Abstract
Although resistance to change is a main feature of autism, the brain processes underlying this aspect of the disorder remain poorly understood. The aims of this study were to examine neural basis of auditory change-detection in children with autism spectrum disorders (ASD; N = 27) through electrophysiological patterns (MMN, P3a) and to test whether these are quantitatively related to intolerance of change (using the BSE-R scale). ASD displayed significantly shorter MMN latency and larger P3a than controls, indicating a greater tendency to switch attention to deviant events. These electrophysiological abnormalities were significantly more marked in children who displayed greater difficulties in tolerating change. The atypical neurophysiological mechanism of change perception identified might thus be associated with one of the hallmark behavioural manifestations of autism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to serious impairments in communication, social interaction, empathy and play, Autism Spectrum Disorders (ASD) involve pathologically high levels of repetitive, stereotypic, ritualistic or compulsive behaviours, together with extreme resistance to change. This was initially described as a ‘need for sameness’ (Kanner 1943). It may be expressed in terms of exaggerated emotional responses to change, or in terms of restricted interests and repetitive or stereotyped behaviours. Although this third dimension of autistic disorder is fundamental, it has received little direct research attention. Yet it results in major difficulties in daily life both for the individual and his or her relatives (Gabriels et al. 2005; Murphy et al. 2005) and remains persistent with age (Kobayashi and Murata 1998; Leekam et al. 2007). This key characteristic of autism points to a lack of cognitive flexibility that may arise from a disorder in processing irregular events. For instance, as they display difficulties in coping with unstructured and unpredictable situations, when confronted with the changeable social world people with autism try to impose predictability, with insistence on repetition and sameness.
Resistance to change is also expressed at the sensory level; individuals with autism—especially children—display unusual behaviours in response to sensory stimuli (Baranek et al. 2005; Liss et al. 2006; Malvy et al. 2004; Rogers et al. 2003). Systematic review of the literature indicates that rates of dysfunction in sensory processing may be as high as 90% in individuals with autism (Baker et al. 2008; Baranek et al. 2006; Tomchek and Dunn 2007). This is especially the case in the auditory modality, where paradoxical responses to sounds and both hyper- and hypo-reactivity to noise are reported (Baranek et al. 2006). Clinical studies have described significant relationships between frequent/severe repetitive behaviours and abnormal sensory responses to environmental stimuli in people with autism (Baranek et al. 1997; Gabriels et al. 2005; Gal et al. 2009). Consistent with the common clinical observation of intense reactions to changes in the environment, experimental studies have also evidenced enhanced perceptual function such as visual hyperacuity (Ashwin et al. 2009; Plaisted et al. 1998), hyperacusis (Khalfa et al. 2004) and acute tactile sensitivity (Baranek et al. 1997; Blakemore et al. 2006). Taken together, these features suggest that resistance to change in people with autism may reflect basic abnormalities in the processing of sensory information, and especially in the automatic processing of changing stimuli.
Although resistance to change is a main feature of autism, the brain processes underlying this aspect of the disorder remain poorly understood. The neural correlates of mismatch detection are commonly studied through the oddball paradigm, which involves the presentation of infrequent stimuli embedded in a sequence of repetitive standard stimuli. The oddball paradigm provides an objective electrophysiological index allowing investigation of central processes of change-detection i.e. Mismatch Negativity (MMN). MMN reflects automatic detection of minor change in the physical features of stimuli and is characterized by a negative deflection that culminates over fronto-central sites at around 150–200 ms after the deviant stimulus (Naatanen et al. 2007). The MMN is measured from difference waves obtained by subtracting the response to the standard stimulus from the response to the deviant stimulus. In response to large deviancy or to novel stimuli, MMN could be followed by a P3a, a positive response culminating at around 300 ms over centro-frontal sites, and thought to reflect attentional switch toward the unusual event (for review see Knight and Scabini 1998).
The MMN matures very early, and its underlying mechanisms are assumed to be similar across the lifespan (Cheour et al. 2000; Gomot et al. 2000). The MMN is very appropriate to study the dynamics of the central auditory processes involved in change detection in children because it does not require the subject’s active participation.
The MMN can also be used to index auditory discrimination of complex stimuli, including speech (Kraus et al. 1995; Naatanen et al. 1997). Many studies have assessed speech discrimination in clinical populations, including children with ASD (Kemner et al. 1995; Kuhl et al. 2005; Lepisto et al. 2008). However, few MMN studies involving more basic auditory stimuli such as tones have been performed in people with autism, and the findings reported are inconsistent and generally address only differences in amplitude. Some studies have indicated that MMN amplitude in individuals with autism is in the normal range (Ceponiene et al. 2003) whereas others have shown a reduced response (Dunn et al. 2008; Lepisto et al. 2006). Both normal and longer latencies (Jansson-Verkasalo et al. 2003; Seri et al. 1999) have also been reported in this population.
