Abstract
Twenty-two children with autism spectrum disorders who had not responded to supported behaviour management strategies for severe dysomnias entered a double blind, randomised, controlled crossover trial involving 3 months of placebo versus 3 months of melatonin to a maximum dose of 10 mg. 17 children completed the study. There were no significant differences between sleep variables at baseline. Melatonin significantly improved sleep latency (by an average of 47 min) and total sleep (by an average of 52 min) compared to placebo, but not number of night wakenings. The side effect profile was low and not significantly different between the two arms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High rates of sleep problems are reported by parents of children with autism (National Autistic Society 2009). Studies report between 44 and 83% of children with autism having sleep difficulties (Richdale 1999), in comparison to 10–20% of typically developing young children (Ramachandani et al. 2000). Krakowiak and colleagues (2008) summarised the literature and compared parent reports of 303 children with autism spectrum disorders (ASD), 63 children with developmental delay (DD), and 163 children with typical development. Of the children with ASD surveyed, 53% were reported to have had at least one frequent sleep problem, in comparison to rates of 44 and 32% in the developmentally delayed and typically developing groups, respectively (Krakowiak et al. 2008). Another large study, assessing 477 children with ASD who had been referred to a diagnostic centre, found that 60% had difficulty falling asleep in autism, 50% woke often in the night and 45% woke too early (Dickerson Mayes and Calhoun 2009). Reports of difficulty falling to sleep have been confirmed objectively by both monitoring of night movement (Allik et al. 2006; O’Connell and Chapparo 2002; Wiggs and Stores 2004) and polysomnography (Malow et al. 2006). Furthermore, there is evidence that in children with autism significant sleep disturbance is associated with problems in daytime social and learning behaviours (Hoshino et al. 1984; Dickerson Mayes and Calhoun 2009; Patzold et al. 1998).
Sleep disorders in children with developmental disorders can cause exhaustion to both the children and their carers, and are a key source of carer stress (Doo and Wing 2006; Norton and Drew 1994). Children who establish normal sleep patterns are considered to be less irritable, calmer and happier (Gordon 2000). Improving severe sleep problems in children with autism has benefits for both the children (Minde et al. 1994) and mothers (Quine 1992). This highlights the importance of strategies to improve sleep. Many services offer behaviour management support, which has been shown to be effective in improving sleep and parental stress (Weiskop et al. 2005; Wiggs and Stores 2001). For reasons which are still uncertain, some children’s sleep will not improve despite these interventions.
There are many hypotheses for poor sleep in autism, including hyperarousal, anxiety, poor understanding of social cues, related brain pathology and inadequate regulation of the sleep hormone melatonin (Richdale 1999). More recent studies have postulated abnormalities in melatonin metabolism in some children with autism (Nir et al. 1995; Tordjman et al. 2005). Melke et al. (2008) recently suggested that the N-Acetylserotonin O-methyltransferase (ASMT) gene, encoding the last enzyme of melatonin synthesis, was significantly less active in some individuals with ASD, and these individuals had correspondingly lower levels of melatonin.
In clinical practice, many professionals have been prescribing melatonin for sleep problems in children with autism despite the fact that it is not licensed (Bramble and Feehan 2005). Case series in healthy children (Ivanenko et al. 2003) or those with developmental disorders (Miyamoto et al. 1999; Niederhofer et al. 2003) suggest that melatonin has been effective for sleep disorders, while randomised controlled studies in typically-developing children have also shown benefits, particularly with sleep onset (Smits et al. 2001, 2003). However, very few studies have examined its effectiveness for children with developmental disorders. A recent review found only three randomised controlled trials with a total of 35 children with developmental disorders (Phillips and Appleton 2004). In the largest of these, Dodge and Wilson (2001) used a randomised, double blind, placebo controlled trial in 20 children with moderate to severe disability. All but two participants improved on sleep latency (time to onset of sleep), but there were no differences in the number of wakenings. In another double blind study, sleep latency also improved significantly in a group of nine children with Rett syndrome following 1 month of melatonin (McArthur and Budden 1998). The third trial, which did not report sleep improvements with melatonin, only included six participants (Camfield et al.1996).
Since Phillips and Appleton’s (2004) review, one further RCT has been published in children with learning disabilities (Wasdell et al. 2008), although other studies have looked at children and adults together (Coppola et al. 2004; Ishizaki et al. 1999). The study trialled a dose of 10 days slow-release melatonin in a sample of children with neurodevelopmental disabilities, including 16 children with ASD. Significant improvements in both sleep latency and total sleep time were observed, although a quarter of these children had been on melatonin prior to the study, which may have led to a selection bias or hormone regulation feedback changes in those children. Some also had other disabilities such as blindness, which is known to affect melatonin release (Lockley et al. 1997).
