Abstract
The electrochemical properties of bare and Co3(PO4)2-coated LiNi0.8Co0.16Al0.04O2 electrodes after high current damage testing were characterized. Damage was induced by cycling with a high current density of 600 m Ag−1. Co3(PO4)2-coated LiNi0.8Co0.16Al0.04O2 electrodes exhibit lower capacity loss and better charge retention than bare LiNi0.8Co0.16Al0.04O2 electrodes after damage testing. The discharge capacity reduction of bare and Co3(PO4)2-coated electrodes after damage testing were ∼27 and 15%, respectively. The impedance of cells containing bare electrodes remarkably increased after high current cycling, which may be induced by damage to the electrode surface. However, damage was successfully suppressed by the Co3(PO4)2 coating. Bare LiNi0.8Co0.16Al0.04O2 electrodes developed large amounts of cracks and other extended defects after high current cycling. In contrast, Co3(PO4)2-coated electrodes maintained stable features after high current cycling, indicating the coating layer effectively protected the surface of the LiNi0.8Co0.16Al0.04O2 powder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the emergence of lithium ion batteries, many efforts have been made to develop good electrode materials with improved electrochemical performance and low material cost. Research has shown that the surface of electrode materials is of great importance to the electrochemical properties of lithium ion batteries. As a result, many groups have focused attention on surface coating the cathode material with metal oxide nanoparticles [1–9]. Coating metal oxides, such as TiO2, Al2O3, SiO2 and AlPO4, on the surface improve capacity retention, rate capability, and, in some cases, thermal stability, without sacrificing the specific capacity of the cathode. Recently, Co3(PO4)2 nanoparticle coatings were introduced for modifying LiCoO2 [10]. Co3(PO4)2 nanoparticles completely reacted with Li in LiCoO2, resulting in the formation of a LiCoPO4 phase and a partially lithium-deficient LixCoO2 phase. The olivine LiCoPO4 phase is very electrochemically and thermally stable, even after full delithiation [11]. Also, a Co3(PO4)2 coating on the surface of LiNi0.8Co0.16Al0.04O2 has been reported [12]. In fact, LiNiO2-based materials, such as LiNi0.8Co0.16Al0.04O2, are very promising cathode materials with higher capacity and lower cost than commercial LiCoO2 for lithium ion batteries [13–18]. However, LiNiO2-based materials rapidly take up moisture and easily form surface film impurities, such as Li2CO3 or LiOH [19–21]. The Co3(PO4)2 coating reacts with such impurities during annealing to form an olivine LiCoPO4 phase on the surface, which improves storage at high temperature [12].
In the present work, the effects of a Co3(PO4)2 nanoparticle coating on Li[Co0.1Ni0.15Li0.2Mn0.55]O2 cathode material are characterized for surface damage caused by high current cycling. Currently, cathode materials with high rate capabilities have attracted attention for their potential application in hybrid electric vehicles (HEV) or electric vehicles (EV) [22, 23]. However, the rapid electrochemical reaction due to high current can induce mechanical stress and strain to the oxide particle, which lead to damage to the structural and electrochemical properties. This damage may concentrate on the surface of the particle, thus the Co3(PO4)2 coating on the surface of the cathode material is expected to effectively depress high current damage to the cathode material itself.
2 Experimental
LiNi0.8Co0.16Al0.04O2 used as starting material and reference samples were purchased from Sumitomo Chemical. (NH4)2HPO4 (0.45 g) and Co(NO3)2 · 6H2O (1.5 g) were dissolved in distilled water for the coating solution. Ammonium hydroxide was added to the solution to increase the pH to 8.5. LiNi0.8Co0.16Al0.04O2 powder (50 g) was then slowly added to the coating solution and mixed to create a uniform slurry. The mixture was dried at 130 °C for 5 h. The dried powder was ground and heat-treated at 700 °C for 5 h. X-ray diffraction (XRD) patterns were obtained on the cathode electrode using a Philips X-ray diffractometer in the 2θ ranges from 15 to 70° with monochromatized Cu-Kα radiation (λ = 1.5406 Å). Bare and coated samples were tested with transmission electron microscopy (TEM) (CM 20, Philips, 200 KV) before and after high current damage.
For preparation of a positive electrode, 0.15 g (3% by wt.) polyvinyl difluoride (Aldrich) was dissolved in about 12 mL of N-methyl-2-pyrrolidone for one hour, and then 4.75 g (94% by wt.) of sample powder and 0.15 g (3% by wt.) of Super P black (MMM Carbon Co.) were added. After 24 h of ball mill processing, the viscous slurry was coated on aluminum foil using a scalpel and dried at 90 °C in an oven. The obtained cathode film was hot-pressed at 100 °C. The thickness of the cathode film was about 30 μm. Electrochemical cells were assembled in a dry room using the positive electrode, lithium, porous polyethylene film and 1 M LiPF6 solution in 1:1:1 weight ratio of ethylene carbonate:dimethyl carbonate:diethyl carbonate. Cells were subjected to galvanostatic cycling using a Toyo (TOSCAT 3000) charge–discharge system. The impedance measurement was carried out by a impedance/grain phase analyzer (Solartron SI 1260) used in conjunction with a potentiostat (Solartron SI 1287) equipped with Z-view software, where an AC voltage with an amplitude of 5 mV was applied over the frequency range from 0.1 Hz to 100 KHz.
