Abstract
Periodic anolyte replacement was investigated as a means of enhancing the performance of a four air–cathodes single chamber microbial fuel cell (4ACMFC) during its long term operation. The 938 cc unit exhibited very good stability over a period of 53 days in continuous mode operation, attributed to reduction of the growth of antagonistic non-electrogenic bacteria in the bulk solution as a result of the periodic anolyte replacement. Moreover, the MnO2 catalyst layer coating remained stable for approximately 105 days of total cell operation, both in batch and continuous mode. During the long term continuous operation at 32 °C, the steady-state cell voltage was equal to 0.413 ± 0.023 V, the coulombic efficiency ranged from 14 to 16.5 %, while the COD removal remained stable at an average value of 80 %. A maximum power density Pmax of 10 W/m3 was achieved. The 4ACMFC was further assessed at different temperatures and conductivities. Its optimum performance was obtained at 32 °C while the ionic strength was not a limiting factor of the cell performance. The aforementioned results highlight periodic anolyte replacement as a potential operation mode for more stable MFC performance over time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microbial fuel cells (MFCs) are devices that convert biomass spontaneously into electricity through the metabolic activity of microorganisms [1]. In the recent years, MFCs have gained a lot of interest because they can generate electric power while treating wastewater simultaneously. Although relatively high MFC performances have been reported in the literature in terms of power output and chemical oxygen demand (COD) removal efficiency [2–4], a decrease of power output has been observed during their long term operation. Several factors, such as the characteristics of the electrode materials, the membranes, the biofilms formed on electrode surfaces as well as on electrolyte separators and the cathodic performance, affect the long term stability of an MFC [5, 6].
The biofilm formation on the cathode electrode over time has been given a notable interest. The biofilm can act as a separator and block the proton transfer to the cathode electrocatalyst which results in a decrease of the power output, while at the same time it can reduce oxygen diffusion to the anode chamber, increasing the coulombic efficiency (CE) [7]. Cheng et al. [8] tested different catalysts (Pt, cobalt tetramethylphenylporphyrin, CoTMPP) and binders (poly(tetrafluoroethylene) (PTFE), Nafion) in single chamber, air–cathode, microbial fuel cells (MFCs). After 31 days of operation, they observed a decrease in the maximum power density and an increase in the CE for the different catalysts and binders. The effect of operation time on maximum power density and CE was attributed to the biofilm formation on the cathode electrodes. Similar observations were made by Yang et al. [9], using a membrane-less air–cathode MFC with Pt cathode catalyst. Kiely et al. [10] reported that removing the biofilm from the cathode of an air cathode MFC with Pt cathode catalyst did not fully restore the MFC performance. Replacing the cathodes with new ones the power output increased by 118 %, while, in comparison, the power output increased only up to 26 % when the biofilm was removed. Using cathode electrocatalysts less expensive than platinum, Zhang et al. [7] as well as Tremouli [11] also observed deterioration in cathode performance over time. Zhang et al. [7] used air–cathode cells and activated carbon (AC) as cathode catalyst. They observed that replacing the cathode after 1 year completely restored the initial power densities. The cathode performance deterioration was attributed to clogging of the AC micropores. Tremouli [11], using a four-air–cathodes MFC and MnO2 as cathode electrocatalyst, also reported an increase in power performance and in CE when replacing the electrodes with new ones, after 118 days of total cell operation. Gonzalez del Campo et al. [12] evaluated the long term performance of a photosynthetic microbial fuel cell using carbon cloth as the electrodes. After 10 months of operation no mechanical degradation of the electrodes was observed while the cell was effectively abating COD and producing electricity during this period.
Santoro et al. [13], using a single chamber MFC with clean or pre-colonized anodes and platinum-based or platinum-free cathodes, investigated the effect of biofilm growth on the anode and cathode surfaces on the power performance over a period of 26 weeks. In addition to the cathodic biofilm formation, the observed power output decrease over time was attributed to formation of a thick anodic biofilm. This biofilm can hinder the diffusion of organic substances from the solution to the reaction sites at the anode and the diffusion to solution of the protons generated by the electrochemical oxidation, at the anodic biofilm, which can lead to a decrease in apparent anodic activity and electron generation. Hu et al. [14] investigated the effect of fouling of the proton exchange membrane (PEM) in a single chamber MFC with air cathode. The formation of the fouling layer, consisting mainly of microorganisms, microbial extracellular polymers and inorganic salts, resulted in deterioration of the MFC performance due to transfer limitation of cations across the membrane and to increase of cathodic overpotential.