Despite various therapeutic papers about its use in children with autism (Gordon 2000; Jan et al. 1999; Sack et al. 1997), high-quality evidence specific to ASD has been scarce. A retrospective study of 107 children with autism taking between 0.75 and 6 mg of melatonin reported that 25% of participants reported no sleep problems following medication, and 60% reported improvements with some ongoing concerns (Andersen et al. 2008). However, the majority of participants studied were also on psychotropic medication. In autism, a number of case reports (Hayashi 2000, 2001) and case series (Giannotti et al. 2006; Jan et al. 1994) have reported use of melatonin for sleep disorders and suggested benefits, but only one published trial with a randomised controlled methodology has been conducted to date (Garstang and Wallis 2006). This study reported some evidence of improvements in sleep latency and total time asleep, but had to be suspended after only seven children had completed due to the discovery that some of the placebo capsules were empty (Garstang and Wallis 2006).
As such, while melatonin is becoming more commonly used in clinical practice with children on the autism spectrum, there is a paucity of strong evidence to support its prescription. Furthermore, whilst many clinicians believe melatonin has a relatively low side-effect profile, theoretical considerations have made some concerned that melatonin in medicinal doses may have effects on seizure threshold (Sheldon 1998; Coppola et al. 2004), thresholds for attacks in asthma sufferers (Sutherland et al. 2002), exacerbation of immunological diseases (Jan et al. 1999) and mood in those at risk of depression (Armour and Paton 2004). Since epilepsy and emotional lability are common associated with autism (Tuchman and Rapin 2002), further studies need to carefully evaluate side-effects in comparison to placebo. In adult studies side-effect profiles appear low (Buscemi et al. 2006; Galli-Carminati et al. 2009) but this has not been sufficiently examined in children.
The main objective of this study was to compare melatonin with placebo used alongside behaviour management in children with autism spectrum disorders for sleep latency, multiple night wakening and/or total sleep time. As behaviour management is usually the first line of treatment, we have only included those in whom this has not been effective.
Methods
Design
The study was a randomised, double blind, crossover trial of melatonin versus placebo control.
Participants
Children with a diagnosed autistic spectrum disorder (ASD) who had been referred for serious sleep problems to paediatricians or specialist CAMHS (child and adolescent mental health services) were eligible for inclusion in the study. ASD diagnoses were made based on World Health Organisation (ICD-10) research diagnostic criteria (WHO 1993) through an established multidisciplinary assessment process, and confirmed if not clear by ADI-R (Lord et al.1994) and ADOS (Lord et al. 2001) diagnostic tools. Children referred were included in the study if they had sleep disorders involving sleeplessness as described by Stores (2003), covering excessive time establishing sleep (sleep latency), excessive night-waking or reduced total sleep time. Other sleep disorders such as night terrors, sleepwalking or night-time hallucinations were not the focus of this research. Children were only invited to participate if behaviour management with parenting support had been provided by an experienced clinician and was not successful. The age range studied was 3–16 years. Exclusion criteria included children previously or currently on melatonin, those currently on psychotropic medication, and those suffering from related developmental or neurological disorders, such as Fragile X syndrome or Rett’s syndrome.
Settings
The study was set in 4 specialist centres experienced in treating children with developmental disabilities and sleep disorders, covering the York and Selby, Bradford and Airedale, Bolton, and Harrogate areas. All investigators and research teams received training to ensure that the study adhered to regulations and principles of good practice established by the International Conference of Harmonization (ICH-GCP), Standard Operating Procedures (SOPs), the EU directive on clinical trials in minors (Directive 2001/20/EC) and local research governance. The centres are all part of a network of child mental health researchers in the North of England.
Sample Size
No previous RCTs of children with autism were available at the beginning of our study. The power calculation made prior to study was for repeated measures analysis of variance comparing two groups and based on a double blind RCT of 20 children with learning disabilities (Dodge and Wilson 2001). The baseline measure accounted for 56% of the variance in the final outcome, and the change in the score on the outcome was found to be 0.49 SDs. The power of a crossover design is determined by the sample size and effect size (as is a parallel groups RCT), but is also determined by the correlation between the outcome scores on each measurement occasion. The higher the correlation between the two measures for each person, the lower the standard error of the difference, and the greater the power of the study will be, assuming that the mean differences are the same. We assumed that a similar correlation would be found as in Dodge and Wilson (2001). This required a sample size of 32 (16 individuals crossing over) to have 80% power to detect an effect of 0.49 SDs (i.e. Sufficient power to detect an effect the same size as that found by Dodge and Wilson). We set out to recruit 32 children and young people to allow for attrition.