In our previous work involving children with autism, we investigated the processes involved in the automatic detection of minor changes occurring in a sequence of sounds, using a similar oddball paradigm in electrophysiological (Gomot et al. 2002) and fMRI studies (Gomot et al. 2006). The electrophysiological results showed significantly shorter latency and abnormal MMN topography in children with autism compared to controls. fMRI confirmed the hypothesis of atypical change processing in autism as it demonstrated unusual activation in the left anterior cingulate (a region mainly involved in the distribution of attentional resources) in children with autism in response to deviant events. However, the involvement of these neurophysiological abnormalities in the behavioural need to preserve sameness in autism remains to be clarified. The investigation reported here examined relationships between electrophysiological findings from a passive auditory oddball paradigm and clinical assessments focused on intolerance of change in order to provide evidence of possible brain-behaviour relationships.
Methods
Subjects
Twenty-seven children with autism spectrum disorders (ASD; 21 boys and 6 girls) and twenty-seven gender- and chronological age-matched normally developing children (CTRL) participated in the experiment. Children were aged 5–11 years (mean ± s.e.m.: ASD, 8 years 4 months ± 4 months; CTRL, 8 years 4 months ± 4 months). The clinical subjects were recruited from patients attending a child psychiatry day-care unit of a University Hospital. All children with autism were diagnosed by two independent experts (a child psychiatrist and a clinical psychologist) and met the DSM-IV-R criteria (APA 2000). The Childhood Autism Rating Scale—CARS (Schopler et al. 1980) was used to confirm the diagnosis (mean score ± s.e.m: 36.7 ± 6.5; threshold for ASD = 30). Developmental quotients of children with autism were evaluated by using tests appropriate to mental age: (1) the Brunet-Lézine-R, a psychomotor developmental test for infants 1–30 months of age (Brunet and Lezine 1976, revised form 1997), and (2) the EDEI-R, a cognitive test for children 30 months–9 years of age (Perron-Borelli 1978, revised form 1996). These two developmental scales provided (mean ± s.e.m.) overall developmental (DQ: 51 ± 4.6), verbal (vDQ: 43 ± 5.0) and non-verbal (nvDQ: 59 ± 4.7) quotients. All subjects were right-handed and had normal hearing as assessed by brainstem auditory evoked responses recorded before investigating late auditory evoked potentials. Children with metabolic or chromosomal disease, history of substantial neurological disorders or seizures, abnormal EEG with either slow waves or epileptiform discharges were excluded. All children were free of psychotropic medication for at least 1 month before the electrophysiological study. The Ethics Committee of the University Hospital of Tours approved the protocol. Signed informed consent was obtained from parents, and assent from the children when possible.
Autistic behaviors were assessed using the Behavior Summarized Evaluation scale (BSE-R (Barthelemy et al. 1997)), a composite scale of 29 items for evaluation of symptoms of autism, including poor social interaction, abnormal eye contact, verbal and non-verbal communication disabilities, bizarre responses to auditory stimuli, and ritual use of objects. The associated features of the disorder, including eating, sleep and motor disorders are also assessed. The BSE-R scale permits the follow-up of the child in his/her daily environment. It is rated every 2 weeks by nurses who care for the children during the day, and thus provides information about the current behavioural difficulties of the patient. Raters are trained on the scale and are experienced with children with autism. For this study we retained the results of the scale rated during the month of the electrophysiological recording. Each of the 29 items is rated from 1 to 5 according to the severity of the disturbance: [1] if the symptom is never observed; [5] if the symptom is always observed. We selected items relevant to our hypothesis, with the aim of identifying bioclinical relationships: (1) item 11 reflects intolerance of change in place, time, people, food, and clothes, for example: disproportionate frustration and anger when activities are interrupted, when an object is forbidden, and when desires or expectations remain unsatisfied; and, (2) item 24 reflects bizarre responses to auditory stimuli.
Stimuli and Procedure
Auditory stimulus sequences consisted of 1,000 Hz standard tones and 1,100 Hz deviant tones (probability of occurrence: p = 0.15) delivered in random order, with the constraint that each deviant tone was preceded by at least three standard tones. All tones had an intensity of 70 dB SPL and duration of 50 ms (5 ms rise/fall). Stimuli were presented monaurally through headphones with a constant stimulus onset asynchrony of 700 ms. A block of 1,000 stimuli was delivered to each ear, and the order of the stimulated ear was counterbalanced across subjects. The subjects watched a silent movie on a TV screen during the recording session that lasted 25 min.