A number of other factors, such as temperature and conductivity, in addition to those mentioned above, can also significantly affect the overall MFC performance. Changes in temperature may affect system kinetics and mass transfer, the MFC thermodynamics, as well as the nature and distribution of the microbial community [15–17]. Moreover, increasing conductivity can improve the slow rate of proton transfer, which often limits the performance of MFC systems [18–20]. Thus, temperature and conductivity were taken under consideration in this study and their effect on the 4ACMFC performance was investigated.
Although, for real world applications MFCs must exhibit effective and stable operation over time, relatively little attention has been given to the study of the long term performance of the MFC systems. This may be due to the fact that it requires significant effort in order to determine all affecting factors and their contribution on the decrease in the overall cell performance over time. The present work focuses on the study of the effect of periodic complete anolyte drainage, during the long term continuous operation of a microbial fuel cell, aiming to maintenance or enhancement of its performance over time. To the best of the authors’ knowledge, this is the first time that such a strategy is applied for the long term continuous operation of an MFC, in an attempt to provide a solution to the problem of gradually reduced MFC performance, which can be partly due to the presence and growth over time of bacteria in the bulk anolyte solution competing with the electrogenic bacteria for the same substrate.
2 Materials and methods
2.1 The 4ACMFC set-up
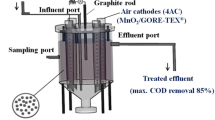
All experiments were performed in a membrane-less single chamber four air–cathode MFC (4ACMFC) [11], shown in Fig. 1. The cell consists of a single cylindrical plexiglas chamber (12.5 cm long, 10 cm diameter and 2 cm thickness) with a conical base (3.5 cm long). Four plexiglas tubes (16.5 cm long, 2 cm diameter and 2 mm thickness) run through the chamber, providing a structural support to the separator. These tubes are uniformly perforated with circular holes (2 mm diameter, approximately 1306 holes/tube) which offers a total geometrical surface area of 164 cm2 (4 tubes) available for proton transport from the anode to the cathode. The graphite granules (type 00514, Le Carbone, Belgium), with diameters ranging between 1.5 and 5 mm, serve as the anodic biofilm support and conducting material, conveying electrons to five graphite rods (13 cm long by 7 mm diameter) inserted into the packed bed of granules. The cathode tubes are open to the atmosphere and no special aeration is employed. GORE-TEX ® cloth is used as separator and as support of the cathode electrocatalyst, which was MnO2. The cloth is tightly bound on the outside wall of each perforated tube, the side covered with the catalyst coating being the air-facing side (cathode). It was made electrically conductive and catalytically active using the methods described by Zhuang et al. [21]. The effective area covered with catalyst coating is 56.25 cm2 (7.5 cm × 7.5 cm), resulting in a MnO2 loading of 142.2 mg cm2.
The volume of the anode chamber is 938 cm3, with approximately 70 % of this volume filled with graphite granules. The bed of the graphite granules has a void fraction of 0.53, resulting in a calculated volume of 348 cm3 available for filling with liquid. Τhis value was in accordance with the volume of the liquid contained in the anode chamber during the experiments, as measured in the process of anolyte replacement. In order to remove the metals from the surface and the inner pores of the granules, the granules were washed for 24 h in 32 % HCl, the process being repeated four times [22]. The wastewater was fed through the influent port located at the top of the cell and connected with a tube ending near the bottom of the cell. The effluent was removed by overflow through the side effluent port located at a distance of 5.4 cm from the top of the chamber.
The anode graphite rods and the cathode were connected via copper wires with a 100 Ω external resistor (unless stated otherwise), forming a closed electrical circuit. The whole MFC was placed in a thermostatic chamber, maintained at 32 °C, unless stated otherwise.
2.2 4ACMFC enrichment and operation
The start-up of the continuous operation of the 4ACMFC was preceded by ten batch cycles. The enrichment of the electrochemically active bacteria in the anode chamber was performed during the first three of these cycles. The inoculum was anaerobic sludge provided by the Wastewater Treatment Plant in Patras, Greece, with the following main characteristics: pH = 7.03 ± 0.15, COD = 0.39 ± 0.13 g COD/L, total suspended solids (TSS) = 29.15 ± 3.74 g/L and volatile suspended solids (VSS) = 13.84 ± 1.75 g/L. In each cycle the active inoculum concentration was 10 % by volume, whereas glucose (0.8 g COD/L) was used as substrate (electron donor). The exact composition of the medium used in the experiments can be found in Ref. [23].
