Abstract
A comparative study of hydrogen evolution in devices based on cadmium chalcogenides quantum dots (CdS, CdSe and CdTe) and its combinations, sensitizing TiO2 was carried out. A maximum photocurrent of 2.7 mA cm−2 at 0 V bias, and a solar-to-hydrogen (STH) conversion efficiency of 0.9%, was obtained with CdSe QDs due to its wide absorption range. The co-sensitized device with CdS–CdSe QDs showed a higher photocurrent of 3.9 mA cm−2 with an STH of 1.2%. The improvement in hydrogen generation for electrodes sensitized with CdS in combination with CdSe or CdTe QDs, was attributed to the increased light absorption and appropriate band alignment for the enhanced charge transport.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solar energy is being considered as one of the most promising alternative renewable energies, however, due to the intermittence of sunlight it must be stored in an appropriate manner. Artificial photosynthesis enables the possibility on photo-assisted water splitting with semiconductor materials for generating hydrogen as an energy carrier [1, 2]. Efficient photocatalytic water splitting requires highly stable and inexpensive photoelectrodes. Control of the semiconducting properties of photoelectrode materials is the primary concern in developing materials for solar water splitting, since they determine how much photoexcitation occurs in a semiconductor under solar illumination and the quantity of photoexcited carriers reaching the surface where water splitting takes place [3].
To absorb a major portion of the solar spectrum for solar water splitting, a semiconductor shall have a conduction band (CB) that is higher than the H2/H2O redox level and a valence band (VB) that is lower than the H2O/O2 redox level [4]. However, no single material has fulfilled all the required conditions. First result on photocatalytic water splitting using a TiO2 electrode was reported by Fujishima and Honda [5]. Along with TiO2 several metal oxides have been extensively studied, such as ZnO, WO3 and α-Fe2O3 since their bands are energetically favorable for such application. Nevertheless, these materials have large band gap, which implies low light absorption [6,7,8,9,10,11]. Recently, researchers put serious effort on addressing these issues by modifying semiconductors with noble metal loading, addition of sacrificial reagent and sensitization [6, 12, 13]. Momeni et al. [14,15,16,17,18,19,20,21,22,23] have made a valiant effort on introducing materials such as Cr, Fe, Cu, WO3, ZnO, Ag2S and CdS to TiO2 photoanode to enhance the photochemical activity of the later. The sensitized wide bandgap semiconductor with dyes or quantum dots (QD) such as CdS, CdSe, CdTe, PbS and PbSe, has demonstrated improvement of solar light absorption [3, 24,25,26,27,28,29,30,31,32,33]. Instead of bulk electrodes, the use of nanocrystalline semiconductors in photoelectrochemical cells (PEC) offer certain advantages such as larger surface area which is beneficial for the catalytic processes, flexibility in design and fabrication of materials, and short distance for charge transport due to quantum confinement effect [34,35,36]. Since the electron mobility of nanostructures such as nanotubes, nanowires and nanorods are better than nanospheres, application of such nanostructures can improve the photoelectrochemical response of a photoanode [37,38,39,40]. Photocorrosion occurs in semiconductors such as CdS and SiC, and hence, it is necessary to add sacrificial reagents (or hole scavengers) that react irreversibly with the photogenerated holes in the VB and can enhance the photocatalytic electron/hole separation, resulting in a higher quantum efficiency and lower charge recombination [6, 37, 41]. Furthermore, CdS–CdSe co-sensitized photoelectrodes have been reported in PECs as tandem or core/shell structures, mainly supported on TiO2 or ZnO nanotubes/nanowires using successive ionic layer adsorption and reaction (SILAR) deposition or chemical bath deposition (CBD) [38, 42,43,44,45,46]. This combination has the ability to produce hydrogen without the assistance of an external bias, placing it as a promising alternative photoelectrodes [38, 47]. However, SILAR or CBD methods have the disadvantage of taking longer deposition time and less control of the size and shape of the QDs compared to colloidal pre-synthesized QDs [48,49,50].
In this work, the photoactivity of sensitized TiO2 semiconductor with cadmium chalcogenides QD: TiO2/CdS/ZnS, TiO2/CdSe/ZnS and TiO2/CdTe/ZnS, as well as electrodes co-sensitized in the configuration TiO2/CdS-CdSe/ZnS and TiO2/CdS–CdTe/ZnS in hydrogen evolution was evaluated. CdS QDs were deposited by SILAR while colloidal CdSe and CdTe pre-synthesized QDs were deposited by electrophoresis. Electrochemical measurements were performed in a two-electrode configuration PEC. Moreover, the role of the CdS in the solar-to-hydrogen (STH) conversion efficiency of co-sensitized photoelectrodes was investigated.