EEG Recording and Data Analysis
Electroencephalogram (EEG) was recorded from 7 Ag/AgCl electrodes referenced to the nose (the ground electrode was placed on Fpz location). Five electrodes were placed according to the international 10–20 system (Fz, Cz, Pz, T3, T4), the remaining electrodes were M1 and M2 (left and right mastoid sites). The impedance value of each electrode was less than 10 kΩ. Horizontal and vertical electro-oculograms (EOG) were recorded differentially from two electrodes located on the outer canthi of the right and left eyes (horizontal bipolar) and two electrodes above and below the right eye (vertical bipolar).
The EEG and EOG were amplified (20000), filtered with an analog bandpass filter (0.5–70 Hz), and digitized at a sampling rate of 256 Hz. Movements artefacts were manually discarded and automatic correction of the deviations due to ocular activity, based on a spatial filter transform, was applied. EEG epochs were averaged separately for the standard and deviant tones over a 500 ms analysis period, including a 100 ms prestimulus baseline, and were digitally filtered (low-pass filter: 30 Hz). The ERPs to deviant tones included at least 120 responses for each subject. MMN and P3a were measured in the difference waveforms obtained by subtracting the responses to the standard tones from responses to the deviant stimuli.
Because no N1 wave is recorded in response to standard tones in children for such stimulus onset asynchrony (Korpilahti and Lang 1994; Sussman et al. 2008), peak amplitude and latency of the most prominent negative deflection occurring over fronto-central sites (N250 wave) were measured in each subject in a 180–280 ms latency range.
MMN peak amplitude and latency were then measured in each subject by locating the most negative deflection within a ±50 ms latency window around the peak of the grand average waveform of each group (i.e.: CTRL, 200 ms; ASD, 170 ms). P3a peak amplitude and latency were measured as the most positive deflection within a ±50 ms latency window around the peak of each group (i.e.: CTRL, 300 ms; ASD, 280 ms). Since latencies and amplitudes did not significantly vary according to the ear stimulated in either group, responses to right and left ears were pooled. This improved the signal to noise ratio and therefore made it possible to extract reliable results.
Amplitudes and latencies were analyzed using repeated measure analysis of variance (ANOVA) with Group (CTRL, ASD) as between-subjects factor and Electrode as within-subjects factor.
For further detailed analysis children with autism were divided into two groups according to their resistance to change (greater or less than a score of three out of five on the rating scale of item 11). The characteristics of the electrophysiological brain responses (amplitude, latency) of each subgroup were then entered in an ANOVA analysis.
Correlations between electrophysiological and behavioural data were performed using Pearson’s correlations which were computed between the AEP indices (amplitude, latency) and clinical data: (1) the cognitive levels (DQ, vDQ and nvDQ); (2) the severity of autistic symptoms as assessed by CARS; (3) item 24 bizarre behaviours in response to auditory stimuli; and, (4) item 11 intolerance of change. The level of significance was set at p < 0.05 for all analyses.
Results
Comparison Between Controls and Children with Autism
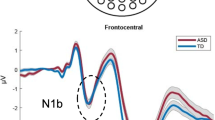
The grand average waveforms in response to standard stimuli for each group at selected electrodes are illustrated in Fig. 1a. The ‘obligatory’ components in children are most clearly observed in the responses to the standard and consisted of fronto-central positivity at 100 ms followed by large negativity peaking at around 240 ms, known as N250 (Ceponiene et al. 1998). These responses were clearly identified at fronto-central electrodes in all children and did not vary significantly in amplitude or latency according to group (N250 response to standard at Fz (mean ± sem): CTRL −6.3 μV ±0.6, 238 ms ±5; ASD −5.9 μV ±0.6, 242 ms ±5).
Figure 1b shows the grand mean difference waveforms for each group at selected electrodes. MMN was evident at Fz and Cz around 150–200 ms after stimulus onset in both groups, with the positive counterpart at mastoid electrodes (M1 and M2), indicating involvement of generators located in the supratemporal cortex. MMN was followed by a P3a response peaking at Cz around 280 ms mostly in ASD.
Statistical analysis showed that MMN peak latency was significantly shorter in ASD than in CTRL at both Fz [F (1,52) = 5.9, p = .018], and Cz sites [F (1,52) = 4.8, p = .034] (Table 1). Similar latency shortening was found on the concomitant positive peak at mastoid sites measured at M1 [F (1, 52) = 12.9, p = .0007], and at M2 [F (1, 52) = 9.6, p = .003].