Following the inoculation (acclimation) period, seven successive batch operation cycles were performed, without further addition of sludge. In order to study the effect of small temperature changes on cell performance, the temperature was set to 27 °C and then to 29.5 °C during the last four batch cycles. In order to ensure the reproducibility of the results, at least two operation cycles were completed at each temperature. In particular, the 7th and 8th cycles were performed at 27 °C while the 9th and 10th cycles were performed at 29.5 °C. The temperature during the 4th, 5th and 6th cycle was maintained at 32 °C.
Following the ten batch cycles, the temperature was switched back to 32 °C and the reactor operation was shifted to continuous mode. Without changing the characteristics of the synthetic wastewater, the MFC was operated continuously for approximately 53 days. Τhe cell was operated with a flow rate of 0.2 mL/min (corresponding to a 15 h hydraulic retention time, HRT) and the anolyte (glucose 0.8 g COD/L) was replaced approximately every 10 days. Each anolyte replacement occurred by completely draining the anode chamber and refilling it with fresh anolyte solution, taking care that no solid fraction was left in the bottom of the chamber. The cell voltage, as well as the effluent COD were followed with time.
In order to investigate the effect of conductivity on cell performance, after 53 days of continuous operation the characteristics of the synthetic wastewater feed were modified by salts additions to five different values. The change of the conductivity was accomplished by adding sodium chloride to the feed, while the decrease by reducing in half or by not adding phosphate buffer in the solution. Specifically, these changes occurred during the time period from 105.4 to 142.5 days (the time t measured from the beginning of the batch cycles) corresponding to the following modifications: addition of NaCl 1 g/L (~15.44 mS/cm) at t = 105.4 days, addition of NaCl 2 g/L (~17.14 mS/cm) at t = 109.5 days, feeding without phosphate buffer (~5.31 mS/cm) at t = 113.3 days, feeding with half the amount of the initial buffer (NaH2PO4: 1.84 g/L, Na2HPO4·2H2O: 1.73 g/L, conductivity: ~9.04 mS/cm) at t = 120 days. After t = 124 days, the feeding was switched to its initial state (conductivity equal to approximately 12.25 mS/cm).
During batch (52 days) and continuous operation (90.5 days) of the 4ACMFC, after replacement of the anolyte, the fresh solution was always sparged with a gas mixture of N2/CO2 (80/20 v/v), for at least 15 min, in order to remove the dissolved oxygen. Additionally, at selected time intervals liquid samples were taken, in order to measure COD and pH.
2.3 Polarization measurements
4ACMFC polarization experiments were carried out by connecting a decade variable resistor box between the anode and cathode electrodes. Polarization data were obtained by varying the external resistance from 0.04 to 80 kΩ and recording the corresponding steady-state cell voltage and current values. Measurements were taken 10 min after changing the external resistance, so as to ensure that the system reached steady state.
2.4 Calculations and analysis
The performance of the 4ACMFC system was evaluated in terms of COD removal efficiency, CE and volumetric power density, normalized for the anodic liquid volume (348 cm3). The COD removal efficiency and the CE were calculated as described in Logan et al. [24]. The measurements of dissolved COD, TSS and VSS were carried out according to Standard methods [25]. The pH and conductivity were measured using a digital pH-meter (HACH) and a conductivity meter (HQ440d multi), respectively.
3 Results and discussion
3.1 4ACMFC start up: temperature effect
Figure 2 shows the changes in the monitored cell voltage Ucell (100 Ω external load) and in COD during the start-up stage (ten batch cycles). From Fig. 2 it can be clearly observed that during the first two batch cycles the peak voltage Upeak remained relatively low, at a value around 125 mV, while the CE was 8 and 11.4 % for the first and the second cycle, respectively. At the third cycle a large increase of the peak voltage to 500 mV and of CE to 23 % occurred, confirming the successful formation of electrogenic biofilm on the graphite granules.
