Abstract
Mothers, fathers, and siblings from 87 multiplex (M-mothers, M-fathers, and M-siblings) and 41 simplex (S-mothers, S-fathers, and S-siblings) Autism spectrum disorder families were assessed using the Broader Phenotype Autism Symptom Scale. S-mothers, S-fathers, and S-siblings showed more social interest and were more expressive in their use of nonverbal communication compared to M-mothers, M-fathers, and M-siblings. Conversational skills were also improved in S-fathers and S-siblings compared to M-fathers and M-siblings. S-siblings showed significantly lower rigidity and intense interests compared to M-siblings. The decreased number and intensity of broader autism phenotype traits observed in parents and siblings within simplex families provide behavioral evidence consistent with findings of increased de novo genetic events in simplex families.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder defined by impairments in social communication and restricted and repetitive interests and behaviors. Genetic influence in ASD is strong. For instance, probandwise concordance rates of 58–77 % were recently observed in monozygotic (MZ) twins versus 21–36 % for dizygotic (DZ) twins (Hallmayer et al. 2011), confirming early reports of discrepant concordance rates between MZ and DZ twins (e.g., Folstein and Rutter 1977). Infant sibling risk rates are similar to concordance rates in DZ twins: approximately 20 % (Ozonoff et al. 2011). These behavioral genetic studies suggest that ASD risk rises as the level of shared genes increases.
Molecular findings suggest that copy number variants (CNVs) in genic regions are more common in individuals with ASD compared to controls (as reviewed in Abrahams and Geschwind 2008). De novo or non-inherited CNVs appear to be particular risk factors in simplex (families with one clinically diagnosed individual) ASD families relative to multiplex (families with more than one clinical diagnosed individual) ASD families, with de novo CNV rates ranging from 5 to 10 % (Pinto et al. 2010; Sanders et al. 2011; Sebat et al. 2007).
In addition to the examination of specific genetic mechanisms in ASD, the familiality of ASD-related traits in family members of individuals with ASD has been examined. Subclinical differences in social skills, communication abilities, and personality traits compared to controls (for review, see Gerdts and Bernier 2011) are generally considered to constitute the broader autism phenotype (BAP). Social traits, such as decreased interest in reciprocal social interactions and a focus on special interests as a conversational topic, identified using clinical interviews have been noted more often in parents and siblings of individuals with ASD compared to parents and siblings in control groups (Bolton et al. 1994; Piven et al. 1997a; Wolff et al. 1988). Like the diagnosis of ASD, BAP traits tend to aggregate more often in male compared to female relatives (Bolton et al. 1994; Pickles et al. 2000; Piven et al. 1997a; Schwichtenberg et al. 2010). Most studies find that at least half of relatives do not have quantifiable differences, suggesting that traits may present in only a subset of family members (e.g., Landa et al. 1992; Piven et al. 1997b).
A greater number of affected children within a family appears to increase the likelihood of observable BAP traits in family members. Improved social responsiveness has been found in siblings from simplex families (S-siblings) versus siblings from multiplex families (M-siblings). Social responsiveness is less consistently found to be increased in parents from simplex families (S-parents) versus multiplex families (M-parents; Constantino et al. 2010; Schwichtenberg et al. 2010; Virkud et al. 2009). Szatmari et al. (2000) found that social impairments, but not communication challenges or presence of restricted behaviors, were more common in immediate and extended family members from multiplex versus simplex families. In the most extensively phenotyped sample to date, Losh et al. (2008) reported that M-parents had more ASD-related personality traits, fewer quality friendships, and decreased pragmatic language skills than S-parents (Losh et al. 2008). Recently, Bernier et al. (2012) reported greater impairment in social communication skills in M-parents compared with S-parents, parents of children with non-ASD developmental delays (DD), and parents of typically developing children. No differences in BAP traits were found among S-parents, DD, and typical parent groups, which may signify that S-parents do not possess a greater number or intensity of social BAP traits than the population at large. If affected children from simplex families are more likely than those from multiplex families to develop ASD as a result of a de novo genetic event (e.g., Sebat et al. 2007), then findings of an increased presence of ASD-related traits in multiplex families may suggest that such family members are more vulnerable to ASD symptoms given shared genetic variance.
