Abstract
A novel bisphenol A (BPA) sensor based on amperometric detection has been developed by using molecularly imprinted polymers (MIPs) and gold nanoparticles. The sensitive layer was prepared by electropolymerization of 2-aminothiophenol on a gold nanoparticles-modified glassy carbon electrode in the presence of BPA as a template. Cyclic voltammetry was used to monitor the process of electropolymerization. The properties of the layer were studied in the presence of Fe(CN)6 3−/Fe(CN)6 4− redox couples. The template and the non-binding molecules were removed by washing with H2SO4 (0.65 mol L−1) solution. The linear response range of the sensor was between 8.0 × 10−6–6.0 × 10−2 mol L−1, with a detection limit of 1.38 × 10−7 mol L−1 (S/N = 3). The proposed MIPs sensor exhibited good selectivity for BPA. The stability and repeatability of the MIPs senor were found to be satisfactory. The results from real sample analysis confirmed the applicability of the MIPs sensor to quantitative analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bisphenol A (BPA) is widely used for the production of epoxy resins and polycarbonate (PC) plastics [1]. It is extensively employed for nursing bottle, food can linings and beverage container [2]. Because BPA is a potential endocrine-disrupting chemical, it can bind to the estrogen receptors and induce proliferation of them [3, 4]. In addition, BPA is postulated to cause a decrease of sperm quality in humans [5], various kinds of cancers, diverse pleiotropic actions in the brain and cardiovascular system [6]. To prevent the noxious effects of BPA, an efficient monitoring system is required, so that immediate remediation can be activated. Until now, quantification of BPA has frequently been performed by high-performance liquid chromatography (HPLC) [7–9], liquid chromatography coupled with electrochemical detection (LC–ED) [10], liquid chromatography coupled with mass spectrometry (LC–MS) [11–13], gas chromatography (GC) [14, 15] and gas chromatography coupled with mass spectrometry (GC–MS) [16–18]. Although these methods can offer good selectivity and detection limit, they often require complicated and expensive instrumentations, professional operators, time-consuming detection process and complex pre-treatment steps [19]. Therefore, there is a demand for new analytical technique with reliable, fast response speed, cheap instrument, low cost, simple operation, time saving and high sensitivity for BPA detection in situ condition. For this purpose, an electrochemical sensor based on molecularly imprinted polymers (MIPs) will be a good alternative [20].
MIPs have been established as a new technique that allows the creation of tailor-made binding sites for certain molecules, such as chiral molecules [21]. It is one of the most efficient strategies to offer a synthetic route to artificial recognition systems by a template polymerization technique [22]. MIPs are usually prepared by polymerizing a mixture of a target molecule (template), functional monomers and an excess of cross-linkers, and then removing the template from the cross-linked polymer network. The synthesis technique of MIPs is simple and cheap. The resultant MIPs materials exhibit high selectivity, excellent mechanical strength, durability to heat, acid and base conditions and better engineering possibility than biological counterparts. Moreover, the introduction of synthetic design into molecular imprinting strategy can even make a host element suitable for the analyte, for which the natural receptor does not exist. These characteristics allow MIPs materials as recognition elements to be used in a wide range of fields. Because of the several advantages of MIPs such as low cost, stability and easy preparation compared with those of natural molecular recognition products (e.g., antibody), MIPs have been used in electrochemical sensor system [23].
The aim of this study was to construct an electrochemical sensor for the detection of BPA by using molecularly imprinted technique and gold nanoparticles (NG). The role of NG was to increase the electrode’s surface area and enhance the current signal. The combination of the nanoparticles with MIPs technique offered an attractive route to enhance the sensitivity of the imprinted sensor. This MIPs sensor was employed to detect BPA in PC water bottle samples successfully.
2 Experiments
2.1 Reagents
Tetrabutylammonium perchlorate, 2-aminothiophenol, chloroauric acid were purchased from Sigma-Aldrich (USA). BPA was obtained from Beijing Chemical Technology Co., Ltd. (China). Samples were obtained from a local market. All other chemicals were analytical reagent grade and all the solutions were prepared with deionized water.