2 Experimental section
2.1 Electrodes preparation
2.1.1 Materials
WER2-0 Reflector and DSL 18NR-T TiO2 paste were obtained from DYESOL. Titanium (IV) isopropoxide (97%), acetylacetone (> 99%), trioctylphosphine (TOP 90%), trioctylphosphine oxide (TOPO 99%), cadmium oxide (CdO 99%), selenium powder (Se 99%), tellurium powder (Te 99%), cadmium acetate dihydrate (Cd(CH3COO)2·2H2O), zinc acetate dihydrate (Zn(CH3COO)2·2H2O), lead acetate dehydrate (Pb(CH3COO)2·2H2O), sodium hydroxide (NaOH), sodium sulfite (Na2SO3·98%) and toluene anhydrous (99%) were obtained from Sigma-Aldrich. 1-tetradecylphosphonic acid (TDPA 99%) was purchased from PCI Synthesis. Sulphur (S), sodium sulfide (Na2S·9H2O), absolute ethanol, and methanol were obtained from KARAL. Fluorine-doped tin oxide (FTO, TEC-15) was obtained from MTI.
2.1.2 TiO2 film preparation
Fluorine-doped tin oxide (FTO) glasses were cleaned with water, acetone and ethanol in an ultrasonic bath for 15 min before use. The electrodes consist of three different TiO2 layers stacked on top of the other. 1) A compact layer of about 150 nm deposited by spray pyrolysis, 2) An active layer of 8 µm composed of particles of 20 nm, and 3) a scattering layer of 7 µm composed of particles of 200 nm deposited by blading. Detailed description of the fabrication of electrodes is in previous work [51, 52].
2.1.3 Synthesis of colloidal CdSe and CdTe
CdSe QDs were synthesized by hot injection method based on the Peng protocol [53], with minor changes [54]. The QDs were dissolved in toluene prior to their use as a sensitizer. The synthesis of CdTe QDs was similar to the CdSe QDs, via a solution of TeTOP instead of SeTOP as tellurium precursor.
2.1.4 QDs deposition
CdS and ZnS were deposited into TiO2 electrodes by SILAR method. This was performed with 0.05 M Cd(CH3COO)2·2H2O dissolved in ethanol and 0.05 M Na2S in methanol:water solution (V:V = 1:1). Seven SILAR cycles were done for CdS QDs [52, 55]. ZnS was obtained using 0.1 M Zn(CH3COO)2·2H2O and 0.1 M Na2S both dissolved in water. The films were dipped in the solutions during two SILAR cycles [56]. ZnS was deposited taking into account that it reduces the recombination process of electrons in the TiO2-electrolyte interface by surface passivation [57, 58].
Since CdSe and CdTe were synthesized as colloidal QDs, these were deposited by electrophoresis. In this method, TiO2 electrodes were inserted face to face with a FTO in a colloidal QDs cuvette and kept at a distance of 0.2 cm. A constant voltage of 200 V was applied for 105 min to allow the deposition of QDs into the electrode [54]. The geometrical active area of the electrodes was 0.196 cm2. For the co-sensitized photoelectrodes, CdS QDs were deposited on TiO2 films by SILAR, followed by the CdSe or CdTe deposition by electrophoresis.
2.1.5 Photoelectrochemical cell assembling
The PEC consists of a two-electrode configuration using the TiO2/QDs/ZnS photoelectrode as working electrode and Pt wire as counter electrode. The electrolyte with sacrificial agent was 0.25 M Na2SO3 and 0.35 M Na2S. The hydrogen obtained under light irradiation was collected in a syringe locked on top.
2.2 Characterization
The scanning electron microscope (SEM), JEOL (JSM-7800F) was used to measure surface morphology of the samples. The UV–Vis absorption measurements were performed using an Agilent Technologies Spectrophotometer (Cary 5000) with an integrating sphere. The linear sweep voltammetry and chronoamperometry measurements were recorded with a Gamry reference 600 potentiostat/galvanostat under 100 mW cm−2 illumination intensity using an Oriel Sol 3A solar simulator. The light intensity was calibrated employing a NREL Si solar cell with a KG-2 filter. The energy levels were measured by Electrochemical Impedance Spectroscopy (EIS), as was reported in our previous work [54], with a three electrodes cell using FTO/QDs as working electrode, Ag/AgCl as reference electrode and Pt wire as counter electrode, while a solution of 1 M Na2S was the electrolyte.