Following the acclimation period (three first batch cycles) the system performance was examined at three different temperatures (32, 27, 29.5 °C). As shown in Fig. 2, the peak voltage was stabilized at relatively high values for the cycles carried out at 32 °C (4th cycle: 567 mV, 5th cycle: 611 mV, 6th cycle: 590 mV), whereas a decrease in Upeak was observed for the cycles performed at lower temperatures (7th cycle (27 °C): 388 mV, 8th cycle (27 °C): 405 mV, 9th cycle (29.5 °C): 439 mV, 10th cycle (29.5 °C): 407 mV). Similar to the peak voltage, the CE values for 4ACMFC operation at 32 °C were higher than those for operation at lower temperatures. Namely, the CE values were 37.3, 29.4 and 24.8 % for the 4th, 5th and 6th cycle, respectively, while they ranged from 20 to 22 % for the cycles carried out at 27 and 29.5 °C.
The higher performance of the 4ACMFC at 32 °C could be attributed to the enhancement of the metabolic activity of the electrogenic bacteria with increasing temperature. The fact that the MFC performance did not change significantly when varying the temperature between 27 and 29.5 °C implies that the metabolic activity of the electrogenic bacteria was not sensitive to relatively small changes in this temperature range. These results are in accordance with previous studies, which also reported higher values of CE at higher temperatures [26–28]. In contrast with the observed change in CE, the COD removal remained relatively stable, in the range 66–74 %, for all batch cycles. This indicates that part of the COD was removed by non-electrogenic bacteria grown in the anodic chamber [29].
In agreement with the aforementioned observations, Jadhav and Ghangrekar [30], using a dual chamber MFC, reported a stable COD removal (90 %) for operation of the cell at different temperatures in the range 20–35 °C. Similar observations were made by Larrosa-Guerrero et al. [16], who studied the effect of temperature for both a dual chamber and a single chamber MFC. In the temperature range 20–35 °C the COD removal remained practically stable (75–82 % for the dual chamber MFC, 90–95 % for the single chamber MFC), whereas the CE values varied in the range 0.7–1.8 % for both cells. Ahn and Logan [31] observed a stable COD removal (88 %) and a relatively high CE value (47–50 %) using a single chamber MFC operated at temperatures 23–30 °C.
3.2 Effect of periodic anolyte replacement on the 4ACMFC performance
Following acclimation, the MFC performance was examined in continuous mode (HRT 15 h) for 53 days, using synthetic wastewater (SW) as substrate (glucose, 0.8 g COD/L). In order to reduce or to avoid the growth of bacteria in the bulk solution, which act competitively to the exoelectrogens, the anolyte was completely removed every 10 days and the anode chamber was refilled with fresh medium. The variation of the cell voltage Ucell and of the organic content of the output (in terms of COD) during continuous operation with periodic anolyte replacement is presented in Fig. 3a, whereas, for comparison, in Fig. 3b is presented the time course of Ucell in case of no periodic anolyte replacement, as discussed below.
4ACMFC voltage, Ucell, and COD of the cell effluent versus time during continuous operation of the cell with synthetic wastewater (glucose 0.8 gCOD/L) with (a) and without (b) periodic anolyte replacement. External load R External load Rext = 100 Ω. Black arrows in (a) indicate the times when anolyte replacement was carried out. Red arrows indicate the times when the polarization experiments were performed. (Color figure online)
The periodic anolyte changes (Fig. 3a) were made at the following times: 1st change: t = 52.4 days, 2nd change: t = 63.9 days, 3nd change: t = 73.5 days, 4th change: t = 83.3 days, 5th change: t = 91.3 days, 6th change: t = 99.1 days. The fresh anolyte solution was then sparged with a gas mixture of N2/CO2 (80/20 v/v), for at least 15 min, in order to remove the dissolved oxygen. The results revealed that the 4ACMFC exhibited very good stability during continuous operation with periodic anolyte replacement. The steady-state cell voltage was equal to 0.413 ± 0.023 V, the CE was in the range 14–16.5 % and the COD removal remained stable at an average value of 80 %. The fact that the CE value was practically constant indicates that the population of non-electrogenic bacteria remained stable due to replacement of the anolyte every 10 days of operation. Moreover, the stable MFC performance in terms of voltage generation indicates that no remarkable changes of the metabolic activity of the electrogenic microorganisms occurred over the operation period of 53 days [18].