Procedure
Families who met inclusion criteria were given information leaflets upon contact with local clinical services. If they wanted to proceed they were referred to a research clinic or they could choose to meet a research assistant for further information. Standard informed consent procedures as defined by article 4 of the GM Directive “Clinical Trials on Minors” (Directive 2001/20/EC) were followed. Previous interventions were discussed with all participants to be sure that they had all been offered adequate behaviour management and parenting support to manage such issues. If they had not then they were referred to the learning disability CAMHS team for this service. All families who had been given appropriate behaviour management advice were given a refresher session with materials to take away afterwards. This included advice about evening nutrition, the environment, bedtime routine and parental positive and clear management of evening and sleep related events. As part of the clinical support they were given sleep diaries for a month to ascertain the level of the difficulty.
A month later the sleep diaries were reviewed and the child was only entered to the medication phase of the study if they met the inclusion criteria and significant sleep problems persisted. They were randomised to one of two arms using double-blind randomisation stratified by treatment unit and age range. The arm was decided by remote randomisation through a trials pharmacist (who had no knowledge of or contact with the children) using patient code numbers, which were then assigned and used on all study materials. The trial design is shown in Fig. 1.
Medication
Medication (standard release) and placebo were purchased for the study and manufactured by DHP pharma on request by the study investigators. Each child’s carers were given a medication regime, an information booklet about the study and a drug pack with instructions. Questionnaire measures were completed at different points in the study. Since melatonin takes approximately 1 h to reach a maximum serum level when taken orally (Mulchahey et al. 2004) it is suggested that it be administered within an h of expected sleep (Gordon 2000). We started with 2 mg, 30–40 min before planned sleep with the sleep routine timed around this. As oral melatonin can have variable and poor bioavailability (DeMuro et al. 2000), upwards titration of dose was allowed. The dose was increased by the parent every three nights by 2 mg to a maximum dose of 10 mg. If “good” sleep was achieved the child was stabilised at that titrated dose. “Good” sleep was defined as an improvement of 50% or better (and illustrations given in the information pack). The matched placebo followed the same protocol. The placebo was manufactured to be identical to the active medication in appearance and constitution other than the active ingredient.
Clinical Trial Authorisation was obtained from the Medicines and Healthcare products Regulatory Agency MHRA. In all centres, the medication was stored under regulated storage conditions, and the temperature of the locked storage unit checked twice weekly to be within manufacturer guidelines. Medication used and returned was monitored through a drug accountability form completed by clinicians. Accountability forms were checked by both the clinician with the family and then again independently by the trials pharmacist. Emergency code-breaking mechanisms and contacts were in place but no urgent events required this to be used.
Measures
Changes in sleep patterns were assessed using sleep diaries, which were completed daily by parents collected by a researcher every month for 9 months. The diaries documented start of bedtime routine, time the capsule was taken, times asleep, night wakenings and times awake. In addition, further aspects of sleep, health and behaviour were assessed using the following questionnaires.
Sleep Difficulties Questionnaire (SDQ: Simonds and Parraga 1982/Quine 2001). A measure of sleep disorders with subscales including dysomnias, parasomnias and other sleep disorders.
Developmental Behaviour Checklist (DBC; Einfield and Tonge 1989). A measure of behavioural difficulties in developmental disorders yielding a total behaviour score and five subscale scores (disruptive behaviour, self-absorption, communicative difficulties, anxiety and social relating).
General Health Questionnaire (GHQ; Goldberg 1992). A scale completed by the parent or primary caregiver concerning their own general health.
Side Effects Questionnaire (SEQ). A study specific questionnaire that included all literature and manufacturer reported side effects for melatonin, theoretical side effects, common generic side effects and any other noted changes in health or wellbeing.
SDQs, DBCs and GHQs were collected at the start of the study, at the end of each 3-month period of medication/placebo, and on completion. The SEQ was completed at the start and end of the study, and at the end of each 3-month period of medication/placebo.
Primary outcome measures were defined as
-
1.
Sleep latency measured by time from starting bedtime routine to sleep (time from taking medication to sleep was also measured).
-
2.
Total sleep time.
-
3.
Number of wakenings.
Secondary outcomes included DBC total and subscale scores, GHQ total score, SDQ subscale scores and side effect counts on the SEQ.
Adverse Events
In addition to the SEQ, adverse events, serious adverse events (SAE) or suspected unexpected serious adverse reactions (SUSAR) were monitored carefully through an available 24 h telephone number and monthly parent reviews. Questionnaires were also monitored independently for any 2-point changes in behaviour scores. An adverse event was defined broadly as any event that was a change from usual behaviour or symptoms. Any adverse events were discussed locally in visits by a study monitoring officer who also scrutinised all case notes, and at regular steering group meetings.