No inter-group difference in MMN peak amplitude was found at Fz (CTRL 3.7 μV ±0.3; ASD 3.7 μV ±0.4). ASD tended to display greater amplitude values for the MMN positive counterpart recorded at mastoid sites than CTRL; however the difference only reached significance in the right hemisphere.
No inter-group differences were found in the P3a latency at Fz or Cz sites. The P3a amplitude recorded at Fz and Cz sites was significantly larger in ASD than in CTRL [F (1,52) = 9.94, p = .0026].
Analysis of Clinical Sub-groups
The ASD group was divided into two subgroups according to the score obtained for item 11 of the BSE-R measuring ‘intolerance of change’, and labelled INT+ (score ≥ to 3) and INT− (<3) (Fig. 2a).
The characteristics of each subgroup are indicated in Table 2. Other than the score on item 11, no clinical differences were evidenced between subgroups. There was a non-significant trend for DQ and nVDQ to be lower in the INT+ subgroup, and Pearson correlation analysis indicated that the more intolerant children had lower DQ (r = −0.43; p < .05). DQ was thus introduced as a covariate in the ANCOVA comparing the two clinical subgroups. Partial Pearson correlations were also computed by taking into account the correlation with DQ.
Statistical analysis indicated that neither MMN nor P3a amplitude varied significantly according to item 11 subgroups.
MMN peak latency was significantly shorter in INT+ than INT− at both M1 [F (1,24) = 7. 6, p = .011] and M2 [F (1,24) = 5.5, p = .027]) sites (Fig. 2b and Table 3). The same tendency was observed at Fz, but comparison failed to reach significance [F (1,24) = 2.5, p = .13]. However, significant Pearson partial correlation indicated that higher scores for item 11 were associated with shorter MMN latencies at Fz (r = −0.40; p < .05).
ANCOVA analysis at Cz revealed that P3a also displayed shorter latency in INT+ than in INT− [F (1,24) = 4.32; p = .048]. The correlation analysis confirmed that shorter P3a latency was associated with greater intolerance of change (Fz: r = −0.43; Cz: r = −0.48; p < .05).
Finally MMN and P3a amplitude and latency were not significantly correlated with DQ, vDQ, nvDQ, severity of autistic behaviours as assessed by the CARS, or with bizarre behaviours in response to auditory stimuli (item 24).
Discussion
These results obtained for a larger sample confirmed previous findings showing significant shortening of MMN latency in ASD compared to typically-developing children (Gomot et al. 2002), and furthermore evidenced greater amplitude of the subsequent P3a in this group. This indicates more rapid and attention-demanding detection of even small changes occurring in the physical features of the environment. These atypical electrophysiological responses might thus constitute an index of abnormal change processing in ASD. Furthermore, shorter latency in both MMN and P3a were correlated with increased intolerance of change as measured by item 11 of the BSE-R, suggesting that dysfunction in automatic deviancy detection in this group is associated with one of the hallmark behavioural manifestations of ASD.
Basic research on MMN has shown that shorter MMN latencies are recorded for greater frequency deviations (Schroger and Winkler 1995; Tiitinen et al. 1994). One explanation of our results might thus be that children with autism detect acoustic changes in their surroundings more rapidly than normally developing children because of a hyper reactivity to the deviancy. The earlier occurrence of MMN could possibly trigger an earlier and larger P3a, thus leading to abnormally intense involuntary switching of attention towards the deviant event. Taken together, these findings strongly suggest unusual processing of auditory stimulus-change in children with autism.
People with autism thus show activation of neuronal networks that correspond to those involved in the processing of highly contrasting stimuli, even for minor changes in stimulus characteristics, and this was more pronounced in children who displayed greater difficulties in tolerating change. Such relationships between electrophysiological indices of regulation of sensory mechanisms and clinical features of inhibitory deficits (such as restricted behaviours) have been previously reported in adults with autism (Perry et al. 2007). These authors studied Prepulse Inhibition (Braff and Geyer 1990), an operational measurement of sensorimotor gating in which a weak auditory prepulse attenuates the subsequent eye-blink response to a loud startling noise. They observed enhanced sensorimotor gating deficiency in patients who displayed increased ratings of restricted and repetitive behaviours, suggesting that inhibitory failure may lead to the behavioural and cognitive impairments observed downstream.
Previous investigations using psychophysiological methods in children with autism have provided evidence of relationships between physiological measurements and responses to novelty. Kootz et al. (1982) studied the influence of familiarity of the environment on cardiovascular system responses during a target detection task. They showed that variations in both reaction times and heart rate according to the environment in which the experiment took place (laboratory vs. school) were significantly higher in children with autism than in the control group and concluded that children with autism are hypersensitive to changes that occur in their surroundings.