For the same conditions and substrate, but without periodic replacement of the anolyte, a declining performance of the 4ACMFC was observed during long term operation. Namely, the 4ACMFC performance showed an overall voltage decrease of 55.5 % after 135.4 days of continuous operation. This decrease was partly attributed to methanogenic inhibition (25.1 %), partly to MnO2 cathode catalyst poisoning (47.9 %) and partly (27 %) to graphite granule clogging [11]. Specifically, during the mentioned 3250 h period, the cell voltage decreased from 0.551 ± 0.019 V to 0.244 ± 0.011 V and the CE reduced from 21 to 8.34 %, whereas the COD removal remained relatively high, increasing from 66–74 to 80–85 % after 2000 h of cell operation. The fact that glucose degradation during continuous operation remained high (70–85 %), while the 4ACMFC performance was reduced implied that during the long term operation of the 4ACMFC, microbes other than exoelectrogens were grown. Similarly to the present study, at t = 40.7 days of total cell operation, the anolyte was completely emptied and replaced, which however had no impact on the cell voltage, indicating either that no microorganisms were grown in the bulk solution or that their population at t = 40.7 days was very small to affect the performance of the 4ACMFC. The corresponding results are presented in Fig. 3b. The anolyte replacement occurred at t = 40.71 days (corresponding to t = 0 day in Fig. 3b) and the procedure was not repeated. After 51 days of cell operation the Ucell decreased by 45.7 %.
The observed in the present study beneficial effect of complete anolyte replacement, which partially resolves the problem of the declining 4ACMFC performance with time, confirms the significant growth of non-electrogenic bacteria in the bulk solution for long operation times.
In this study, the MnO2 catalyst layer remained stable after approximately 105 days of total operation in batch and continuous mode, in contrast with the results of the aforementioned previous study of the 4ACMFC operation [11], which indicated that the catalyst layer was deactivated after approximately 118 days of cell operation. Further work is needed in order to specify the exact contribution of MnO2 cathode electrode assembly in the 4ACMFC decreasing performance.
Moreover, the steady-state voltage and the practical constant CE values observed in the present study indicate that no clogging (or no increasing clogging with time) of the graphite granules due to biofilm formation during the continuous operation period of 53 days. The voltage drop percentage of 27 %, attributed in the aforementioned previous study [11] to clogging of graphite granules, was not observed. This result indicates that the washing up of the graphite granules with HCl, before the start up of the system, had a positive effect to the long term operation of the 4ACMFC. The washing of granules removed the unwanted residues from their surface and improved their electrical contact [5].
3.2.1 Effect of time on polarization and power output of the 4ACMFC
In order to determine the effect of operation time on polarization and power output of the 4ACMFC, three polarization experiments were conducted during the continuous operation of the cell, at the following times: 1st at 70.6 days, 2nd at 75.4 days and 3nd at 84.8 days. The corresponding results, namely Ucell versus I and and Pv versus I are presented in Fig. 4, where I denotes the current and PV the power output normalized per volume of the liquid (volumetric power output).
The polarization experiments showed that the maximum power density Pv,max was 7.6, 10 and 7.4 W/m3 for the 1st, 2nd and 3nd polarization experiment, respectively. The open circuit potential (OCP) of the 4ACMFC ranged from 0.51 to 0.55 V. These results reveal a stable power density performance during the 53 days of continuous operation. It is noted that the anolyte volume, which was used for normalization of the power output, did not decrease with operation time, remaining equal to 348 cm3, as measured in the process of anolyte replacement. This corroborates the absence of progressive development of biofilm on the graphite granules, which in the case of operation of the 4ACMFC without periodic anolyte displacement [11] was presumably responsible for the observed gradual reduction of the anolyte volume from an initial value of 350 cm3 to approximately 180 cm3. These findings indicate that replacing the anolyte periodically can be as effective as other maturing techniques for improved MFC performance. In any case, the removed anolyte could be used in a secondary MFC treatment system as, e.g. part of a cascade.
3.3 Effect of conductivity on the 4ACMFC performance
After 53 days of stable continuous operation, the effect of the conductivity on the 4ACMFC performance was examined. The increase of the conductivity was accomplished by adding sodium chloride to the feed, while its decrease was realized by reducing in half (or not adding at all) the phosphate buffer in the solution. In particular, the conductivity of the influent was switched from 12.25 mS/cm to the values ~15.44, ~17.14, ~5.31 and ~9.04 mS/cm, at 105.4, 109.5, 113.3 and 120 days, respectively. Following these changes, the conductivity of the anolyte was switched back to its initial value at t = 124 days.