Data Analysis
The sleep diary data was analysed by taking the average sleep latency, average time asleep, and average number of awakenings. These were calculated over the baseline month (month 1), and for the whole 3 months of medication or placebo. Baseline means between the randomisation groups were compared using an independent groups t-test. The primary analysis for comparing sleep latency time when on melatonin vs. latency when on placebo was a repeated measures analysis of variance, covarying out the measures from month 1 (no medication). For the count data (sleep wakenings) we used a repeated measures analysis embedded within a multilevel modelling framework. This technique is equivalent to a repeated measures analysis of variance, but can employ a model which is appropriate for count data. We checked for order effects using a time × group interaction test. For data from the DBC, GHQ, SDQ and SEQ, baseline scores were compared using a Mann–Whitney U Test. Melatonin and placebo scores were compared using a Wilcoxon Signed Ranks Test. A repeated measures ANOVA was used to look at crossover effects. All analyses were performed on SPSS (Version 17). A p-value of < .05 was considered to indicate statistical significance.
Results
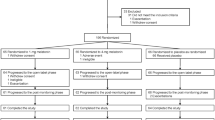
Twenty patients were randomised to the study (16 male, 4 female). The mean age was 9 years (2.9 SD; range = 4–16). Of the 20 children, 14 had autism, 4 had atypical autism and 2 had Asperger syndrome. Four patients withdrew from the study. One because it was too difficult for parents to administer the medication, another withdrew as the family were unable to complete the sleep diaries questionnaire measures. One withdrew because of very significant benefits in sleep early in the first arm and one withdrew because of the apparent ineffectiveness of the medication. This latter participant completed one arm of the trial and therefore, has been included in some of the analysis (but not including melatonin-placebo comparisons). It transpired that they were in the melatonin first arm and this was complicated by the prescription of Risperidone by another clinician, which was the only child where additional medication was added.
In the final analysis dataset of 17 patients, seven were randomised to receive melatonin first and then placebo (Group A), and 10 patients received placebo first and then melatonin (Group B). There was no difference in age [M (SD) group A: 8.9 (3.0), group B: 8.5 (2.3), t (15) = 0.328, p = .747] or gender (6/7 males vs. 3/10 males, chi (1) = 0.565, p = .452) between the two groups. One participant out of the 17 completed only one arm of the trial (Fig. 2).
Sleep Diary Data
The baseline average sleep latency (SL), from the point of starting the bedtime routine to sleep, was 135.0 (63.0) min. The average total sleep time was 499.7 (66.4) min (see Table 1). The average number of night wakenings was 0.5 (0.5). There was no difference at baseline between the two randomisation groups.
Irrespective of starting group, there was a significant difference between melatonin and placebo for sleep latency (t (15) = 3.394), p = .004). There was a reduction in sleep latency whilst on treatment compared to placebo by an average of 46.7 (55.0) min (p = .004). There was no crossover effect for sleep latency (p = 0.922). Figure 3 shows the average sleep latency by month, and by randomisation group.
There was also a significant difference between melatonin and placebo for the time from taking the medication to sleep (an alternative measure of sleep latency—SL2) (t (15) = 2.877, p = .012). There was a decrease on average of 51.7 (71.9) min whilst on treatment compared to placebo. There was no crossover effect (p = 0.981).
There was a significant difference between melatonin and placebo for total sleep (t (15) = 3.75, p = .002) There was an increase in total sleep by an average of 52.3 (55.1) min whilst on treatment compared to placebo. There was no crossover effect (p = .834). There were no significant differences in the number of night wakenings (t (15) = 1.313, p = .209) comparing active treatment to placebo.
There was a significant difference between the maximum dose in milligrams (mg) reached on the melatonin arm compared to the placebo arm of the trial (W = −2.356, n = 16, p = .018). For the 16 participants completing both arms of the trial, 12 participants reached the maximum dose of 10 mg when on the placebo arm compared to six participants when on melatonin. The mean final dose when on the melatonin arm was 7 mg and ranged from 2 to 10 mg (SD = 3.01 mg).
Developmental Behaviour Checklist (DBC)
There were no differences between the two groups at baseline for the DBC scores.
When comparing the treatment and placebo groups, there was a statistically significant difference for the change in total score (Wilcoxon test (Z = −1.964) p = 0.05), with a mean difference of 6.0 (10.8). There were no differences for the other subscales. Repeated measures ANOVA showed there was no crossover effect (all p-values > .05) for any of the subscales.
Sleep Difficulties Questionnaire
There was no difference in the baseline sub-scales scores between the two randomisation groups (asymp. Sig [2 tailed]: dysomnia z = −.647, p = .518; parasomnia z = −.174, p = .862; sleep apnoea z = −.526, p = .599).
There was a significant difference between the dysomnia subscale between treatment and placebo, with lower scores for the treatment group compared to placebo [M (SD) treatment = 22.2 (6.6), placebo = 27.8 (6.4), p = 0.041]. There was no difference for any of the other subscales (all p-values > .05; see Table 1).
We monitored daytime naps and the use of a satisfactory bedtime routine, using a scale from 1–6, where 1 is ‘never’ and 6 is ‘every night.’ There was no significant difference in the reported frequency of daytime naps between placebo and treatment [M (SD) treatment = 1.56(1.03), placebo = 1.86(1.60)]. There was also no significant difference in the reported use of a bedtime routine between placebo and treatment [M (SD) treatment = 5.87 (0.35), placebo = 6.00 (0.00)].