More recently the relationship between repetitive behaviours and a possible neuroanatomical underpinning has been investigated in autism. Sears et al. (1999) examined the relationship between structural abnormalities based on MRI findings and repetitive behaviours in individuals with autism. Involvement of the basal ganglia—in particular the caudate nuclei—was evident and negatively correlated with ADI-R items (compulsions/rituals, difficulties with minor changes, and complex motor mannerisms). Yet our study is the first to identify a clear relationship between functional neurophysiological abnormalities associated with change detection and intolerance of change in children with autism. It has to be noticed that the age range of our subjects is quite broad. However, the effects of interest (group differences in brain evoked responses and correlations with behavioural measures) were not related to age.
Our findings are in accordance with the theory of weak central coherence in autism (Frith and Happe 1994) that states that people with autism have a particular cognitive style, which has been proposed to result in weakening of the tendency to integrate sensory stimuli into a ‘perceptual whole’, while the ability to focus on stimulus details is preserved. Rather than a deficit in global processing, it was proposed that low level information processing systems for sensory stimuli are over developed in autism (Mottron et al. 2000; Plaisted et al. 2003), consistent with studies of auditory attention processes that revealed enhanced pitch processing (Bonnel et al. 2003; Heaton 2003). Further conceptualisation has been proposed with the Enhanced Perceptual Processing Model (Mottron et al. 2006) that has been mostly associated with hyperfunctioning in people with autism. Our results in children with autism support this idea of auditory processing facilitation, but the association we found with greater difficulties in tolerating change suggests that this particular processing of unusual sensory events represents more of a disadvantage than an advantage for patients in their daily lives.
In the long term these electrophysiological markers (MMN and P3a latency shortening) might be used as pre-behavioural neurophysiological phenotype, as long as further research establishes that they fit with the criteria for determining endophenotypes. According to these criteria, a valid endophenotype must be reliable, heritable, causal, and associated with the behaviour of interest (de Geus et al. 2001; Gottesman and Gould 2003). From this standpoint, MMN is a good candidate.
Previous studies have investigated the test–retest reliability and heritability of MMN in healthy monozygotic and dizygotic twins. The heritability of MMN peak amplitude has been estimated at 63% and its reliability as reflected by an ICC (intra-class correlation) of 0.67 was also acceptable (Hall et al. 2006). Moreover, MMN is an electrophysiological response which is fairly reliable throughout development.
Causality is the most challenging criterion, and more work remains to be undertaken in this direction. One possible hypothesis involves the NMDA/Glutamate pathway and is based on genetic studies that have demonstrated mutations of neuroligin genes in autism (Jamain et al. 2003; Laumonnier et al. 2004), a family of genes encoding the neuroligin proteins that are mostly found in excitatory synapses. An explanatory model of the MMN has been proposed based on animal research, (Javitt et al. 1996) involving glutamatergic neurotransmission. This AEP response is thus a promising candidate to investigate the gene/behaviour relationships involving the glutamatergic pathway in individuals with autism.
Finally, the findings of the present study suggest that abnormalities in MMN characteristics in ASD are associated with resistance to change. Moreover, although MMN characteristics have been found to be abnormal (atypical scalp distribution or smaller amplitude) in other developmental disorders such as dyslexia (Hommet et al. 2009) and in children born preterm (Gomot et al. 2007; Jansson-Verkasalo et al. 2004), the latency shortening evidence here appears to be specific to autism, and this again supports the validity of such an electrophysiological index as a possible mediator of gene/behaviour relationships.
Our findings support the existence of electrophysiological markers reflecting the physiopathological mechanisms underlying intolerance to change in ASD, and that might be envisaged as early indicators for diagnosis. It appears that the effects observed are quite robust. By instance, among the 27 children with ASD, 21 had shorter MMN latency than their age-matched control. However lots of work remains to be done—for instance study of the evolution of these electrophysiological markers throughout development and under treatment—before to establish their diagnostic and potential prognostic value.
The association between MMN and P3a latency shortening and intolerance of change is partly based on correlation and does not address causality. However, it is conceivable that MMN abnormalities can be interpreted as indicators of malfunction of change processing and may be indirectly linked to the hallmark feature of disease related to such brain processes—namely resistance to change. Piven (Piven et al. 1996) has demonstrated that the social and communication domains show significant changes from 5 years to adulthood, whereas significant change was not detected in the ritualistic/repetitive domain over time. It therefore seems crucial to improve understanding of the underpinning cerebral bases of impairment related to ritualistic/repetitive behaviours that, in comparison with social and communicative behaviours, are less likely to improve over time.