2.2 Apparatus
All of the electrochemical experiments were carried out with a model VersaSTAT 3 electrochemical workstation (Princeton Applied Research, USA). Electrochemical measurements were performed with a three-electrode system composed of platinum wire as auxiliary electrode, Ag/AgCl electrode as reference electrode and modified glassy carbon electrode (GCE) as working electrode. Scanning electron microscope (SEM) images were obtained using field emission SEM (SUPRA®55, Germany).
2.3 Preparation of NG–MIP–modified GCE
Before modification, the bare GCE was polished to a mirror-like finish with 0.3- and 0.05-μm alumina slurry on micro-cloth pads. Then, washed successively with deionized water and ethanol in an ultrasonic bath and dried in air before use. For preparation of the NG-GCE, 1 mg mL−1 HAuCl4 solution was prepared in deionized water. Modification with NG on the electrode was performed by immersing the electrode into the 1 mg mL−1 HAuCl4 solution and applying a potential of −200 mV during 10 min.
The modified NG-GCE was dipped into a self-assembled solution (20 mL) of tetrabutylammonium perchlorate (5 mmol L−1), 2-aminothiophenol (20 mmol L−1), BPA (10 mmol L−1) and CH2Cl2 for 18 h. Polymerization of MIPs was performed by cyclic voltammetry (30 scans) in the range −0.4 to 1.2 V (scan rate 50 mV s−1). After that, the polymer film was subjected to a washing procedure in H2SO4 (0.65 mol L−1) to remove the BPA template entrapped in the polymeric matrix (Fig. 1). So, the BPA-imprinted polymer (MIP)–NG–GCE was obtained.
For comparison, non-imprinted polymer (NIP)-NG-GCE was fabricated with the similar procedures without the template. All the modified electrodes were stored at 4 °C in a refrigerator before use.
2.4 Preparation of sample solution
A kind of plastic product was purchased from a local supermarket. This sample was cut into pieces by scissors and washed twice with deionized water. Then, the plastic product was treated as the following process to obtain sample solution. About 1.00 g plastic product pieces and 30 mL ethanol were added into a beaker. After heating in water bath for 4 h at 50 °C, sealed with fresh-keeping film and put it aside for 5 days. After filtrated, the liquid phase was collected in a 50 mL volumetric flask and diluted to volume with ethanol. Otherwise, the spiked sample solution was prepared as the same method after adding the known amount of BPA standard solution.
3 Results and discussion
3.1 Preparation of polymeric film
A typical cyclic voltammogram (CV) was shown in Fig. 2, which was recorded during the electropolymerization in the presence of 2-aminothiophenol and BPA. With increasing the number of cycles, width of current in CV curve gradually narrowed, which indicated that the MIPs layer was formed on the surface of the electrode. Aniline of 2-aminothiophenol could be used as a polymerizing unit in polymerization. And there was an amino in 2-aminothiophenol, which could form hydrogen bonds with the phenolic hydroxyl in BPA. So we chose 2-aminothiophenol as a functional monomer. During polymerization, it interacted with hydrogen atoms in the poly(2-aminothiophenol) network through hydrogen bonding. The thickness of the polymer was easily adjusted by controlling the scan rate and the number of cycles during electropolymerization.
3.2 Characterization of the MIP–NG–modified electrode
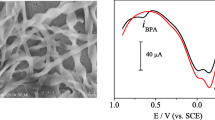
To characterize the prepared electrodes, CV was performed with a supporting electrolyte containing 5 mmol L−1 K3Fe(CN)6 and 0.1 mol L−1 KCl. As shown in Fig. 3, compared with curve b, the peak current of curve a was higher, which indicated that the NG layer could enhance the current response. Moreover, the morphology of the NG-GCE surface was characterized by SEM technique. As shown in Fig. 4, the NG was homogeneously distributed on the GCE surface with a diameter in the range of 40–80 nm. Because the MIPs polymeric layer was insulated, the redox peak current decreased obviously (curve d). After removing the template BPA, peak currents of the CV increased again (curve c). This result demonstrated that there were many empty binding sites on the electrode after removing the BPA, the redox couple could direct access to the surface of the electrode.