Side Effects Questionnaire
Comparing treatment and placebo, there were no statistically significant differences in the frequencies of any of the side effects reported on the side effects questionnaire (all p > 0.05). This includes daytime drowsiness, dizziness, headaches, vomiting, tummy aches, reduced appetite, low mood, anxiety, irritability, reduced alertness, confusion, tearfulness, diarrhoea, constipation, rashes, sore throat, earaches, asthma, fit or seizure, mild tremor or ‘other’. There were four side effects which appeared ‘never present’ more often in the placebo arm than the melatonin arm: daytime drowsiness (melatonin 35.3%, placebo 68.8%); reduced appetite (melatonin 35.3%, placebo 50%); reduced alertness (melatonin 58.8%, placebo 81.3%); diarrhoea (melatonin 70.6%, placebo 87.5%). These differences were not statistically significant but we report them here for larger studies to consider. There were no significant differences between reports of bedwetting in the sleep difficulties questionnaire between melatonin and placebo.
There were no serious adverse events. One child stopped medication during an episode of influenza and since his sleep continued to be good his mother decided not to restart. None of the participants were on anti-epileptic medication during the trial, although one participant had experienced seizures in the past. There were no known reports of seizures or asthmatic attacks during the trial. Family reports of side effects at meetings with clinicians were of similar frequency throughout no matter which arm of the study they were in. No clear patterns emerged. The only side effect that was reported as thought to be potentially related to medication was tearfulness in two children but on breaking the code neither of these effects began during the melatonin arm. During the melatonin arm and then ongoing there were reports of one child hurting people, increased moodiness and self injurious behaviour. Clinicians reported that this child had long standing problems in all these areas.
General Health Questionnaire
The main carer General Health Questionnaire total scores showed no differences (p = 0.120) between treatment and placebo groups at baseline (Table 1). The mean GHQ score at the end of the melatonin period was 1.4 (2.7 SD) and at the end of placebo was 3.2 (4.6 SD). The mean difference in the total score between the treatment and placebo was 1.8 (4.3 SD). This was not a statistically significant change (Wilcoxon test, p = 0.273). There was no cross-over effect, as confirmed using the repeated measures ANOVA (p = 0.293).
Discussion
Despite small numbers, we found high levels of significance showing the benefits of melatonin over placebo for improving sleep in patients with autism spectrum disorders. Significant changes were specifically observed for sleep latency and total sleep time, but not number of night wakenings.
Primary Outcomes
The improvement of approximately 45 min in total sleep time and sleep latency compared to placebo is higher than the 30-minute improvement reported by Wasdell et al. (2008). A possible explanation for this is that their study used a standard dose of 5 mg slow-release melatonin, whereas the final doses in our study ranged from 2 to 10 mg (M = 7 mg). This maximum is greater than that used in most other studies of children with neurodevelopmental disorders (range = 1–6 mg: Camfield et al. 1996, Dodge and Wilson 2001; Andersen et al. 2008, 7.5 mg McArthur and Budden 1998). This suggests that some children may require larger doses than those being suggested in the literature, and that there may be considerable variability in dose needed. Large variability in bioavailability and metabolism between individuals is a possible reason for this (DeMuro et al. 2000) which could be subject to further research. The fact that the placebo reached the maximum dose in 75% of the 16 children is likely to be because of its therapeutic inactivity, but also suggests that a “placebo effect” may be limited for children with autism.
The sleep diary findings are corroborated by the sleep difficulties questionnaire, which shows significant improvement for questions related to sleep onset. The SDQ scores also showed significant improvements for the question related to night wakening (p = 0.046) even though no significant differences were found in wakening rates in the sleep diaries. (The average night wakenings in the placebo group was 0.58 per night compared to 0.44 when participants were taking melatonin). The lack of a significant difference in these scores may reflect a ceiling effect on this measure, or inadequate power in the sample, but it is also possible that the questionnaire-based results actually reflect parents’ perceived changes in sleep patterns. Success in one area (e.g. Sleep latency or time asleep) may affect the perception of night wakening.
The study showed improvements in total sleep time by just over three quarters of an h on average over a 3 month course of treatment. The improvements in total sleep time appeared to be largely as a result of improved sleep onset. There does not appear to be a loss in effect over a 3 month period of treatment. In fact there is a slight improvement as the 3 months progress suggesting limited tolerance effects in this period. Any sustained improvement after medication with good habit formation is not as evident as hoped for: once medication was stopped there was a rapid return to poor sleep. This may be because of suppression of natural melatonin release when taken exogenously or it may be because of a fundamental problem with melatonin release in autism. Tordjman and colleagues (2005) suggested that melatonin levels may simply be lower in children and adolescents with autism, while in contrast Nir et al. (1995) have suggested equivalent total melatonin release but lower night time amplitudes. Furthermore there may be some children with autism who have melatonin synthesis problems (Melke et al. 2008), as discussed above. It is clear that further research needs to be done in this area to clarify any systematic melatonin metabolism problems in autism.