Important work in this area has established the possibility that the BAP is increased in families with more children with ASD. However, it is difficult to interpret some of the previous literature because exclusionary criteria regarding family history in simplex families has generally not been described and the majority of measures have either been questionnaire-based or have relied on clinical interview with parents only. In this study, we intend to augment findings of differences in BAP traits in simplex versus multiplex families by addressing these potential limitations in the current literature.
The Broader Phenotype Autism Symptom Scale (BPASS; Dawson et al. 2007) was used to directly assess, by clinical examination and interview, social and communication skills and range of interests and flexibility in mothers, fathers, and siblings with no diagnosis of ASD. The BPASS uses behavioral observation in addition to interview, is designed to assess both children and adults, and has demonstrated behavioral-genetic relations in prior research (Sung et al. 2005). One study to date has demonstrated differences in the BAP between S-parents and M-parents using the BPASS (Bernier et al. 2012). However, this study focused on mothers and did not include siblings. Since there are sex differences in the BAP, a larger and more representative sample is needed to take gender into account. To our knowledge, this is the first investigation using direct clinical assessment to compare the BAP in S-siblings versus M-siblings.
Methods
Participants
Participants were recruited from two genetic studies of ASD conducted at the University of Washington (UW) Autism Center: the Family Study of Autism (FSA; 1998–2007) and the Simons Simplex Collection (SSC; 2007–2011). Study procedures, protocols, clinical staff, measures, and inclusionary/exclusionary criteria were comparable for the FSA and SSC. Exceptions include age requirements (minimum of 3 years for FSA versus minimum of 4 years and maximum of 17 years, 11 months for SSC) and minimal level of functioning for affected children (SSC required a nonverbal mental age of at least 18 months). Additionally, while it was preferred that both parents participate in the FSA, both biological parents were required to participate in the SSC. Exclusionary criteria regarding ASD diagnosis in undiagnosed family members were also different for the studies and were more stringent for simplex than multiplex families (explained below). For both studies, exclusionary criteria included presence of a known genetic condition (e.g., Fragile X), significant birth complications, history of serious head injury or neurological disease, significant sensory or motor impairment affecting measure completion, and language other than English as a primary language. Studies were approved by the University of Washington institutional review board and appropriate informed consent was obtained from all participants.
Demographic information for the subset of families included in these analyses was similar (Table 1) in terms of age at evaluation, race, and ethnicity with two exceptions: (1) mothers and fathers in the SSC were slightly (but significantly) older than in the FSA, t(125) = 3.49, p < 0.001 and t(110) = 2.21, p = 0.03, respectively, and (2) fathers in the SSC obtained a higher level of education, χ 2(4, N = 109) = 15.98, p = .003. BPASS scores have not been found to relate to parent education level in prior studies and, thus, were not entered as covariates in analyses for this study (Dawson et al. 2007).
Multiplex Families
Five hundred fifty families were screened for the FSA and self-identified as having more than one child with ASD. These families were then interviewed with the Autism Diagnostic Interview-Revised (ADI-R; Lord et al. 1994) to identify families with a high probability of having two children with ASD. A direct assessment of all affected family members, parents, and at least one unaffected sibling, if available, was then conducted, including the BPASS. Additional measures consisted of a diagnostic evaluation with affected children and a neuropsychological assessment, blood sample, and physical measurements from each of the participating family members.