3.3 Effects of MIPs content
The molar ratio of template to monomer was found to play a key role in the sensor performance as the molar ratio of MIPs determined the number of binding sites available for selective rebinding of BPA. Addition of appropriate template led to optimum physical properties and ensured high mobility of BPA in the membrane. So, the effect of different molar ratio of template to monomer on the performance of sensor was investigated. Fig. 5 showed the current response of sensor with different ratio of template to monomer. The results demonstrated that the membrane had the best performance when the ratio of template to monomer was 1:2. In the case of 1:4, the total numbers of binding site for rebinding BPA were relatively lower for the membrane. On the other hand, when the ratio was 1:1, template and monomer were dispersed non-uniformly; this would result in poor performance of MIPs.
3.4 Amperometric detection based on MIP and NIP electrodes
To study the BPA recognition ability of MIPs, MIP–NG–GCE and NIP–NG–GCE were prepared and inserted into the BPA-containing solutions. Amperometric measurement was carried out and all sensing potentials were set at 0.4 V. The result of the amperometric detection was shown in Fig. 6. The equilibrium time of each curve was set at 100 s and the concentration of BPA was 50 μmol L−1. The result of amperometric curve suggested that the current response of the MIP–NG–GCE was much higher as compared with that of the NIP-NG-GCE. The reason of this phenomenon was related to the coating of the MIPs film. This MIPs film could leave plentiful selective binding sites after removing template molecules. Therefore, BPA was absorbed easily to the surface of imprinted electrode through the imprinted cavities.
3.5 Amperometric response
Amperometry was used to estimate the detection limit of BPA. In this study, potassium ferricyanide was used as a probe to detect the surface signal of electrode. Figure 7 showed a typical amperometric response of the imprinted sensor under the optimum experimental conditions. With the increase of BPA concentration, the sensor achieved the steady state current within less than 100 s, which indicated a fast detection process [24]. The linear range spanned the concentration of BPA from 8.0 × 10−6 to 6.0 × 10−2 mol L−1. The detection limit was estimated to be 1.38 × 10−7 mol L−1 (S/N = 3). The BPA detection performances of the proposed sensor were compared with those of other sensors. The results were shown in Table 1. It can be seen that the MIP–NG–GCE offered more reasonable linear range for BPA detection and the detection limit was lower than some of previous reports. These indicated that MIP–NG–GCE was an excellent platform for the detection of BPA.
3.6 Analysis of real samples
For ascertain the potential application in real sample analysis, this proposed method was used to detect BPA in real samples, such as PC water bottle. Under the optimized conditions, a modified electrode was placed into the sample solution, and then analyzed according to the above-described procedure. It was discovered that the PC water bottle had 25 μmol L−1 BPA. To study the accuracy of the proposed method, recovery experiments were carried out by standard addition method. The average recovery value of this sensor was 95.6%. This value showed a good agreement with those obtained by HPLC [25] and other analytical method [26, 27, 28]. The results indicated that this method had acceptable accuracy. Thus, the sensor may be satisfactorily applied to the determination of BPA levels in practical samples.
3.7 Interference, repeatability and stability
The specificity of binding mechanism in imprinted sensor has been also taken into account by comparing its response to analyte with some similar molecular structures. In particular, 4-tert-butylphenol, phenol and ο-nitrophenol were considered. As shown in Fig. 8, the sensor could also detect a little amperometric response for the phenol within same concentration level. It can be tentatively explained that phenol had the analogous structure with BPA, but their molecule size are not identical. Consequently, the phenol molecule could not easily enter the recognition sites applicable for the BPA. However, no remarkable amperometric response was observed for other compounds. It confirmed that the sensor based on the MIPs had a good selectivity of recognition to BPA.
To investigate the repeatability of the MIPs sensor, the experiments were performed in replicates of five in a 5.0 × 10−4 mol L−1 BPA solution using the same sensor, the RSD of currents was 5.0%. The good repeatability revealed that BPA could be reversible with the binding sites. The stability of the electrode was investigated by measuring the electrode response with 5.0 × 10−4 mol L−1 BPA. The response current of the electrode decreased to 90% after 5 days, whereas 85% of the original response retained after 10 days. The good stability of the modified electrode attributed to the stability of MIPs film on electrode. The sensors can be stored for more than 2 months at 4 °C without remarkable deterioration in its analytical performance. So the MIPs sensor was expected to be regenerated and used repeatedly.