3 Results and discussion
Figure 1 shows the SEM images of the TiO2, TiO2/CdS, TiO2/CdSe and TiO2/CdTe films. The TiO2 film is shown as reference in Fig. 1A. We can observe that the TiO2 nanoparticles are completely covered with the CdS QDs deposited by SILAR (Fig. 1B). In the films deposited with CdSe and CdTe by electrophoresis it is possible to observe the TiO2 nanoparticles, suggesting a less saturated but uniform coverage, since the QDs are considerably smaller than the TiO2 nanoparticles top layer, thus allowing the QDs to penetrate deeper into the TiO2 layer (Fig. 1C, D).
The cross-sectional SEM image of photoelectrode and the EDS analysis of the QD-sensitized TiO2 are presented in Fig. 2. It is possible to observe the two TiO2 layers mentioned in the experimental section, active in the bottom and scattering in the top of the photoelectrode (Fig. 2A). The EDS distribution of the different QDs is shown in Fig. 2B. As shown in the Fig. 2B, the concentration profile of the CdS QDs is higher in the active layer than in the scattering layer. However, the difference in the distribution of the CdSe concentration between the two layers is not so high. The CdTe QDs showed a high concentration at the bottom of the photoelectrode (active layer), but decreases considerably over a short distance and remains uniform on the rest of the substrate. This difference in the concentration profile between CdS and CdSe (or CdTe) can be attributed to the sensitization technique used, since it is well known that SILAR produce high surface coverage and denser distribution of the QDs deposited, as demonstrated in Fig. 1B, while the ex situ deposition methods for colloidal QDs usually suffers from lower coverage of the QDs on the TiO2 surface [55, 59, 60].
The UV–Vis absorption spectra of the TiO2 films with QDs semiconductors deposited are shown in Fig. 3. The TiO2/CdS film has a wide absorption, as far as 550 nm, which is in good agreement with previous reports for this kind of material [27, 39, 45]. TiO2/CdSe film, has a wider absorption spectrum (from 300 to 650 nm), with three absorption bands at 350 nm, 420 nm and 575 nm corresponding to the TiO2 absorption (350 nm) [61, 62] and to the CdSe QDs (420 and 575 nm) [29, 46, 63]. The TiO2/CdTe film has very weak visible absorption (Fig. 3A), that may be due to the degradation of CdTe QDs in the films. When CdS and CdSe are used together, the obtained absorption spectra is similar to that of TiO2/CdSe films but with a more pronounced peak at 450 nm, indicating the presence of CdS. In the case of TiO2/CdS/CdTe the absorption spectra is similar to that of CdS sensitized films, but with a long tail that reaches 800 nm (Fig. 3B).
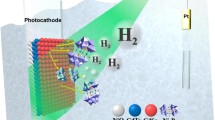
The PEC is illustrated in Fig. 4. It contains the photoanode which absorbs the light and generates the electrons that are used to split the water from the electrolyte solution. The hydrogen is generated on the Pt surface and collected in the syringe container. The photoanode performance was characterized in a two-electrode configuration because the application of a potential bias versus the reference electrode excludes the second half-reaction at the counter electrode [3, 10].
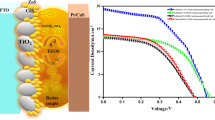
The J-V curves for the photoelectrodes are presented in Fig. 5A. The linear sweep voltammetry of different electrodes in the range of water decomposition shows that, at 0 V the photocurrent rises from 0.3 mA cm−2 obtained with TiO2/CdTe/ZnS to more than 2.3 mA cm−2 and 3.7 mA cm−2 obtained with TiO2/CdS/ZnS and TiO2/CdSe/ZnS, respectively. The photocurrent transient response measurements for the photoanodes with different QDs semiconductors at 0 V bias applied under intermittent illumination are shown in Fig. 5B. It can be observed that for each switch-on and switch-off operation, the resulting current of TiO2/CdTe/ZnS was small, 0.1 mA cm−2. The photocurrent corresponding to TiO2/CdS/ZnS was reached 1.8 mA cm−2 and this remained uniform. The highest photocurrent achieved was 2.7 mA cm−2 with TiO2/CdSe/ZnS, slightly decreasing after some cycles. The difference between the current obtained with CdS and CdSe could be related to the wider light absorption range of the CdSe (Fig. 3).
The CdTe was used in the photoanodes to absorb light in the longer wavelength region of the solar spectrum. However, as observed in the photocurrent-transient response (Fig. 5B), the photoelectrodes with these QDs resulted in low current and instability with the sulfide electrolyte similar to that observed in previous studies [61, 64].