Following the diagnostic evaluation, 311 families had at least two children who met the research diagnostic criteria specified below. In addition to general FSA requirements, specific conditions were implemented to address the research questions in this project. First, only families with at least one child without a diagnosis of ASD (full biological siblings only, verified by genotyping), in addition to two children with ASD, were analyzed to assess the BAP in undiagnosed siblings (n = 97). Further exclusionary criteria were applied in families with more than one unaffected sibling (n = 26). In seven of these families, the “least affected” sibling, as determined by parent-report during the screening process, was directly assessed. This procedure was implemented due to time constraints during evaluations conducted out-of-state in order to maximize the difference in genetic effects between “affected” and “unaffected” children. Since this procedure would produce a systematic bias for the purposes of this study, these seven families with more than one unaffected child were excluded from analyses. Lastly, an additional three families were excluded because the two affected children in the family were MZ twins.
Thus, of the 311 multiplex families, 87 met additional inclusion criteria for this study. These families contained 117 unaffected children (58 males and 59 females) and 184 affected children (149 males and 35 females) diagnosed with autistic disorder (n = 137), Asperger’s disorder (n = 23), or pervasive developmental disorder not otherwise specified (PDD-NOS, n = 24). Families had two (n = 79), three (n = 7), or five (n = 1) affected children and one (n = 68), two (n = 12), three (n = 5), four (n = 1), or six (n = 1) unaffected children. The most common family constellation was two affected children and one unaffected child (n = 62).
Simplex Families
The UW was one of 12 SSC sites with a goal of examining the genetic risk associated with the single occurrence of ASD in the family. Families were recruited if they reported having one child in the family with diagnosed or suspected ASD. Four hundred fifty-three families were screened at UW and 269 families met study criteria. Families were required to have exactly one child who met research diagnostic criteria outlined below.
Families were carefully screened for a history of ASD via parent interview, and were excluded if any first, second, or third degree relative had a diagnosis of ASD. Additionally, families with siblings and parents who were suspected to have a diagnosis of ASD or intellectual disability were excluded from study participation. Participating siblings had to have a composite score of 70 or above on the Vineland Adaptive Behavior Scales- 2nd Edition (Sparrow et al. 2005) to demonstrate adaptive abilities that were within two standard deviations of the norm. During the course of the study, new criteria were implemented to exclude families containing parents and siblings with significant subclinical ASD-related traits. Four families out of 453 screened at UW were excluded for this criterion and the majority of families invited back to participate in the current project were evaluated early in SSC data collection, during which time this criterion had not been established universally.
Like the multiplex sample, only those families with at least one other unaffected full biological sibling (n = 227) were recruited to participate. Because unaffected SSC siblings and parents did not participate in a behavioral assessment, they were recontacted to participate in a second evaluation. Of the SSC families with an additional sibling who were recontacted, 67 % (n = 47) agreed to participate and 59 % (n = 41) completed research measures. 34 % either declined to participate (largely because of time constraints) or did not return recruitment calls. Affected children (35 males and 6 females) had diagnoses of autistic disorder (n = 27), Asperger’s disorder (n = 9), or PDD-NOS (n = 5). There were no MZ twins in this subset of families.
We focused recruitment efforts on those families who had multiple unaffected siblings in the family. This was done to: (1) better account for systematic differences in family size across simplex and multiplex families who, by definition, needed to have a minimum of three children (two affected and one unaffected) to be included in these analyses, and (2) theoretically increase the chance that ASD occurred in the affected child as a result of a sporadic genetic mutation. 73 % (n = 30) of the simplex sample included multiple unaffected children while the remaining 27 % (n = 11) had one unaffected sibling. Families had exactly one affected child and either one (n = 11), two (n = 21), three (n = 8), or five (n = 1) unaffected children. If there was more than one unaffected sibling, the closest in age to the affected child was seen for evaluation (20 males and 21 females).
