruta, L., & Reale, L. (2012). Psychiatric comorbidities in asperger syndrome and high functioning autism: diagnostic challenges. Annals of General Psychiatry, 11(1), 16.
Mehler, P. S., & Andersen, A. E. (1999). Eating disorders: A guide to medical care and complications. Baltimore: Johns Hopkins University Press.
Miller, J. S., Bilder, D., Farley, M., Coon, H., Pinborough-Zimmerman, J., Jenson, W., et al. (2012). Autism spectrum disorder reclassified: A second look at the 1980s Utah/UCLA autism epidemiologic study. Journal of Autism and Developmental Disorders, 43, 200–210.
Miller, K. K., Grinspoon, S. K., Ciampa, J., Hier, J., Herzog, D., & Klibanski (2005). A. medical findings in outpatients with anorexia nervosa. Archives of Internal Medicine, 165, 561–566.
Mondschein, E. R., Adolph, K. E., & Tamis-LeMonda, C. S. (2000). Gender bias in mothers’ expectations about infant crawling. Journal of Experimental Child Psychology, 77(4), 304–316.
Oldershaw, A., Hambrook, D., Tchanturia, K., Treasure, J., & Schmidt, U. (2010). Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosomatic Medicine, 72(1), 73–79.
Pooni, J., Ninteman, A., Bryant-Waugh, R., Nicholls, D., & Mandy, W. (2012). Investigating autism spectrum disorder and autistic traits in early onset eating disorder. International Journal of Eating Disorders 45,4 583–591.
Postorino, V., Fatta L. M., De Peppo L., Giovagnoli G., Armando M., Vicari S., et al. (2015). Longitudinal comparison between male and female preschool children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(7), 2046–2055.
Råstam, M. (1992). Anorexia nervosa in 51 Swedish adolescents: Premorbid problems and comorbidity. Journal of the American Academy of Child and Adolescent Psychiatry, 31, 5.
Råstam, M., Gillberg, C., & Wentz, E. (2003). Outcome of teenage onset anorexia nervosa in a Swedish-community based sample. European Child and Adolescent Psychiatry, 12, 78–90.
Raven, J. C. (2008a). Standard Progressive Matrices. Firenze: Giunti Organizzazioni Speciali.
Raven, J. C. (2008b). Colored Progressive Matrices. Firenze: Giunti Organizzazioni Speciali.
Rhind, E., Bonfioli, R., Hibbs, E., Goddard, P., Macdonald, S., Gowers, U., et al. (2014). An examination of autism spectrum traits in adolescents with anorexia nervosa and their parents. Molecular Autism, 5, 56.
Roberts, M. E., Tchanturia, K., Stahl, D., Southgate, L., & Treasure, J. (2007). A systematic review and meta-analysis of set-shifting ability in eating disorders. Psychological Medicine, 37(8), 1075–1084.
Russell, G., Steer, C., & Golding, J. (2011). Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Social Psychiatry and Psychiatric Epidemiology, 46, 1283–1293.
Russell, T. A., Schmidt, U., Doherty, L., Young, V., & Tchanturia, K. (2009). Aspects of social cognition in anorexia nervosa: affective and cognitive theory of mind. Psychiatry Research, 168(3), 181–185.
Ruta, L., Mazzone, D., Mazzone, L., Wheelwright, S., & Baron-Cohen, S. (2012). The Autism-Spectrum Quotient-Italian version: a cross-cultural confirmation of the broader autism phenotype. Journal of Autism and Developmental Disorders, 42, 625–633.
Scahill, L., Riddle, M. A., McSwiggin-Hardin, M., Ort, S. I., King, R. A., Goodman, W. K., et al. (1997). Children’s Yale- Brown Obsessive Compulsive Scale: reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry, 36(6), 844–852.
Solomon, M., Ozonoff, S. J., Cummings, N., & Carter, C. S. (2008). Cognitive control in autism spectrum disorders. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 26, 239–247.