In Fig. 5C, the J–V curves of the photoanodes with CdS QDs combined with other QDs semiconductor showed that the current at 0 V increased in comparison to the electrodes with one cadmium chalcogenide QDs semiconductor. The observed current for TiO2/CdS/CdTe/ZnS (3 mA cm−2) was higher than that obtained for TiO2/CdS/ZnS and TiO2/CdTe/ZnS configurations. Similarly, the current (4.2 mA cm−2) obtained for TiO2/CdS/CdSe/ZnS configuration is 23% higher than that of either TiO2/CdS/ZnS or TiO2/CdSe/ZnS configurations. The photocurrent transient measurements confirm that the photocurrent generated has been improved (Fig. 5D) to 2.7 mA cm−2 with the configuration TiO2/CdS/CdTe/ZnS and 3.9 mA cm−2 with TiO2/CdS/CdSe/ZnS.
The value of the photocurrent obtained in the PEC cell is equivalent to the amount of H2 generated [9, 65]. Figure 6 shows the hydrogen generated with the different photoanodes under illumination at 0 V external bias voltage. Note that the electrode with TiO2/CdTe/ZnS has a very low rate of hydrogen generation (RH2), 0.06 nmol s−1 (see Table 1). It increased to 1.38 nmol s−1 with TiO2/CdS/ZnS. The RH2 of TiO2/CdSe/ZnS is as high as 2.11 nmol s−1. For the photoelectrodes with two kinds of cadmium chalcogenide QDs semiconductors, the RH2 increased to a maximum of 2.33 nmol s−1 for TiO2/CdS/CdSe/ZnS.
Moreover, the STH conversion efficiency for a PEC system at short circuit conditions could be estimated as the fraction of incident solar energy photoconverted into chemical energy as follows [1, 66, 67]:
where Jsc is the short-circuit current density, E° is the thermodynamic reaction potential taking into account the oxidation reaction caused by the sacrificial agent in the electrolyte [1]:
ηF is the faradaic efficiency of hydrogen generation in standard conditions (ηF = 1) and Pi is the incident solar irradiance. The STH of TiO2/CdTe/ZnS was small (∼ 0.07%), while with TiO2/CdS/ZnS and TiO2/CdSe/ZnS films, the STH is more than eight times higher (0.61 and 0.93% repectively). Nevertheless, when the CdSe and CdS QDs were deposited in the photoelectrode, the STH increased to 1.2% (a 23% relative increase compared to CdSe QDs alone). The combination of CdS and CdTe QDs also produce an increase of STH, reaching 0.79% (a relative increase of 29%).
Figure 7 presents the energy diagram of the QDs semiconductors used in this study. These were obtained by EIS measurements (Fig. 7A) and fitted with an equivalent circuit to obtain the Mott–Schottky plot (Fig. 7B) [54, 68,69,70]. It is worth mentioning that the EIS measurements were carried out in a range of 0–0.6 V bias for all samples, however we only included representative data of each QDs in this manuscript. The CB of all the QDs are above the potential necessary to generate hydrogen. For the hydrogen generation system, the effective voltage is the thermodynamic potential of the water splitting reaction, while the optimization criteria is to lower the band gap as much as possible to obtain as high photocurrent as possible without losing the necessary overpotential required to drive the reaction. The band gap corresponding to the QDs here analyzed were 2.45 eV for CdS, 1.83 eV for CdTe, 2.13 eV for CdSe and 3.2 eV for TiO2. The TiO2 photoelectrode has a wide band gap. Additionally, the overpotential given by the incorporation of QD and the band levels arrangement provide the necessary driving force to separate the photogenerated charge carriers, and the increase of light absorption helps to improve the generation of hydrogen.
The co-sensitization of the electrodes with CdS and CdSe or CdTe allows the synergistic effect for the improvement of light absorption, and thus the photocurrent enhancement [38]. Furthermore, the CdS QDs offer protection to the CdSe and CdTe QDs from corrosion or instability due to their compatibility with the sulfide electrolyte and provides suitable energy levels that allow the improvement of the injection of photon-generated electrons from the QDs to the TiO2, producing an increase in the photocurrent [24].
4 Conclusions
TiO2 thin films were sensitized with different QDs like CdS, CdSe, CdTe and its combinations in order to enhance its photo-absorption, and such films were analyzed in PEC. A comparative study among single-sensitized photoanodes reveals that CdSe QDs sensitization could increase the range of optical absorption and hence, results a higher photocurrent and a higher rate of hydrogen evolution (STH = 0.9). As compared to the single sensitized photoelectrodes, co-sensitized photoelectrodes showed higher photocurrent due to the combined effect of larger optical absorption and cascade effect of energy levels that enhance the transport of photogenerated electrons. Furthermore, the rate of generation of hydrogen was improved with co-sensitized systems TiO2/CdS/CdSe/ZnS (STH = 1.2). These results suggest that such co-sensitized TiO2 photoanodes are promising candidates for hydrogen generation systems.