In conclusion, the findings reported here strongly suggest atypical processing of auditory stimulus change in children with autism that might be related to their behavioural need to preserve sameness. However, further studies are needed to investigate the strength of the relationship between dysfunction of basic sensory processing and cognitive and behavioural difficulties related to resistance to change in autism.
References
APA. (2000). Diagnostic and statistical manual-IV-text revision. Washington, DC: American Psychiatric Association.
Ashwin, E., Ashwin, C., Rhydderch, D., Howells, J., & Baron-Cohen, S. (2009). Eagle-eyed visual acuity: An experimental investigation of enhanced perception in autism. Biological Psychiatry, 65(1), 17–21.
Baker, A. E., Lane, A., Angley, M. T., & Young, R. L. (2008). The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism and Developmental Disorders, 38(5), 867–875.
Baranek, G. T., Barnett, C. R., Adams, E. M., Wolcott, N. A., Watson, L. R., & Crais, E. R. (2005). Object play in infants with autism: Methodological issues in retrospective video analysis. American Journal of Occupational Therapy, 59(1), 20–30.
Baranek, G. T., David, F. J., Poe, M. D., Stone, W. L., & Watson, L. R. (2006). Sensory experiences questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, 47(6), 591–601.
Baranek, G. T., Foster, L. G., & Berkson, G. (1997). Tactile defensiveness and stereotyped behaviors. American Journal of Occupational Therapy, 51(2), 91–95.
Barthelemy, C., Roux, S., Adrien, J. L., Hameury, L., Guerin, P., Garreau, B., et al. (1997). Validation of the revised behavior summarized evaluation scale. Journal of Autism and Developmental Disorders, 27(2), 139–153.
Blakemore, S. J., Tavassoli, T., Calo, S., Thomas, R. M., Catmur, C., Frith, U., et al. (2006). Tactile sensitivity in Asperger syndrome. Brain and Cognition, 61(1), 5–13.
Bonnel, A., Mottron, L., Peretz, I., Trudel, M., Gallun, E., & Bonnel, A. M. (2003). Enhanced pitch sensitivity in individuals with autism: A signal detection analysis. Journal of Cognitive Neuroscience, 15(2), 226–235.
Braff, D. L., & Geyer, M. A. (1990). Sensorimotor gating and schizophrenia. Human and animal model studies. Archives of General Psychiatry, 47(2), 181–188.
Brunet, O., & Lezine, I. (1976). Echelle de developpement psychomoteur de la premiere enfance, 2nd edn 1997. Paris: PUF.
Ceponiene, R., Cheour, M., & Naatanen, R. (1998). Interstimulus interval and auditory event-related potentials in children: Evidence for multiple generators. Electroencephalogr Clin Neurophysiol, 108(4), 345–354.
Ceponiene, R., Lepisto, T., Shestakova, A., Vanhala, R., Alku, P., Naatanen, R., et al. (2003). Speech-sound-selective auditory impairment in children with autism: They can perceive but do not attend. Proceedings of the National Academy of Sciences of the United States of America, 100(9), 5567–5572.
Cheour, M., Leppanen, P. H., & Kraus, N. (2000). Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology, 111(1), 4–16.
de Geus, E. J., Wright, M. J., Martin, N. G., & Boomsma, D. I. (2001). Genetics of brain function and cognition. Behavior Genetics, 31(6), 489–495.
Dunn, M. A., Gomes, H., & Gravel, J. (2008). Mismatch negativity in children with autism and typical development. Journal of Autism and Developmental Disorders, 38(1), 52–71.
Frith, U., & Happe, F. (1994). Autism: Beyond “theory of mind”. Cognition, 50(1–3), 115–132.
Gabriels, R. L., Cuccaro, M. L., Hill, D. E., Ivers, B. J., & Goldson, E. (2005). Repetitive behaviors in autism: Relationships with associated clinical features. Research in Developmental Disabilities, 26(2), 169–181.
Gal, E., Dyck, M. J., & Passmore, A. (2009). The relationship between stereotyped movements and self-injurious behavior in children with developmental or sensory disabilities. Research in Developmental Disabilities, 30(2), 342–352.
Gomot, M., Bernard, F. A., Davis, M. H., Belmonte, M. K., Ashwin, C., Bullmore, E. T., et al. (2006). Change detection in children with autism: An auditory event-related fMRI study. Neuroimage, 29(2), 475–484.