4 Conclusions
A sensitive electrochemical method was proposed for determination of BPA in plastic products using the excellent properties of MIPs. The NG-modified electrode significantly enhanced the current response of sensor. The fabricated MIP–NG sensor not only exhibited strong specific binding activity toward BPA but also provided a broad linear range, remarkable stability and quantitatively repeatable analytical performance. We believe that the MIP–NG–modified electrochemical sensor will have promising application for BPA detection.
References
Yin HS, Zhou YL, Xu J (2010) Anal Chim Acta 659:144–150
Prieto AG, Lunar L, Rubio S et al (2008) Anal Chim Acta 617:51–58
Dekant W, Völkel W (2008) Toxicol Appl Pharmacol 228:114-134
Bredhult C, Sahlin L, Olovsson M (2009) Reprod Toxicol 28:18–25
Gómez AB, Rubio S, Bendito DP (2009) J Chromatogr A 1216:449–469
Völkel W, Kiranoglu M, Fromme H (2008) Toxicol Lett 179:155–162
Rezaee M, Yamini Y, Shariati S et al (2009) J Chromatogr A 1216:1511–1514
Jiang M, Zhang JH, Mei SR et al (2006) J Chromatogr A 1110:27–34
Ou JJ, Hu LH, Hu L et al (2006) Talanta 69:1001–1006
Sajiki J (2003) J Chromatogr B 783:367–375
Hernández1 EH, Martínez RC, Gonzalo ER (2009) Anal Chim Acta 650:195–201
Yan W, Li Y, Zhao LX et al (2009) J Chromatogr A 1216:7539–7545
Gómez AZ, Ballesteros O, Navalón A et al (2008) Microchem J 88:87–94
Shin HS, Park CH, Park SJ et al (2001) J Chromatogr A 912:119–125
Santos JM, Fagelman K, Guthrie JT (2002) J Chromatogr A 969:119–132
Kawaguchi M, Ito R, Okanouchi N et al (2008) J Chromatogr B 870:98–102
Brunete CS, Miguel E, Tadeo JL (2009) J Chromatogr A 1216:5497–5503
Geens T, Neels H, Covaci A (2009) J Chromatogr B 877:4042–4046
March C, Manclús JJ, Jiménez Y (2009) Talanta 78:827–833
Yin HS, Zhou YL, Ai SY (2009) J Electroanal Chem 626:80–88
Janiak DS, Kofinas P (2007) Anal Bioanal Chem 389:399–404
Mosbach K (2006) Sci Am 295:86–91
Lee HY, Kim BS (2009) Biosens Bioelectron 25:587–591
Huang JD, Zhang XM, Liu S et al (2011) Sens Actuators B 152:292–298
Yoon Y, Westerhoff P, Snyder SA (2003) Water Res 37:3530–3537
Chauke V, Matemadombo F, Nyokong T (2010) J Hazard Mater 178:180–186
Modaressi K, Taylor KE, Bewtra JK et al (2005) Water Res 39:4309–4316
Notsu H, Tatsuma T, Fujishima A (2002) J Electroanal Chem 523:86–92
Acknowledgments
This work was supported by the National Natural Science Foundation of the People’s Republic of China (No. 30972056 and 30911140278), the Foundation for Outstanding Young Scientist in Shandong Province (No. BS2009NY001), the Scientific and Technological Development Plan in Shandong Province (No. 2010GNC10960), the Natural Science Foundation of Shandong Province (No. ZR2010DQ025) and the Shandong Province Higher Educational Science and Technology Program (No. J10LB14).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Zhang, X., Liu, S. et al. Electrochemical sensor for bisphenol A detection based on molecularly imprinted polymers and gold nanoparticles. J Appl Electrochem 41, 1323–1328 (2011). https://doi.org/10.1007/s10800-011-0350-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-011-0350-8