References
González-Pedro V, Zarazua I, Barea EM et al (2014) Panchromatic solar-to-H2 conversion by a hybrid quantum dots–dye dual absorber tandem device. J Phys Chem C 118:891–895. https://doi.org/10.1021/jp4109893
Turner J, Sverdrup G, Mann MK et al (2008) Renewable hydrogen production. Int J Energy Res 32:379–407. https://doi.org/10.1002/er.1372
Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 43:7520–7535. https://doi.org/10.1039/C3CS60378D
Khan SUM (2002) Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297:2243–2245. https://doi.org/10.1126/science.1075035
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Ni M, Leung MKH, Leung DYC, Sumathy K (2007) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sustain Energy Rev 11:401–425. https://doi.org/10.1016/j.rser.2005.01.009
Sun J, Zhong DK, Gamelin DR (2010) Composite photoanodes for photoelectrochemical solar water splitting. Energy Environ Sci 3:1252. https://doi.org/10.1039/c0ee00030b
Gonell F, Haro M, Sánchez RS et al (2014) Photon up-conversion with lanthanide-doped oxide particles for solar H2 generation. J Phys Chem C 118:11279–11284. https://doi.org/10.1021/jp503743e
Han W, Ren L, Qi X et al (2014) Synthesis of CdS/ZnO/graphene composite with high-efficiency photoelectrochemical activities under solar radiation. Appl Surf Sci 299:12–18. https://doi.org/10.1016/j.apsusc.2014.01.170
Pihosh Y, Turkevych I, Mawatari K et al (2015) Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci Rep 5:11141. https://doi.org/10.1038/srep11141
Li X, Wang Z, Zhang Z et al (2015) Light illuminated α-Fe2O3/Pt nanoparticles as water activation agent for photoelectrochemical water splitting. Sci Rep 5:9130. https://doi.org/10.1038/srep09130
Haro M, Abargues R, Herraiz-Cardona I et al (2014) Plasmonic versus catalytic effect of gold nanoparticles on mesoporous TiO2 electrodes for water splitting. Electrochim Acta 144:64–70. https://doi.org/10.1016/j.electacta.2014.07.146
Simon T, Bouchonville N, Berr MJ et al (2014) Redox shuttle mechanism enhances photocatalytic H2 generation on Ni–decorated CdS nanorods. Nat Mater 13:1013–1018. https://doi.org/10.1038/nmat4049
Momeni MM, Ghayeb Y, Shafiei M (2017) Preparation and characterization of CrFeWTiO2 photoanodes and their photoelectrochemical activities for water splitting. Dalton Trans 46:12527–12536. https://doi.org/10.1039/c7dt01596h
Sharifi T, Ghayeb Y, Mohammadi T, Momeni MM (2018) Enhanced photoelectrochemical water splitting of CrTiO2 nanotube photoanodes by the decoration of their surface via the photodeposition of Ag and Au. Dalton Trans 47:11593–11604. https://doi.org/10.1039/c8dt02383b
Momeni MM, Mozafari AA (2016) The effect of number of SILAR cycles on morphological, optical and photo catalytic properties of cadmium sulfide–titania films. J Mater Sci Mater Electron 27:10658–10666. https://doi.org/10.1007/s10854-016-5163-4
Momeni MM, Ghayeb Y, Menati M (2018) Fabrication, characterization and photoelectrochemical properties of cuprous oxide-reduced graphene oxide photocatalysts for hydrogen generation. J Mater Sci Mater Electron 29:4136–4146. https://doi.org/10.1007/s10854-017-8358-4
Momeni MM, Ghayeb Y (2015) Visible light-driven photoelectrochemical water splitting on ZnO–TiO2 heterogeneous nanotube photoanodes. J Appl Electrochem 45:557–566. https://doi.org/10.1007/s10800-015-0836-x
Momeni MM, Ghayeb Y, Mozafari AA (2016) Optical and photo catalytic characteristics of Ag2S/TiO2 nanocomposite films prepared by electrochemical anodizing and SILAR approach. J Mater Sci Mater Electron 27:11201–11210. https://doi.org/10.1007/s10854-016-5240-8
Momeni MM, Ghayeb Y (2015) Fabrication, characterization and photoelectrochemical behavior of Fe-TiO2 nanotubes composite photoanodes for solar water splitting. J Electroanal Chem 751:43–48. https://doi.org/10.1016/j.jelechem.2015.05.035