Measures
ASD Diagnosis
Children were considered to be affected if they met diagnostic cutoffs on the revised algorithm of the Autism Diagnostic Observation Schedule (ADOS; Gotham et al. 2007), met clinical cutoffs using the Collaborative Programs of Excellence in Autism (CPEA) criteria on the ADI-R (see Schellenberg et al. 2006), and received a clinical diagnosis of either autistic disorder, Asperger’s disorder, or PDD-NOS. In both the FSA and SSC, clinicians were reliably trained on the ADOS and ADI-R. Reliability checks were performed with local site supervisors who were research reliable with the developers of the measures.
Assessment of Family Members
The BPASS (Dawson et al. 2007) was administered by ADOS- and ADI-R-reliable clinicians to measure autism-related traits in family members across four domains of functioning using clinician ratings via both observation and interview. The coding system of the BPASS is based on trained examiner judgment as to what behavior is normative and what is outside of the normal range. In general, codes of 1 or 2 on individual BPASS items are considered to be normative and scores of 3, 4, or 5 are considered to be below the normal range. BPASS composite scores are calculated by taking the average of individual items within domains. To insure consistency and prevent rater drift, 10 % of BPASS administrations were coded from videotape for reliability by a group of experienced BPASS clinicians, and percent agreements were greater than 80 %.
The Social Interest domain assesses social motivation via questions regarding interest in peers and groups and self-perception of social comfort in groups. A summary score capturing both child- and adulthood preferences (before and after having children) is coded for each item. The Expressiveness domain assesses social expressivity and is based on clinical ratings of nonverbal social communication observed during the interview (e.g., integrated gaze, social smiling, and facial expressions). The Conversation domain uses clinical observations of conversation skills, such as excessive detail and decreased sensitivity to the interviewer (e.g., making comments without adequate background information). The Flexibility/Restricted Interests domain of the BPASS assesses flexibility in physical space and daily schedule as well as interests in both child- and adulthood. The breadth, type, and intensity of interests are assessed through descriptions of preferences in daily schedule and physical environment. Scores range from extreme flexibility in routine and physical space to marked rigidity in these areas causing impairment in relationships or emotional distress if disrupted.
The proportion of family members receiving one of the two highest codes (suggestive of BAP) on the Social Interest and Flexibility/Range of Interests BPASS domains was assessed. In the Social Interest domain, these codes suggest low to very low interest in interacting with others and substantial apprehension and rare initiation of social contact in groups. In the Flexibility/Range of Interests domain, these codes suggest unusual or impairing interests, a strong preference for predictability and routine that is bothersome to others, and limited adaptability and functionality in terms of preference for order in the physical environment.
Statistical Analyses
Data from mothers, fathers, and undiagnosed siblings in multiplex and simplex families were analyzed. MANOVAs were computed for the Social Interest and Flexibility/Range of Interests domains with simplex versus multiplex status included as a fixed factor. Planned contrasts using independent samples t tests compared S-mothers to M-mothers, S-fathers to M-fathers, and S-siblings to M-siblings. Because scores from the Expressiveness and Conversation domains were positively skewed and did not follow a normal distribution, we conducted a Mann–Whitney for those comparisons. Proportions of family members with elevated scores were also compared among simplex and multiplex families using Chi square analyses.
Results
Table 2 presents descriptive statistics for BPASS scores across the four domains. MANOVAs for the BPASS Social Interest and Flexibility/Range of Interest domains yielded significant main effects of simplex versus multiplex status on Social Interest, F(1, 331) = 14.90, p < .001 and Range of Interests/Flexibility, F(1, 331) = 4.07, p = .04. Mann–Whitney U analyses indicated significant differences on Expressiveness, p < .001 and Conversation domains, p = .001.