Figure 5 shows the changes in the monitored cell voltage Ucell (100 Ω external load) and in COD at different conductivities of the anolyte. It can be clearly observed that the change of conductivity did not affect the COD removal efficiency, which remained approximately constant at 80 %. Furthermore, the induced changes in cell voltage Ucell and in CE by increasing the conductivity of the anolyte to approximately 15.44 and 17.14 mS/cm were not significant, as Ucell changed from 0.413 ± 0.023 V to 0.390 ± 0.014 V whereas CE ranged approximately from 14 to 15 %. These results imply that the conductivity of the liquid was not a performance limiting factor for the long term operation of the 4ACMFC, taking also into account that the conductivity was approximately constant at 12.25 mS/cm except for the operation period from 105.4 to 124 days.
4ACMFC voltage, Ucell, and COD of the cell effluent versus time during continuous operation of the cell with synthetic wastewater (glucose 0.8 gCOD/L). External load Rext = 100 Ω. Black arrows indicate the times when anolyte replacement was carried out. Red arrows indicate the times when the polarization experiments were performed. Double end arrows show the time periods corresponding to operation of the MFC with anolytes of different conductivity. (Color figure online)
The addition of the phosphate buffer in the synthetic wastewater during the long term operation of the MFC contributed to maintenance of a constant pH of the anodic liquid (pH 7.7). pH gradually reduced to 6.4 when the 4ACMFC was operated without phosphate buffer (pH values: 7.70 at t = 123.7 days; 7.33 at t = 114.9 days; 6.85 at t = 118 days; 6.44 at t = 120 days). Presumably, this is due to the production of protons in the reaction of electrochemical oxidation of the substrate, which gradually causes acidification of the anolyte (pH = 6.44), without excluding the possibility of a protons production related with a small acidogenic activity of the fermentative colonies. Accumulation of protons in the anolyte has been attributed to slow and incomplete diffusion to the cathode side [15], through the separator cloth in the present case. In parallel with the decrease in pH, the cell voltage and CE also decreased. Specifically, when the conductivity was reduced to 5.3 mS/cm, the cell voltage was sharply decreased by 10 % (to 0.356 V at t = 113.3 days), while a further gradual decrease occurred, to a value of 0.243 V at t = 120 days (Fig. 5). Similarly to the cell voltage, CE sharply decreased by 26.6 % (to 11.4 %) while a further gradual decrease occurred, to a value of 8 %. These results highlight the effect of decreasing conductivity on the 4ACMFC performance, since both Ucell and CE decreased sharply after reducing conductivity from 17.14 to 5.3 mS/cm. This sharp decrease occurred at pH = 7.7, i.e. before acidification of the anodic liquid. However, the following further decrease of Ucell and CE can be also attributed to acidification of the anolyte which affected the electrocatalytic activity of the electrogenic bacteria [15, 32, 33]. The fact that 4ACMFC performance did not change significantly by increasing the anolyte conductivity from 12.25 to 17.14 mS/cm via NaCl addition, contrary to the effect of decreasing conductivity to values lower than 12.25 mS/cm via reducing or not adding the phosphate buffer, could be partly explained by the possible negative effect of NaCl addition on the metabolic activity of the exoelectrogens.
Following the operation of the cell without phosphate buffer, the conductivity was increased to ~9.04 mS/cm by adding half the amount of the initial phosphate buffer into the anolyte. This change resulted in increase of the cell voltage by 37.9 % (to 0.335 V) within 3.9 days. The CE reached a value of approximately 11 % whereas the pH gradually increased from 7.09 at t = 122 days to 7.40 at t = 123.1 days. Subsequently, at t = 124 days, the conductivity of the liquid was switched back to ~12.25 mS/cm. This change was followed by stabilization of the cell voltage at the value Ucell = 0.374 ± 0.008 V and the increase of pH and CE to the values ~7.70 and 12–13 %, respectively. However, the cell voltage did not reestablish its initial value (Ucell = 0.413 ± 0.023 V) before the onset of the changes in conductivity. It is not clear whether this is due to the change of the metabolic mechanism of the bacteria, caused by the modifications of the anolyte in order to accomplish the conductivity changes, or to the deactivation of the cathode catalyst, which might have started occurring during this period. Further study is needed in order to determine the factors which caused this performance decrease.