Gomot, M., Bruneau, N., Laurent, J. P., Barthelemy, C., & Saliba, E. (2007). Left temporal impairment of auditory information processing in prematurely born 9-year-old children: An electrophysiological study. International Journal of Psychophysiology, 64(2), 123–129.
Gomot, M., Giard, M. H., Adrien, J. L., Barthelemy, C., & Bruneau, N. (2002). Hypersensitivity to acoustic change in children with autism: Electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology, 39(5), 577–584.
Gomot, M., Giard, M. H., Roux, S., Barthelemy, C., & Bruneau, N. (2000). Maturation of frontal and temporal components of mismatch negativity (MMN) in children. Neuroreport, 11(14), 3109–3112.
Gottesman, I. I., & Gould, T. D. (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry, 160(4), 636–645.
Hall, M. H., Schulze, K., Rijsdijk, F., Picchioni, M., Ettinger, U., Bramon, E., et al. (2006). Heritability and reliability of P300, P50 and duration mismatch negativity. Behavior Genetics, 36(6), 845–857.
Heaton, P. (2003). Pitch memory, labelling and disembedding in autism. Journal of Child Psychology and Psychiatry, 44(4), 543–551.
Hommet, C., Vidal, J., Roux, S., Blanc, R., Barthez, M. A., De Becque, B., et al. (2009). Topography of syllable change-detection electrophysiological indices in children and adults with reading disabilities. Neuropsychologia, 47(3), 761–770.
Jamain, S., Quach, H., Betancur, C., Rastam, M., Colineaux, C., Gillberg, I. C., et al. (2003). Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nature Genetics, 34(1), 27–29.
Jansson-Verkasalo, E., Ceponiene, R., Kielinen, M., Suominen, K., Jantti, V., Linna, S. L., et al. (2003). Deficient auditory processing in children with Asperger Syndrome, as indexed by event-related potentials. Neuroscience Letters, 338(3), 197–200.
Jansson-Verkasalo, E., Korpilahti, P., Jantti, V., Valkama, M., Vainionpaa, L., Alku, P., et al. (2004). Neurophysiologic correlates of deficient phonological representations and object naming in prematurely born children. Clinical Neurophysiology, 115(1), 179–187.
Javitt, D. C., Steinschneider, M., Schroeder, C. E., & Arezzo, J. C. (1996). Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: Implications for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 93(21), 11962–11967.
Kanner, L. (1943). Autistic disturbances of affective contact. Nervous Child, 2, 217–250.
Kemner, C., Verbaten, M. N., Cuperus, J. M., Camfferman, G., & van Engeland, H. (1995). Auditory event-related brain potentials in autistic children and three different control groups. Biological Psychiatry, 38(3), 150–165.
Khalfa, S., Bruneau, N., Roge, B., Georgieff, N., Veuillet, E., Adrien, J. L., et al. (2004). Increased perception of loudness in autism. Hearing Research, 198(1–2), 87–92.
Knight, R. T., & Scabini, D. (1998). Anatomic bases of event-related potentials and their relationship to novelty detection in humans. Journal of Clinical Neurophysiology, 15(1), 3–13.
Kobayashi, R., & Murata, T. (1998). Behavioral characteristics of 187 young adults with autism. Psychiatry and Clinical Neurosciences, 52(4), 383–390.
Kootz, J. P., Marinelli, B., & Cohen, D. J. (1982). Modulation of response to environmental stimulation in autistic children. Journal of Autism and Developmental Disorders, 12(2), 185–193.
Korpilahti, P., & Lang, H. A. (1994). Auditory ERP components and mismatch negativity in dysphasic children. Electroencephalography and Clinical Neurophysiology, 91(4), 256–264.
Kraus, N., McGee, T., Carrell, T. D., & Sharma, A. (1995). Neurophysiologic bases of speech discrimination. Ear and Hearing, 16(1), 19–37.
Kuhl, P. K., Coffey-Corina, S., Padden, D., & Dawson, G. (2005). Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Developmental Science, 8(1), F1–F12.
Laumonnier, F., Bonnet-Brilhault, F., Gomot, M., Blanc, R., David, A., Moizard, M. P., et al. (2004). X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. American Journal of Human Genetics, 74(3), 552–557.
Leekam, S., Tandos, J., McConachie, H., Meins, E., Parkinson, K., Wright, C., et al. (2007). Repetitive behaviours in typically developing 2-year-olds. Journal of Child Psychology and Psychiatry, 48(11), 1131–1138.
Lepisto, T., Kajander, M., Vanhala, R., Alku, P., Huotilainen, M., Naatanen, R., et al. (2008). The perception of invariant speech features in children with autism. Biological Psychology, 77(1), 25–31.