Planned contrasts using independent t tests indicated significant differences in Social Interest between mothers, t(123) = −2.51, p = .01, Cohen’s d = 0.48, and fathers, t(109) = −2.15, p = .03, Cohen’s d = 0.42, with a trend in differences observed in siblings, t(96) = −1.81, p = .07, Cohen’s d = 0.38, from simplex and multiplex families. Findings suggested increased social interest in S-mothers, S-fathers, and S-siblings versus M-mothers, M-fathers, and M-siblings. S-mothers were also significantly more expressive in their use of nonverbal communication versus M-mothers, p = .006, as were S-fathers, p = .006, and S-siblings, p < .001. Conversational skills were also improved in S-fathers, p = .04, and S-siblings, p = .008, compared to M-fathers and M-siblings. The significant overall MANOVA result for the Range of Interests/Flexibility domain appeared to have been driven by siblings: S-siblings showed significantly lower levels of rigidity and intense interests compared to M-siblings, t(96) = −2.30, p = .02, Cohen’s d = 0.48. Mothers and fathers did not differ significantly on this domain. Figures 1, 2, 3, 4 depict the distribution of BPASS scores across domains and family members.
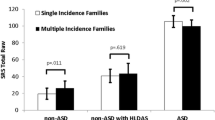
Distribution of social interest domain scores for mothers, fathers, and unaffected siblings in simplex (shown in black) and multiplex (shown in grey) families. Domain scores range from 1 to 5 and are based on codes from semi-structured BPASS interview questions regarding social interest and social apprehension. Lower scores indicate greater social interest and motivation
Distribution of expressiveness domain scores for mothers, fathers, and unaffected siblings in simplex (shown in black) and multiplex (shown in grey) families. Domain scores range from 1 to 3 and are based on codes from observational clinician ratings of nonverbal expressiveness (e.g., facial expressions, social smiling, and eye contact). Lower scores indicate more integrated and appropriate nonverbal expression
Distribution of conversation domain scores for mothers, fathers, and unaffected siblings in simplex (shown in black) and multiplex (shown in grey) families. Domain scores range from 1 to 4 and are based on codes from observational clinician ratings of conversation skills in terms of flexibility and appropriateness of content. Lower scores indicate on-topic conversations that are appropriate in content
Distribution of range of interests/flexibility domain scores for mothers, fathers, and unaffected siblings in simplex (shown in black) and multiplex (shown in grey) families. Domain scores range from 1 to 5 and are based on codes from semi-structured BPASS interview questions regarding preference for sameness in schedule and physical space as well as range of interests. Lower scores indicate flexibility in environment and larger and more flexible range of interests
The percentage of elevated scores (suggesting greater BAP) on the Social Interest and Flexibility/Range of Interests domains were compared across family types. A significantly greater percentage of family members from multiplex versus simplex families were rated as having low to very low social interest, χ 2(1, N = 334) = 14.42, p < .001. 66 % of M-fathers received at least one high score on the Social Interest domain compared to 33 % of S-fathers, χ 2(1, N = 111) = 11.70, p = .001. 39 % of M-siblings were rated highly compared to 22 % of S-siblings, χ 2(1, N = 98) = 3.06, p = .08, which suggests a trend for M-siblings to show lower levels of social interest compared to S-siblings. S-mothers did not differ from M-mothers in the proportion of individuals showing low social interest (32 vs. 44 %, respectively). In the Range of Interests/Flexibility domain, M-siblings versus S-siblings more often had impairing repetitive behaviors, 21 % versus 5 %, χ 2(1, N = 98) = 5.10, p = .02. Mothers and fathers did not differ in their flexibility and range of interests.
Discussion
The BPASS (Dawson et al. 2007) was used to assess social communication skills and the presence of restricted interests and behaviors in undiagnosed first-degree family members in simplex and multiplex ASD families. The BPASS has the benefit of combining responses from a semi-structured clinical interview with behavioral observations. Overall, M-mothers and M-fathers were significantly more likely than S-mothers and S-fathers to demonstrate decreased interest in interacting with peers (both in childhood and adulthood) and to show low initiation of social interactions in group social situations (BPASS Social Interest domain). Additionally, 66 % of M-fathers were rated as having low to very low social interest in at least one domain compared to 33 % of S-fathers. M-siblings showed a trend for decreased social interest, and were significantly more likely to be rated as having very low levels of social interest compared to S-siblings (39 vs. 22 %).