Secondary Measures
The main function of the secondary outcome measures was to document possible side effects and more general impacts on the health and behaviour of participating children and families. Despite reports of epileptic seizures and bedwetting in previous studies of melatonin treatment in children with developmental disorders (Wasdell et al. 2008), no such problems were observed in the present study. Other open label studies have reported very low side effect profiles in children with autism (Giannotti et al. 2006) and this appears to be confirmed by our study. Wasdell et al. also (2008) found reduced stress levels in parents in the melatonin arm of their study, while we did not. Here it is likely that their generic instrument to measure stress was more sensitive than the GHQ, which covers a wider range of health related items than stress.
In addition, it is interesting to note that the daytime behaviour of children with autism improved during the melatonin arm of our study (as measured on the DBC). This confirms previous studies suggesting that healthy sleep has daytime benefits in typically developing children (Gordon 2000) in those with developmental disorders (Wasdell et al. 2008) and in autism (Minde et al. 1994).
Limitations
Some studies have used actigraphy as an objective measure of sleep. We considered this but given that children with autism might find an arm or wrist band interesting or bothersome, we believed it would be likely to interfere with sleep. Given that previous studies show very high correlation between actigraphy and parent report diaries (Wasdell et al. 2008), we made the decision not to use it.
Despite considerable efforts and networking, only 20 participants were recruited. Recruitment was difficult because of the large number of children with autism who were already taking melatonin. Children previously on melatonin were not entered into the trial because we believed that this would represent a selection bias, with the potential to selectively recruit those in whom melatonin had been successful previously, since families where it had been unsuccessful might be less likely to opt for the study. The high use of melatonin in the absence of RCTs may be because clinicians are finding it to be effective, but may also be related to the high levels of stress facing families and the desire for the clinician to try something to support the family. Poor recruitment in some of the original eight centres was also because adequate high level behaviour management support was not always available because of staff shortages and differences in service provision resulting in some children not meeting the study eligibility criteria.
Behaviour therapy is effective for sleep problems in children with autism (Weiskop et al. 2005), but there are some children where it does not work. The children entered for study were those with the most severe difficulties as evidenced by the fact that their sleep had not improved despite behaviour management support (in some instances with intensive support). This study shows that melatonin improves sleep significantly in a group where behaviour therapy has not been effective. Further work on the synthesis and bioavailability of melatonin in children with autism is clearly required.
References
Allik, H., Larson, J. O., & Smedje, H. (2006). Sleep patterns of school age children with asperger syndrome or high functioning autism. Journal of Autism and Developmental Disorders, 36, 585–595.
Andersen, I. M., Kaczmarska, J., McGrew, S. G., & Malow, B. A. (2008). Melatonin for insomnia in children with autism spectrum disorders. Journal of Child Neurology, 23(5), 482–485.
Armour, D., & Paton, C. (2004). Melatonin in the treatment of insomnia in children and adolescents. Psychiatric Bulletin, 28, 222–224.
Bramble, D., & Feehan, C. (2005). Psychiatrists’ use of melatonin with children. Child and Adolescent Mental Health, 10(3), 145–149.
Buscemi, N., Vandermeer, B., Hooton, N., Pandya, R., Tjosvold, L., Hartling, L., et al. (2006). Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. British Medical Journal, 332, 385–388.
Camfield, P., Gordon, K., & Dooley, J. (1996). Melatonin appears ineffective in children with intellectual deficits and fragmented sleep: six ‘N of 1’ trials. Journal of Child Neurology, 11, 341–343.
Coppola, G., Iervolino, G., Mastrosimone, M., La Torre, G., Ruiu, F., & Pascotto, A. (2004). Melatonin in wake sleep disorders in children, adolescents and young adults with mental retardation with and without epilepsy: A double blind, cross over placebo controlled trial. Brain Development, 26(6), 373–376.
Council Directive (EC) 2001/20/EC of the European parliament and of the council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use.
DeMuro, R. L., Nafziger, A. N., Blask, D. E., Menhinick, A. M., & Bertino, J. S. (2000). The absolute bioavailability of oral melatonin. The Journal of Clinical Pharmacology, 40, 781–784.
Dickerson Mayes, S., & Calhoun, S. L. (2009). Variables related to sleep problems in children with autism. Research in Autism Spectrum Disorders, 3(4), 931–941.
Dodge, N. N., & Wilson, G. A. (2001). Melatonin for treatment of sleep disorders in children with developmental disabilities. Journal of Child Neurology, 16, 581–584.