Lepisto, T., Silokallio, S., Nieminen-von Wendt, T., Alku, P., Naatanen, R., & Kujala, T. (2006). Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clinical Neurophysiology, 117(10), 2161–2171.
Liss, M., Saulnier, C., Fein, D., & Kinsbourne, M. (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10(2), 155–172.
Malvy, J., Barthelemy, C., Damie, D., Lenoir, P., Bodier, C., & Roux, S. (2004). Behaviour profiles in a population of infants later diagnosed as having autistic disorder. European Child and Adolescent Psychiatry, 13(2), 115–122.
Mottron, L., Dawson, M., Soulieres, I., Hubert, B., & Burack, J. (2006). Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders, 36(1), 27–43.
Mottron, L., Peretz, I., & Menard, E. (2000). Local and global processing of music in high-functioning persons with autism: Beyond central coherence? Journal of Child Psychology and Psychiatry, 41(8), 1057–1065.
Murphy, G. H., Beadle-Brown, J., Wing, L., Gould, J., Shah, A., & Holmes, N. (2005). Chronicity of challenging behaviours in people with severe intellectual disabilities and/or autism: A total population sample. Journal of Autism and Developmental Disorders, 35(4), 405–418.
Naatanen, R., Lehtokoski, A., Lennes, M., Cheour, M., Huotilainen, M., Iivonen, A., et al. (1997). Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature, 385(6615), 432–434.
Naatanen, R., Paavilainen, P., Rinne, T., & Alho, K. (2007). The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology, 118(12), 2544–2590.
Perron-Borelli, M. (1978). Les échelles différentielles d’efficiences intellectuelles EDEI, manuel. Paris: Editions Scientifiques et Psychotechniques.
Perry, W., Minassian, A., Lopez, B., Maron, L., & Lincoln, A. (2007). Sensorimotor gating deficits in adults with autism. Biological Psychiatry, 61(4), 482–486.
Piven, J., Harper, J., Palmer, P., & Arndt, S. (1996). Course of behavioral change in autism: A retrospective study of high-IQ adolescents and adults. Journal of the American Academy of Child and Adolescent Psychiatry, 35(4), 523–529.
Plaisted, K., O’Riordan, M., & Baron-Cohen, S. (1998). Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. Journal of Child Psychology and Psychiatry, 39(5), 765–775.
Plaisted, K., Saksida, L., Alcantara, J., & Weisblatt, E. (2003). Towards an understanding of the mechanisms of weak central coherence effects: Experiments in visual configural learning and auditory perception. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1430), 375–386.
Rogers, S. J., Hepburn, S., & Wehner, E. (2003). Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders, 33(6), 631–642.
Schopler, E., Reichler, R. J., DeVellis, R. F., & Daly, K. (1980). Toward objective classification of childhood autism: Childhood autism rating scale (CARS). Journal of Autism and Developmental Disorders, 10(1), 91–103.
Schroger, E., & Winkler, I. (1995). Presentation rate and magnitude of stimulus deviance effects on human pre-attentive change detection. Neuroscience Letters, 193(3), 185–188.
Sears, L. L., Vest, C., Mohamed, S., Bailey, J., Ranson, B. J., & Piven, J. (1999). An MRI study of the basal ganglia in autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 23(4), 613–624.
Seri, S., Cerquiglini, A., Pisani, F., & Curatolo, P. (1999). Autism in tuberous sclerosis: Evoked potential evidence for a deficit in auditory sensory processing. Clinical Neurophysiology, 110(10), 1825–1830.
Sussman, E., Steinschneider, M., Gumenyuk, V., Grushko, J., & Lawson, K. (2008). The maturation of human evoked brain potentials to sounds presented at different stimulus rates. Hearing Research, 236(1–2), 61–79.
Tiitinen, H., May, P., Reinikainen, K., & Naatanen, R. (1994). Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature, 372(6501), 90–92.
Tomchek, S. D., & Dunn, W. (2007). Sensory processing in children with and without autism: A comparative study using the short sensory profile. American Journal of Occupational Therapy, 61(2), 190–200.
Acknowledgments
This research was supported by a grant from the “Fondation d’Entreprise France Telecom”, and by the CHU Bretonneau, Tours (CRC). We thank all the subjects and their parents for their time and effort spent participating in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomot, M., Blanc, R., Clery, H. et al. Candidate Electrophysiological Endophenotypes of Hyper-Reactivity to Change in Autism. J Autism Dev Disord 41, 705–714 (2011). https://doi.org/10.1007/s10803-010-1091-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-010-1091-y