Momeni MM, Ghayeb Y (2015) Photoelectrochemical water splitting on chromium-doped titanium dioxide nanotube photoanodes prepared by single-step anodizing. J Alloys Compd 637:393–400. https://doi.org/10.1016/j.jallcom.2015.02.137
Momeni MM, Ghayeb Y, Davarzadeh M (2015) Single-step electrochemical anodization for synthesis of hierarchical WO3-TiO2 nanotube arrays on titanium foil as a good photoanode for water splitting with visible light. J Electroanal Chem 739:149–155. https://doi.org/10.1016/j.jelechem.2014.12.030
Momeni MM, Ghayeb Y, Ezati F (2018) Fabrication, characterization and photoelectrochemical activity of tungsten-copper co-sensitized TiO2 nanotube composite photoanodes. J Colloid Interface Sci 514:70–82. https://doi.org/10.1016/j.jcis.2017.12.021
Trevisan R, Rodenas P, Gonzalez-Pedro V et al (2013) Harnessing infrared photons for photoelectrochemical hydrogen generation. A PbS quantum dot based “quasi-artificial leaf”. J Phys Chem Lett 4:141–146. https://doi.org/10.1021/jz301890m
Tanaka K, Jin-nouchi Y, Fujishima M, Tada H (2015) Lead selenide–titanium dioxide heteronanojunction formation by photocatalytic current doubling-induced two-step photodeposition technique. J Colloid Interface Sci 457:248–253. https://doi.org/10.1016/j.jcis.2015.03.008
Yu Z, Li F, Sun L (2015) Recent advances in dye-sensitized photoelectrochemical cells for solar hydrogen production based on molecular components. Energy Environ Sci 8:760–775. https://doi.org/10.1039/C4EE03565H
Jang JS, Li W, Oh SH, Lee JS (2006) Fabrication of CdS/TiO2 nano-bulk composite photocatalysts for hydrogen production from aqueous H2S solution under visible light. Chem Phys Lett 425:278–282. https://doi.org/10.1016/j.cplett.2006.05.031
Seabold J, Shankar K, Wilke RHT et al (2008) Photoelectrochemical properties of heterojunction CdTe/TiO2 electrodes constructed using highly ordered TiO2 nanotube arrays. Chem Mater 20:5266–5273. https://doi.org/10.1021/cm8010666
Sreedhar G, Sivanantham A, Venkateshwaran S et al (2015) Enhanced photoelectrochemical performance of CdSe quantum dot sensitized SrTiO3. J Mater Chem A 3:13476–13482. https://doi.org/10.1039/C5TA00304K
Cui W, Ma S, Liu L, Liang Y (2012) PbS-sensitized K2Ti4O9 composite: preparation and photocatalytic properties for hydrogen evolution under visible light irradiation. Chem Eng J 204–206:1–7. https://doi.org/10.1016/j.cej.2012.07.075
Chen HM, Chen CK, Chang Y-C et al (2010) Quantum dot monolayer sensitized ZnO nanowire-array photoelectrodes: true efficiency for water splitting. Angew Chem 122:6102–6105. https://doi.org/10.1002/ange.201001827
Liu L, Hensel J, Fitzmorris RC et al (2010) Preparation and photoelectrochemical properties of CdSe/TiO2 hybrid mesoporous structures. J Phys Chem Lett 1:155–160. https://doi.org/10.1021/jz900122u
Jin-Nouchi Y, Hattori T, Sumida Y et al (2010) PbS quantum dot-sensitized photoelectrochemical cell for hydrogen production from water under illumination of simulated sunlight. ChemPhysChem 11:3592–3595. https://doi.org/10.1002/cphc.201000593
Vaneski A, Schneider J, Susha AS, Rogach AL (2014) Colloidal hybrid heterostructures based on II–VI semiconductor nanocrystals for photocatalytic hydrogen generation. J Photochem Photobiol C 19:52–61. https://doi.org/10.1016/j.jphotochemrev.2013.12.001
Peng X (2009) An essay on synthetic chemistry of colloidal nanocrystals. Nano Res 2:425–447. https://doi.org/10.1007/s12274-009-9047-2
Gür TM, Bent SF, Prinz FB (2014) Nanostructuring materials for solar-to-hydrogen conversion. J Phys Chem C 118:21301–21315. https://doi.org/10.1021/jp500966u
Shin K, Yoo J-B, Park JH (2013) Photoelectrochemical cell/dye-sensitized solar cell tandem water splitting systems with transparent and vertically aligned quantum dot sensitized TiO2 nanorod arrays. J Power Sources 225:263–268. https://doi.org/10.1016/j.jpowsour.2012.10.036