Doo, S., & Wing, Y. K. (2006). Sleep problems of children with pervasive developmental disorders: Correlation with parental stress. Developmental Medicine and Child Neurology, 48, 650–655.
Einfield, S. L., & Tonge, B. J. (1989). The Developmental Behaviour Checklist. Melbourne Australia: Monash University Centre For Developmental Psyhciatry.
Galli-Carminati, G., Deriaz, N., & Bertschy, G. (2009). Melatonin in the treatment of chronic sleep disorders in adults with autism: A retrospective study. Swiss med weekly, 139(19–20), 293–296.
Garstang, J., & Wallis, M. (2006). Randomised controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child: Care, Health and Development, 32, 585–589.
Giannotti, F., Cortesi, F., Cerquiglini, A., & Bernabei, P. (2006). An open label study of controlled release melatonin in treatment of sleep disorders in children with autism. Journal of Autism and Developmental Disorders, 36, 741–752.
Goldberg, D. (1992). General health questionnaire (GHQ-12). Windsor, UK: Nfer-Nelson.
Gordon, N. (2000). The therapeutics of Melatonin: a paediatric perspective. Brain and Development, 22, 213–217.
Hayashi, E. (2000). Effect of melatonin on sleep-wake rhythm: The sleep diary of an autistic male. Psychiatry and Clinical Neurosciences, 54(3), 383.
Hayashi, E. (2001). Seasonal changes in sleep and behavioural problems in a prepubescent case with autism. Psychiatry and Clinical Neurosciences, 55, 223–224.
Hoshino, Y., Watanabe, H., Yashima, Y., Kaneko, M., & Kumashiro, H. (1984). An investigation on the sleep disturbance of autistic children. Folia Psychiatrica et Neurologica Japonica, 38, 45–51.
Ishizaki, A., et al. (1999). Usefulness of melatonin for developmental sleep and emotional/behavior disorders-studies of melatonin trial on 50 patients with developmental disorders. No To Hattatsu, 31(5), 428–437.
Ivanenko, A., Crabtree, V. M., Tauman, R., et al. (2003). Melatonin in children and adolescents with insomnia: a retrospective study. Clinical Paediatrics, 42, 51–58.
Jan, M. S., et al. (1994). The treatment of sleep disorders with melatonin. Developmental Medicine and Child Neurology, 36, 97–107.
Jan, J. E., Freeman, R. D., & Fast, D. K. (1999). Melatonin treatment of sleep-wake cycle disorders in children and adolescents. Developmental Medicine and Child Neurology, 41, 491–500.
Lockley, S. W., Skene, D. J., Arendt, J., Tabandeh, H., Bird, A. C., & Defrance, R. (1997). Relationship between melatonin rhythms and visual loss in the blind. The Journal of Clinical Endocrinology and Metabolism, 82, 3763–3770.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism diagnostic interview—revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685.
Lord, C., Risi, S., Lambrecht, L., Cook, E. H., Leventhal, B. L., DiLavore, P. C., et al. (2001). The autism diagnostic observation schedule—generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223.
Malow, B. A., Marzec, M. L., McGrew, S. G., Wang, L., Henderson, L. M., & Stone, W. L. (2006). Characterising sleep in children with autism spectrum disorders: A multidimensional approach. Sleep, 29, 1563–1571.
McArthur, A. J., & Budden, S. S. (1998). Sleep dysfunction in Rett syndrome: A trial of exogenous melatonin treatment. Developmental Medicine and Child Neurology, 40(3), 186–192.
Melke, J., Goubran-Botros, H., Chaste, P., Betancur, C., Nygren, G., Anckarsäter, H., et al. (2008). Abnormal melatonin synthesis in autism spectrum disorders. Molecular Psychiatry, 13(1), 90–98.
Minde, K., Faucon, A., & Faulkner, S. (1994). Sleep problems in toddlers, effects of treatment on their daytime behaviour. Journal of American Academy of Child and Adolescent Psychiatry, 33, 1114–1121.
Miyamoto, A., Oki, J., Takahashi, S., et al. (1999). Serum melatonin kinetics and long-term melatonin treatment for sleep disorders in Rett syndrome. Brain and Development, 21, 59–62.
Mulchahey, J. J., Goldwater, D. R., & Zemlan, F. P. (2004). A single blind placebo controlled across groups dose escalation study of the safety, tolerability, pharmacokinetics, pharmacodynamics of the melatonin analog Beta-methyl-6-chloromelatonin. Life Sciences, 75, 1843–1856.
National Autistic Society (2009, December 15th). Sleep and autism: Helping your child. Retrieved from http://www.nas.org.uk/nas/jsp/polopoly.jsp?d=528&a=3376.
Niederhofer, H., et al. (2003). Brief report: Melatonin facilitates sleep in individuals with mental retardation and insomnia. Journal of Autism and Developmental Disorders, 33(4), 469–472.