Storch, E. A., Murphy, T. K., Adkins, J. W., Lewin, A. B., Geffken, G. R., Johns, N. B., et al. (2006). The children’s Yale-brown obsessive–compulsive scale: Psychometric properties of child-and parent report formats. Journal of Anxiety Disorders, 20(8), 1055–1070.
Szatmari, P., Liu, X. Q., Goldberg, J., Zwaigenbaum, L., Paterson, A. D., Woodbury-Smith, M., et al. (2012). Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. American. Journal of Medical Genetics. Part B. Neuropsychiatric Genetics, 159(B1), 5–12.
Tchanturia, K., Davies, H., Harrison, A., Roberts, M., Nakazato, M., Schmidt, U., et al. (2012). Poor cognitive flexibility in eating disorders: examining the evidence using the Wisconsin Cart Sorting Task. PLoS ONE, 7(1), e28331.
Tchanturia, K., Doris, E., & Fleming, C. (2014). Effectiveness of cognitive remediation and emotion skills training (CREST) for anorexia nervosa in group format: a naturalistic pilot study. European Eating Disorders Review, 22(3), 200–205.
Tchanturia, K., Happé, F., Godley, J., Treasure, J., Bara-Carril, N., & Schmidt, U. (2004). “Theory of mind” in anorexia nervosa. European Eating Disorder Review, 12, 361–366.
Tchanturia, K., Harrison, A., Davies, H., Roberts, M., Oldershaw, A., Nakazato, M., et al. (2011). Cognitive flexibility and clinical severity in eating disorders. PLoS ONE, 6(6), e20462.
Tchanturia, K., Smith, E., Weineck, F., Fidanboylu, E., Kern, N., Treasure, J., et al. (2013). Exploring autistic traits in anorexia: A clinical study. Molecular Autism, 4, 44.
Urgesi, C., Campanella, F., & Fabbro, F. (2011). NEPSY–II. Second edition. Contributo alla taratura italiana (NEPSY–II. Second edition. Italian Standardization). Firenze. Italy: Giunti O.S. Organizzazioni Speciali.
Van Wijngaarden-Cremers, P.J., van Eeten, E., Groen, W.B., Van Deurzen, P.A., Oosterling, I.J., & Van der Gaag, R.J. (2013). Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic Review and meta-analysis. Journal of Autism and Developmental Disorders, 30.
Wentz Nilsson, E., Gillberg, C., & Råstam, M. (1998). Familial factors in anorexia nervosa: A community-based study. Comprehensive Psychiatry, 39, 392–399.
Wentz Nilsson, E., Lacey, J. H., Waller, G., Rastam, M., Turk, J., & Gillberg, C. (2005). Childhood onset neuropsychiatric disorders in adults eating disorder patients. A pilot study. European Child and Adolescent Psychiatry, 14, 431–437.
Westwood, H., Eisler, I., Mandy, W., Leppanen, J., Treasure, J., & Tchanturia, K. (2015). Using the autism-spectrum quotient to measure autistic traits in anorexia nervosa: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 46(3), 964–977.
Whitehouse, A. J. O., Hickey, M., & Ronald, A. (2011). Are autistic traits in the general population stable across development? PLoS ONE, 6(8), e23029.
Author Contribution
VP designed, conducted, supervised the study and wrote the manuscript, LS interpreted the data, corrected drafts, and helped in the writing process, LDP, LMF, VZ, and MCC helped in the recruitment and in the data collection process, SG performed the analysis, interpreted the data and helped with the revisions of the manuscript; SV assisted in the write up of the study; LM designed and supervised the project, and revised the draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Postorino, V., Scahill, L., De Peppo, L. et al. Investigation of Autism Spectrum Disorder and Autistic Traits in an Adolescent Sample with Anorexia Nervosa. J Autism Dev Disord 47, 1051–1061 (2017). https://doi.org/10.1007/s10803-016-3023-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-016-3023-y