Wang G, Yang X, Qian F et al (2010) Double-sided CdS and CdSe quantum dot co-sensitized ZnO nanowire arrays for photoelectrochemical hydrogen generation. Nano Lett 10:1088–1092. https://doi.org/10.1021/nl100250z
Kim DH, Han HS, Cho IS et al (2015) CdS-sensitized 1-D single-crystalline anatase TiO2 nanowire arrays for photoelectrochemical hydrogen production. Int J Hydrogen Energy 40:863–869. https://doi.org/10.1016/j.ijhydene.2014.09.174
Hensel J, Wang G, Li Y, Zhang JZ (2010) Synergistic effect of CdSe quantum dot sensitization and nitrogen doping of TiO2 nanostructures for photoelectrochemical solar hydrogen generation. Nano Lett 10:478–483. https://doi.org/10.1021/nl903217w
Berr MJ, Wagner P, Fischbach S et al (2012) Hole scavenger redox potentials determine quantum efficiency and stability of Pt-decorated CdS nanorods for photocatalytic hydrogen generation. Appl Phys Lett 100:223903. https://doi.org/10.1063/1.4723575
Seol M, Jang J, Cho S et al (2013) Highly efficient and stable cadmium chalcogenide quantum Dot/ZnO nanowires for photoelectrochemical hydrogen generation. Chem Mater 25:184–189. https://doi.org/10.1021/cm303206s
Kim H, Seol M, Lee J, Yong K (2011) Highly efficient photoelectrochemical hydrogen generation using hierarchical ZnO/WOx nanowires cosensitized with CdSe/CdS. J Phys Chem C 115:25429–25436. https://doi.org/10.1021/jp2093115
Ali Z, Shakir I, Kang DJ (2014) Highly efficient photoelectrochemical response by sea-urchin shaped ZnO/TiO2 nano/micro hybrid heterostructures co-sensitized with CdS/CdSe. J Mater Chem A 2:6474. https://doi.org/10.1039/c3ta15439d
Wang H, Zhu W, Chong B, Qin K (2014) Improvement of photocatalytic hydrogen generation from CdSe/CdS/TiO2 nanotube-array coaxial heterogeneous structure. Int J Hydrog Energy 39:90–99. https://doi.org/10.1016/j.ijhydene.2013.10.048
Gao X-F, Sun W-T, Ai G, Peng L-M (2010) Photoelectric performance of TiO2 nanotube array photoelectrodes cosensitized with CdS/CdSe quantum dots. Appl Phys Lett 96:153104. https://doi.org/10.1063/1.3386525
Rodenas P, Song T, Sudhagar P et al (2013) Quantum dot based heterostructures for unassisted photoelectrochemical hydrogen generation. Adv Energy Mater 3:176–182. https://doi.org/10.1002/aenm.201200255
Zeng T-W, Liu S, Hsu F-C et al (2010) Effects of bifunctional linker on the performance of P3HT/CdSe quantum dot-linker-ZnO nanocolumn photovoltaic device. Opt Express 18:A357. https://doi.org/10.1364/OE.18.00A357
Zhao Y, Yan Z, Liu J, Wei A (2013) Synthesis and characterization of CdSe nanocrystalline thin films deposited by chemical bath deposition. Mater Sci Semicond Process 16:1592–1598. https://doi.org/10.1016/j.mssp.2013.04.027
Emin S, Singh SP, Han L et al (2011) Colloidal quantum dot solar cells. Sol Energy 85:1264–1282. https://doi.org/10.1016/j.solener.2011.02.005
De La Fuente MS, Sánchez RS, González-Pedro V et al (2013) Effect of organic and inorganic passivation in quantum-dot-sensitized solar cells. J Phys Chem Lett 4:1519–1525. https://doi.org/10.1021/jz400626r
Cerdán-Pasarán A, Esparza D, Zarazúa I et al (2016) Photovoltaic study of quantum dot-sensitized TiO2/CdS/ZnS solar cell with P3HT or P3OT added. J Appl Electrochem 46:975–985. https://doi.org/10.1007/s10800-016-0972-y
Peng ZA, Peng X (2002) Nearly monodisperse and shape-controlled CdSe nanocrystals via alternative routes: nucleation and growth. J Am Chem Soc 124:3343–3353
Cerdán A, López-Luke T, Esparza D et al (2015) Photovoltaic properties of multilayered quantum dot/quantum rod-sensitized TiO2 solar cells fabricated by SILAR and electrophoresis. Phys Chem Chem Phys. https://doi.org/10.1039/C5CP02541A
Esparza D, Zarazúa I, López-Luke T et al (2015) Effect of different sensitization technique on the photoconversion efficiency of CdS quantum dot and CdSe quantum rod sensitized TiO2 solar cells. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.5b01525