Nir, I., Meir, D., Zilber, N., Knobler, H., & Hadjez, J. (1995). Brief report: Circadian melatonin, thyroid stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. Journal of Autism and Developmental Disorders, 25, 641–654.
Norton, P., & Drew, C. (1994). Autism and potential family stressors. The American Journal of Family Therapy, 22(1), 67–76.
O’Connell A, Chapparo C (2002) The trouble with sleeping can a waterbed help? Inaugural World Autism Conference.
Patzold, L. M., Richdale, A. L., & Tonge, B. J. (1998). An investigation into the sleep characteristics of children with autism and Asperger’s disorder. Journal of Paediatrics and Child Health, 34, 528–533.
Phillips, L., & Appleton, R. (2004). Systematic review of melatonin treatment in children with neurodevelopmental disabilities and sleep impairment. Developmental Medicine and Child Neurology, 46(11), 771–775.
Quine, L. (1992). Severity of sleep problems in children with severe learning disabilities: Description and correlates. Journal of Community and Applied Social Psychology, 2, 247–268.
Quine, L. (2001). Sleep problems in primary school children: Comparison between mainstream and special school children care. Health and Development., 37(3), 201–221.
Ramachandani, P., Wiggs, L., Webb, V., & Stores, G. (2000). A systematic review of treatments for settling problems and night waking in young children. BMJ, 320, 209–213.
Richdale, A. L. (1999). Sleep problems in autism: Prevalence, cause and intervention. Developmental Medicine and Child Neurology, 41, 60–66.
Sack, R. L., Hughs, R. J., Edgar, D. M., & Lewy, A. J. (1997). Sleep-promoting effects of melatonin. At what dose, in whom, under what circumstances, and by what mechanisms? Sleep, 20, 908–915.
Sheldon, S. (1998). Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet, 351, 1254.
Simonds, J. F., & Parraga, H. (1982). Prevalence of sleep disorders and sleep behaviours in children and adolescents. Journal of the American Academy of Child Psychiatry, 21, 383–388.
Smits, M. G., Nagtegaal, E. E., Van Der Heijden, R., et al. (2001). Melatonin for chronic onset sleep disorder in children: A randomised placebo-controlled trial. Journal of Child Neurology, 16, 86–92.
Smits, M. G., Van Stel, H. F., Van Der Heijden, R., et al. (2003). Melatonin improves health status and sleep in children with idiopathic chronic sleep-onset insomnia: A randomised placebo-controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry, 42, 1286–1293.
Stores, G. (2003). Medication for sleep wake disorders. Archives of Disease in Childhood, 88, 899–903.
Sutherland, E. R., Martin, R. J., Ellison, M. C., & Kraft, M. (2002). Immunomodulatory effects of melatonin in asthma. American Journal of Respiratory Critical care Medicine, 166, 1055–1061.
Tuchman, R., & Rapin, I. (2002). Epilepsy in autism. Lancet Neurology, 1(6), 352–358.
WHO (1993). The ICD—10 Classification of mental and behavioural disorders:- Diagnostic Criteria for Research: General World Health Organisation.
Wasdell, M. B., Jan, J. E., Bomben, M. M., Freeman, R. D., Rietveld, W. J., Tai, J., et al. (2008). A randomised, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. Journal of Pineal Research, 44, 57–64.
Weiskop, S., Richdale, A., & Matthews, J. (2005). Behavioural treatment to reduce sleep problems in children with autism or fragile X syndrome. Developmental Medicine and Child Neurology, 47, 94–104.
Wiggs, L., & Stores, G. (2001). Behavioural treatment for sleep problems in children with severe intellectual disabilities and daytime challenging behaviour: Effect on mothers and fathers. British Journal of Health Psychology, 6(3), 257–269.
Wiggs, L., & Stores, G. (2004). Sleep patterns and sleep disorders in children with autism spectrum disorders: Insights using parent report and actigraphy. Developmental Medicine and Child Neurology, 46, 372–380.
World Health Organisation (1993). The ICD – 10 Classification of Mental and Behavioural Disorders:- Diagnostic Criteria for Research: General World Health Organisation.
Acknowldgments
This research was supported by grants from the York Innovations Fund and the London Law Trust. It was given ethical approval by York Research Ethics Committee and sponsored by Selby and York (North Yorkshire and York) PCT.Thanks to Than Lwin, Anne Worrall-Davies, Clare Dover, Helen Pearce and Greg Richardson for their support on the trial steering groups. Thanks also to Clive Nicholson, the trial monitor and to Chris Davey for her support with data entry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, B., Sims, D., Smart, S. et al. Melatonin Versus Placebo in Children with Autism Spectrum Conditions and Severe Sleep Problems Not Amenable to Behaviour Management Strategies: A Randomised Controlled Crossover Trial. J Autism Dev Disord 41, 175–184 (2011). https://doi.org/10.1007/s10803-010-1036-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-010-1036-5