Clinical observation of nonverbal social communication skills (BPASS Expressiveness domain) confirmed self- and parent-report of decreased social interest in multiplex versus simplex families. Mothers, fathers, and siblings from multiplex families less frequently used integrated eye gaze, social smiling, directed facial expressions, and typical vocal prosody compared to family members from simplex families. Further, M-fathers and M-siblings versus S-fathers and S-siblings offered excessive detail in conversation and failed to provide sufficient information in their verbal exchanges (BPASS Conversation Domain) more often, suggesting more impaired conversation skills. Finally, M-siblings had, on average, more patterns of restricted behavior (e.g., preference for sameness in their schedule and physical space) and intense interests compared to S-siblings and were more likely to be rated as having clinically impairing behaviors in these areas (21 vs. 5 %, respectively). This pattern of increased repetitive behaviors and interests was not observed in M-mothers and M-fathers. The lack of difference may be due to the fact that parenting a child with a disability often requires increased structure; this may have overpowered underlying preferences in both family types, despite efforts to disentangle behaviors specific to child-rearing. Findings were similar to reports by Bernier et al. (2012) in which parents of children with ASD and parents of children with non-ASD developmental disabilities showed similar preferences for increased structure in schedule and physical environment compared to parents of typically developing children.
Effect sizes were moderate to large across the four BPASS domains and were most consistently observed in siblings, followed by fathers, and then mothers. In general, differences in the BPASS were more likely to occur in fathers compared to mothers, supporting the role of sex differences in the differential BAP presentation in simplex and multiplex families. There were not enough siblings in the current study to allow for valid comparisons of the BAP separately by sex. M-siblings more consistently presented with decreased social motivation, lower observed verbal and nonverbal communication skills, and impairing repetitive behavior patterns. Thus, undiagnosed children from multiplex families more closely modeled their siblings with ASD whose symptoms involve impairment in these same three domains. This suggests that ASD-related traits in children from multiplex families may lie more on a continuum than in simplex families.
Findings from this study replicate previous reports using clinical interview and questionnaire-based methods (Bernier et al. 2012; Constantino et al. 2010; Losh et al. 2008; Schwichtenberg et al. 2010; Virkud et al. 2009). However, to our knowledge, this is the first report involving a clinical observation and interview of undiagnosed siblings in simplex and multiplex families.
Etiological and Clinical Implications
The decreased number and intensity of BAP traits observed in S-parents and S-siblings provide behavioral support to findings of increased de novo genetic events in simplex families not shared by other family members since ASD-related behavioral traits were found less frequently in simplex compared to multiplex families. Thus, these behaviorally based findings suggest that genetic causes of ASD may vary between single-incidence and multiple-incidence families and that family members in multiplex families are more vulnerable to ASD symptoms given shared genetic variance. An alternative explanation could be that shared, contributory environmental effects, such as stress of multiple children with disabilities, play a significant role in the behavioral presentation of family members of multiple families. Further, it is possible that individual, causal environmental effects, such as exposure to toxic substances in utero (as reviewed in Landrigan 2010), are present in simplex families that are not shared by relatives and this contributes to the observed differences.
In addition to offering insight into ASD etiology, consideration of BAP traits in family members has important clinical implications in terms of treatment planning. An understanding of parent and family factors is crucial to the development of any intervention plan for a child. In ASD, awareness that BAP traits are present in some family members, particularly in families who have multiple children with ASD, should be part of overall family considerations. Additionally, a careful family history prior to treatment onset may be helpful in determining the potential for BAP expression in multiplex families, and prove helpful in clinical settings to recommend the most appropriate treatments for a child.
There were a number of limitations to the current study. The primary drawback was that simplex and multiplex samples were collected as part of separate research projects conducted over nonoverlapping time periods. Although study protocols, measures, clinical raters, and inclusionary/exclusionary criteria were similar across studies, it is nonetheless possible that cohort effects exist. However, given observed differences on particular outcome variables in some groups and not others (e.g., in siblings but not mothers), cohort effects are unlikely to fully account for the results.