Furthermore, starting after 109.2 days of total cell operation, three polarization experiments were conducted for three different conductivities of the anolyte, as follows (Fig. 5): 1st: at 109.2 days (15.44 mS/cm); 2nd: at 113.3 days (17.14 mS/cm); 3nd: at ~124 days (9.04 mS/cm). The results of these polarization experiments are shown in Fig. 6. As shown in the figure, a maximum power density Pv,max of 6.33 and 4.30 W/m3 was obtained for conductivities 15.44 and 7.14 mS/cm, respectively. These results imply that the reduction of the maximum power density could be attributed to the increase in the amount of sodium chloride (1–2 g/L) added to increase the conductivity from 15.44 to 17.14 mS/cm, presuming that it had a negative effect on the electrocatalytic activity of the bacteria. When the conductivity was decreased to 9.04 mS/cm the maximum power density Pv,max was further decreased to 3.94 W/m3 while the internal resistance, Rin was increased to ~20 Ω, which implies that the decrease of conductivity itself (per se) has also certain contribution to the observed decrease of Pv,max. The enhanced performance of the MFCs at higher conductivity values of the anolyte has been also reported in previous studies [34–36]. Additionally, the open circuit potential (OCP) of the 4ACMFC was ~0.5 V for conductivities 15.44 or 17.14 mS/cm and ~0.4 V for 9.04 mS/cm. The change of the OCP value could be attributed to the change of the metabolic mechanism of the anodophilic bacteria in the presence or absence of NaCl. These observations are consistent with previous findings [18].
4 Conclusions
A four air–cathodes MFC (4ACMFC) was operated in batch and continuous flow mode, for ca. 142.5 days using synthetic wastewater (glucose 0.8 g COD/L) feed. During continuous flow mode of operation, the anolyte was replaced every 10 days. Assessment of the MFC performance on the basis of cell voltage, CE and COD removal showed that periodic anolyte replacement contributes to a more stable performance of the 4ACMFC during long term operation, which can be attributed to inhibition of the growth of antagonistic non-electrogenic bacteria in the bulk solution. The effect of temperature and anolyte conductivity of the 4ACMFC performance was also studied. The highest performance was observed at 32 °C and for an anolyte conductivity value of 12.25 mS/cm. A maximum power density Pmax of 10 W/m3 was achieved. From a practical viewpoint, the problem of requiring a short interruption of the MFC operation for anolyte replacement may be easily handled as the interruption has only a very short duration. The addition of a small volume stabilization tank before the MFC could receive the incoming flow while anolyte replacement is taking place. Alternately, a properly designed system of two or more MFCs (it is standard practice in wastewater treatment systems to have multiple parallel treatment lines for maintenance purposes) could also be used so that the wastewater to be treated is fed to the other MFC(s) during anolyte replacement in one of them. The results of the present work indicate that periodic replacement of the anolyte could be used as a means of ensuring stable performance of MFCs during long term operation. Further study is needed in order to identify the factors contributing to the stable performance of the cell as a result of periodic anolyte replacement.
References
Pant D, Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543
Dekker A, Heijne AT, Saakes M, Hamelers HVM, Buisman CJN (2009) Analysis and improvement of a scaled-up and stacked microbial fuel cell. Environ Sci Technol 43:9038–9042
Ieropoulos I, Greenman J, Melhuish C (2010) Improved energy output levels from small-scale microbial fuel cells. Bioelectrochemistry 78:44–50
Heijne AT, Liu F, Van Rijnsoever LS, Saakes M, Hamelers HVM, Buisman CJN (2011) Performance of a scaled-up microbial fuel cell with iron reduction as the cathode reaction. J Power Sour 196:7572–7577
Logan BE (2008) Microbial fuel cells. Wiley, New Jersey
Zhang G, Wang K, Zhao Q, Jiao J, Lee D-J (2012) Effect of cathode types on long-term performance and anode bacterial communities in microbial fuel cells. Bioresour Technol 118:249–256
Zhang F, Pant D, Logan BE (2011) Long term performance of activated carbon air cathodes with different diffusion layer porosities in microbial fuel cells. Biosens Bioelectron 30:49–55
Cheng S, Liu H, Logan BE (2006) Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ Sci Technol 40:364–369