Diguna LJ, Shen Q, Kobayashi J, Toyoda T (2007) High efficiency of CdSe quantum-dot-sensitized TiO2 inverse opal solar cells. Appl Phys Lett 91:023116. https://doi.org/10.1063/1.2757130
Giménez S, Lana-Villarreal T, Gómez R et al (2010) Determination of limiting factors of photovoltaic efficiency in quantum dot sensitized solar cells: correlation between cell performance and structural properties. J Appl Phys 108:064310. https://doi.org/10.1063/1.3477194
Jung SW, Kim JH, Kim H et al (2012) ZnS overlayer on in situ chemical bath deposited CdS quantum dot-assembled TiO2 films for quantum dot-sensitized solar cells. Curr Appl Phys 12:1459–1464. https://doi.org/10.1016/j.cap.2012.04.012
Lee H, Wang M, Chen P et al (2009) Efficient CdSe quantum dot-sensitized solar cells prepared by an improved successive ionic layer adsorption and reaction process. Nano Lett 9:4221–4227. https://doi.org/10.1021/nl902438d
Liu Y, Li Z, Yu L, Sun S (2015) Effect of the nature of cationic precursors for SILAR deposition on the performance of CdS and PbS/CdS quantum dot-sensitized solar cells. J Nanoparticle Res 17:132. https://doi.org/10.1007/s11051-015-2940-6
Ai G, Mo R, Xu H et al (2015) Vertically aligned TiO2/(CdS, CdTe, CdSTe) core/shell nanowire array for photoelectrochemical hydrogen generation. J Power Sources 280:5–11. https://doi.org/10.1016/j.jpowsour.2015.01.071
Luo J, Karuturi SK, Liu L et al (2012) Homogeneous photosensitization of complex TiO2 nanostructures for efficient solar energy conversion. Sci Rep 2:451. https://doi.org/10.1038/srep00451
Seol M, Kim H, Kim W, Yong K (2010) Highly efficient photoelectrochemical hydrogen generation using a ZnO nanowire array and a CdSe/CdS co-sensitizer. Electrochem Commun 12:1416–1418. https://doi.org/10.1016/j.elecom.2010.07.035
Yue D, Qian X, Zhang Z et al (2016) CdTe/CdS Core/shell quantum dots cocatalyzed by sulfur tolerant [Mo3S13]2-nanoclusters for efficient visible-light-driven hydrogen evolution. ACS Sustain Chem Eng 4:6653–6658. https://doi.org/10.1021/acssuschemeng.6b01520
Banerjee S, Mohapatra SK, Das PP, Misra M (2008) Synthesis of coupled semiconductor by filling 1D TiO2 nanotubes with CdS. Chem Mater 20:6784–6791. https://doi.org/10.1021/cm802282t
Li Z, Luo W, Zhang M et al (2013) Photoelectrochemical cells for solar hydrogen production: current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ Sci 6:347–370. https://doi.org/10.1039/C2EE22618A
Chen X, Shen S, Guo L, Mao SS (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev 110:6503–6570. https://doi.org/10.1021/cr1001645
Cendula P, Tilley SD, Gimenez S et al (2014) Calculation of the energy band diagram of a photoelectrochemical water splitting cell. J Phys Chem C 118:29599–29607. https://doi.org/10.1021/jp509719d
Gelderman K, Lee L, Donne SW (2007) Flat-band potential of a semiconductor: using the Mott–Schottky equation. J Chem Educ 84:685. https://doi.org/10.1021/ed084p685
Mora-Seró I, Bisquert J (2012) Impedance characterization of quantum dot sensitized solar cells. In: Frontiers of Quantum Dot Solar Cells. CMC Publishing Co., Ltd, Japan, pp 162–175
Acknowledgements
We acknowledge financial support to CONACYT through Grant 259192 and CEMIE-Solar (207450) consortium projects P27 and P28. A. Cerdán-Pasarán acknowledges to CONACYT for the Doctoral fellowship. We thank to Christian Albor for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cerdán-Pasarán, A., López-Luke, T., Zarazúa, I. et al. Co-sensitized TiO2 electrodes with different quantum dots for enhanced hydrogen evolution in photoelectrochemical cells. J Appl Electrochem 49, 475–484 (2019). https://doi.org/10.1007/s10800-019-01299-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-019-01299-x