Although there were sufficient numbers of participating fathers and mothers to allow for separate analyses in parents, there was an inadequate number of siblings to conduct separate analyses by sex. Since the BAP likely presents differently in males versus females, this warrants further study. Additionally, it is possible that the findings of increased BAP traits in multiplex families could be due to greater parental stress related to the demands of raising multiple children with developmental disabilities. The BPASS is intended to assess and take into account current and past (meaning both childhood and early adulthood) preferences in coding decisions. Nonetheless, it is possible that parents struggled to disentangle current from earlier preferences. Future studies may benefit from including measures of parenting-related stress to address this possible confound. Although it would be challenging, an ideal comparison group would consist of families who had multiple children with non-ASD developmental disabilities.
This study originated from genetic findings of an increased prevalence of de novo CNVs in simplex versus multiplex families. However, many simplex families do not have currently identifiable de novo genetic events. To decrease variability, future research may benefit from focusing on those simplex families who have known de novo mutations to determine the presenting phenotype in the affected child and family.
Conclusion
In general, findings support a differential presentation of the BAP in family members from multiplex compared to simplex families. M-mothers, M-fathers, and M-siblings showed decreased social interest, more impaired nonverbal communication abilities, and less flexible conversation skills compared to S-mothers, S-fathers, and S-siblings. In the area of restricted and repetitive behaviors, M-siblings showed more impairing and repetitive patterns of behavior than S-siblings. Because of the pervasiveness of BAP traits in multiplex families, this pattern of results supports genetic findings of increased rates of de novo genetic mutations in affected children from simplex compared to multiplex families.
References
Abrahams, B. S., & Geschwind, D. H. (2008). Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics, 9(5), 341–355.
Bernier, R., Gerdts, J., Munson, J., Dawson, G., & Estes, A. (2012). Evidence for broader autism phenotype characteristics in parents from multiple incidence autism families. Autism Research, 5(1), 13–20.
Bolton, P., Macdonald, H., Pickles, A., & Rios, P. (1994). A case-control family history study of autism. Journal of Child Psychology and Psychiatry, 35(5), 877–900.
Constantino, J. N., Zhang, Y., Frazier, T., Abbacchi, A. M., & Law, P. (2010). Sibling recurrence and the genetic epidemiology of autism. American Journal of Psychiatry, 167(11), 1349–1356.
Dawson, G., Estes, A., Munson, J., Schellenberg, G., Bernier, R., & Abbott, R. (2007). Quantitative assessment of autism symptom-related traits in probands and parents: Broader Phenotype Autism Symptom Scale. Journal of Autism and Developmental Disorders, 37(3), 523–536.
Folstein, S., & Rutter, M. (1977). Infantile autism: A genetic study of 21 twin pairs. Journal of Child Psychology and Psychiatry, 18(4), 297–321.
Gerdts, J., & Bernier, R. (2011). The broader autism phenotype and its implications on the etiology and treatment of autism spectrum disorders. Autism Research and Treatment, 2011, 545901.
Gotham, K., Risi, S., Pickles, A., & Lord, C. (2007). The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37(4), 613–627.
Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68, 1095–1102.
Landa, R., Piven, J., Wzorek, M. M., & Gayle, J. O. (1992). Social language use in parents of autistic individuals. Psychological Medicine, 22(1), 245–254.
Landrigan, P. J. (2010). What causes autism? Exploring the environmental contribution. Current Opinion in Pediatrics, 22(2), 219–225.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685.
Losh, M., Childress, D., Lam, K., & Piven, J. (2008). Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics, Part B Neuropsychiatric Genetics, 147B(4), 424–433.
Ozonoff, S., Young, G. S., Carter, A., Messinger, D., Yirmiya, N., Zwaigenbaum, L., et al. (2011). Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics, 128(3), e488–e495.