Yang S, Jia B, Liu H (2009) Effects of the Pt loading side and cathode-biofilm on the performance of a membrane-less and single-chamber microbial fuel cell. Bioresour Technol 100:1197–1202
Kiely PD, Rader G, Regan JM, Logan BE (2011) Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation end products. Bioresour Technol 102:361–366
Tremouli A (2013) Development of an innovative fuel cell for energy valorisation of wastewaters. Dissertation, University of Patras, Greece
Gonzalez del Campo A, Perez JF, Canizares P, Rodrigo MA, Fernandez FR, Lobato J (2015) Characterization of light/dark cycle and long-term performance test in a photosynthetic microbial fuel cell. Fuel 140:209–216
Santoro C, Lei Y, Li B, Cristiani P (2010) Power generation from wastewater using single-chamber microbial fuel cells (MFCs) with platinum-free cathodes and pre-colonized anodes. Biochem Eng J 62:8–16
Xu J, Sheng G-P, Luo H-W, Li W-W, Wang L-F, Yu H-Q (2012) Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res 46:1817–1824
Oliveira VB, Simões M, Melo LF, Pinto AMFR (2013) Overview on the developments of microbial fuel cells. Biochem Eng J 73:53–64
Larrosa-Guerrero A, Scott K, Head IM, Mateo F, Ginesta A, Godinez C (2010) Effect of temperature on the performance of microbial fuel cells. Fuel 89:3985–3994
Wei L, Han H, Shen J (2013) Effects of temperature and ferrous sulfate concentrations on the performance of microbial fuel cell. Int J Hydrog Energ 38:11110–11116
Lefebvre O, Tan Z, Kharkwal S, Ng HY (2012) Effect of increasing anodic NaCl concentration on microbial fuel cell performance. Bioresour Technol 112:336–340
Fan Y, Sharbrough E, Liu H (2008) Quantification of the internal resistance distribution of microbial fuel cells. Environ Sci Technol 42:8101–8107
Gil GC, Chang IS, Kim BH, Kim M, Jang JK, Park HS, Kim HJ (2003) Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens Bioelectron 18:327–334
Zhuang L, Zhou S, Wang Y, Liu C, Geng S (2009) Membrane-less cloth cathode assembly (CCA) for scalable microbial fuel cells. Biosens Bioelectron 24:3652–3656
Freguia S, Rabaey K, Yuan Z, Keller J (2007) Non-catalyzed cathodic oxygen reduction at graphite granules in microbial fuel cells. Electrochim Acta 53:598–603
Tremouli A, Antonopoulou G, Bebelis S, Lyberatos G (2013) Operation and characterization of a microbial fuel cell fed with pretreated cheese whey at different organic loads. Bioresour Technol 131:380–389
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40(2006):5181–5192
APHA, AWWA, WEF (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Del Campo AG, Lobato J, Cañizares P, Rodrigo MA, Fernandez Morales FJ (2013) Short-term effects of temperature and COD in a microbial fuel cell. Appl Energ 101:213–217
Patil SA, Harnisch F, Kapadnis B, Schröder U (2010) Electroactive mixed culture biofilms in microbial bioelectrochemical systems: the role of temperature for biofilm formation and performance. Biosens Bioelectron 26:803–808
Wang X, Feng YJ, Qu YP, Li DM, Li H, Ren NQ (2008) Effect of temperature on performance of microbial fuel cell using beer wastewater. Huanjing Kexue/Environ Sci 29:3128–3132
He Z, Minteer S, Angenent L (2005) Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ Sci Technol 39:5262–5267
Jadhav GS, Ghangrekar MM (2009) Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour Technol 100:717–723
Ahn Y, Logan BE (2010) Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour Technol 101:469–475
Rozendal RA, Hamelers HVM, Rabaey K, Keller J, Buisman CJN (2008) Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol 26:450–459
Harnisch F, Schröder U, Scholz F (2008) The suitability of monopolar and bipolar ion exchange membranes as separators for biological fuel cells. Environ Sci Technol 42:1740–1746
Liu H, Cheng S, Logan BE (2005) Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ Sci Technol 39:5488–5493
Nam JY, Kim HW, Lim KH, Shin HS, Logan BE (2010) Variation of power generation at different buffer types and conductivities in single chamber microbial fuel cells. Biosens Bioelectron 25:1155–1159
Cheng S, Logan BE (2011) Increasing power generation for scaling up single-chamber air cathode microbial fuel cell. Bioresour Technol 102:4468–4473
Acknowledgments
This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tremouli, A., Intzes, A., Intzes, P. et al. Effect of periodic complete anolyte replacement on the long term performance of a four air cathodes single chamber microbial fuel cell. J Appl Electrochem 45, 755–763 (2015). https://doi.org/10.1007/s10800-015-0842-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0842-z