Pickles, A., Starr, E., Kazak, S., Bolton, P., Papanikolaou, K., Bailey, A., et al. (2000). Variable expression of the autism broader phenotype: Findings from extended pedigrees. Journal of Child Psychology and Psychiatry, 41(4), 491–502.
Pinto, D., Pagnamenta, A. T., Klei, L., Anney, R., Merico, D., Regan, R., et al. (2010). Functional impact of global rare copy number variation in autism spectrum disorders. Nature, 466(7304), 368–372.
Piven, J., Palmer, P., Jacobi, D., Childress, D., & Arndt, S. (1997a). Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. American Journal of Psychiatry, 154(2), 185–190.
Piven, J., Palmer, P., Landa, R., Santangelo, S., Jacobi, D., & Childress, D. (1997b). Personality and language characteristics in parents from multiple-incidence autism families. American Journal of Medical Genetics, 74(4), 398–411.
Sanders, S. J., Ercan-Sencicek, A. G., Hus, V., Luo, R., Murtha, M. T., Moreno-De-Luca, D., et al. (2011). Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron, 70(5), 863–885.
Schellenberg, G. D., Dawson, G., Sung, Y. J., Estes, A., Munson, J., Rosenthal, E., et al. (2006). Evidence for multiple loci from a genome scan of autism kindreds. Molecular Psychiatry, 11(11), 1049–1060.
Schwichtenberg, A. J., Young, G. S., Sigman, M., Hutman, T., & Ozonoff, S. (2010). Can family affectedness inform infant sibling outcomes of autism spectrum disorders? Journal of Child Psychology and Psychiatry, 51(9), 1021–1030.
Sebat, J., Lakshmi, B., Malhotra, D., Troge, J., Lese-Martin, C., Walsh, T., et al. (2007). Strong association of de novo copy number mutations with autism. Science, 316(5823), 445–449.
Sparrow, S. S., Cicchetti, D. V., & Balla, D. A. (2005). Vineland adaptive behavior scales (2nd ed.). Minneapolis, MN: NCS Pearson, Inc.
Sung, Y. J., Dawson, G., Munson, J., Estes, A., Schellenberg, G. D., & Wijsman, E. M. (2005). Genetic investigation of quantitative traits related to autism: Use of multivariate polygenic models with ascertainment adjustment. American Journal of Human Genetics, 76(1), 68–81.
Szatmari, P., MacLean, J. E., Jones, M. B., Bryson, S. E., Zwaigenbaum, L., Bartolucci, G., et al. (2000). The familial aggregation of the lesser variant in biological and nonbiological relatives of PDD probands: A family history study. Journal of Child Psychology and Psychiatry, 41(5), 579–586.
Virkud, Y. V., Todd, R. D., Abbacchi, A. M., Zhang, Y., & Constantino, J. N. (2009). Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics), 150B(3), 328–334.
Wolff, S., Narayan, S., & Moyes, B. (1988). Personality characteristics of parents of autistic children: A controlled study. Journal of Child Psychology and Psychiatry, 29(2), 143–153.
Acknowledgments
This work was supported in part by the Simons Foundation, the National Institute of Child Health and Human Development, and the National Institution Deafness and Communication Disability (PO1HD34565). We thank research study staff at the UW Autism Center, particularly Katy Ankenman, Susan Faja, Rachel Lowy, and Tracey Ward, who completed evaluations for this project and Ellen Wijsman who aided in analyses and subject selection for the FSA sample. We also greatly thank the families who devoted considerable time and energy to our research. A version of this paper was presented at the International Meeting for Autism Research in May, 2012 in Toronto, Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerdts, J.A., Bernier, R., Dawson, G. et al. The Broader Autism Phenotype in Simplex and Multiplex Families. J Autism Dev Disord 43, 1597–1605 (2013). https://doi.org/10.1007/s10803-012-1706-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-012-1706-6