3 Results and discussion
A damage testing was carried out by high current cycling. The cells were initially charged and discharged at a low current rate (40 mA g−1) and then cycled 100 times at a current rate of 600 mA g−1 for high current cycling. The voltage range was 4.3–3.0 or 4.5–3.0 V. Figure 1 shows the charge–discharge profiles of the cells containing bare and Co3(PO4)2-coated LiNi0.8Co0.16Al0.04O2 electrodes during high current cycling damage testing. Considering that the electrode was made with only 3% of carbon (Super P), a current density of 600 mA g−1 seemed to be sufficient to produce damage on the surface of the LiNi0.8Co0.16Al0.04O2 powder. In the initial charge–discharge profile before high current cycling, the bare LiNi0.8Co0.16Al0.04O2 electrode displays larger polarization than that of the Co3(PO4)2-coated electrode. This indicates that the coating treatment is beneficial for intercalation/deintercalation of lithium ions during cycling and decreases the internal resistance of the cells. During high current cycling, the voltage profile steeply increased and decreased. Although the voltage range was set on the cycler to be between 4.3–3.0 or 4.5–3.0 V, the voltage profile of the bare electrode dropped below 0 V and went above 5 V. However, the Co3(PO4)2-coated electrode showed a relatively narrower out-of-range profile with voltages between 2 and 4.5 V. The charge and discharge capacity of the bare electrode during high current cycling was almost zero, but the Co3(PO4)2-coated electrode delivered capacity, even though it was very small, when high current cycling was in the voltage range of 4.5–3.0 V, which indicates that the Co3(PO4)2 coating increased the rate capability of the LiNi0.8Co0.16Al0.04O2 electrode. The effect of the Co3(PO4)2 coating on rate capability was reported in detail in previous work [24].
The charge–discharge profiles of cells containing LiNi0.8Co0.16Al0.04O2 electrodes at an initial 50 mA g−1 and a subsequent cycling process at a current density of 600 mA g−1. (a) Bare electrode, voltage range of 4.3–3.0 V; (b) bare electrode, voltage range of 4.5–3.0 V; (c) Co3(PO4)2-coated electrode, voltage range of 4.3–3.0 V; (d) Co3(PO4)2-coated electrode, voltage range of 4.5–3.0 V
After high current cycling, the voltage profiles of the cells containing the bare and the Co3(PO4)2-coated electrodes were observed, as shown in Fig. 2. Initially five cycles were tested at a current density of 20 mA g−1 (rate of ∼0.1 C), and then the current density was increased to 40, 100, 200, 400, and 600 mA g−1 for subsequent cycles. The profile of the bare electrode showed high polarization even though a relatively low current density (20 mA g−1) was supplied, and the profile rapidly became steeper with increasing current density. When the current density was increased over 100 mA g−1, capacity dropped to almost zero. The degradation of the charge–discharge profile shows that the bare electrode was seriously damaged by high current cycling. In contrast, the polarization of the voltage profile for the Co3(PO4)2-coated electrode was much smaller, and the change of the profile with increasing current density was more gradual. Even though it was relatively weak, capacity was detected up to the higher current density level of 400 mA g−1. Figure 3 displays the discharge capacity and cyclic performance of the cells containing bare and Co3(PO4)2-coated electrodes at different current densities after high current cycling (after damage testing as shown in Fig. 1). Before the high current cycling, the bare and Co3(PO4)2-coated electrodes exhibited a discharge capacity of ∼190 and ∼200 mAh g−1, respectively, at a current density of 20 mA g−1 in the voltage range of 4.5–3.0 (not included in Fig. 3) [24]. However, after high current cycling, the capacities were decreased to ∼140 and ∼170 mAh g−1 under the same conditions, indicating both samples were damaged. However, the coated sample showed less of a capacity loss during high current cycling. With increasing current density (from 20 to 400 mA g−1), the Co3(PO4)2-coated electrode showed higher discharge capacity and better rate capability than the bare electrode. Considering Figs. 2 and 3, it is obvious that the Co3(PO4)2 coating can decrease damage caused by high current cycling.
Voltage profiles of cells containing LiNi0.8Co0.16Al0.04O2 electrodes after high current cycling (damage test) at different current densities. Current density of 20 mA g−1 for cycles 1–5, 40 mA g−1 for cycles 6–10, 100 mA g−1 for cycles 11–15, 200 mA g−1 for cycles 16–20, 400 mA g−1 for cycles 21–25, and 600 mA g−1 for cycles 26–30. (a) Bare electrode, measuring voltage range is 4.3–3.0 V; (b) bare electrode, measuring voltage range is 4.5–3.0 V; (c) Co3(PO4)2-coated electrode, measuring voltage range is 4.3–3.0 V; (d) Co3(PO4)2-coated electrode, measuring voltage range is 4.5–3.0 V
AC impedance analysis has been known as a powerful technique to determine the kinetic parameters of the electrode process [25–27]. To confirm the effects of Co3(PO4)2 coatings on LiNi0.8Co0.16Al0.04O2 electrodes, impedace spectra of the cells containing bare and coated electrodes were obtained. Figure 4 shows the Nyquist plot of the cells before and after the high current damage testing. The measurement was carried out at a charged state to 4.3 or 4.5 V. The semicircle of the Nyquist plot of the bare electrode was dramatically increased after the damage test. It is presumed that structural changes of the electrode, including chemical dissolution of the transition metal ion and/or surface film formation, may result in a remarkable increase of impedance during the damage test. In contrast, it is interesting that the size of the semicircle for the Co3(PO4)2-coated electrode did not increase; instead, another small semicircle appeared during the damage test.
The electrochemical reaction of the positive electrode proceeds as an insertion/extraction reaction of lithium ions, which leads to changes in the molar volume of the materials. Thus, the reaction induces mechanical stress and strain to the oxide particles [28]. As the current density is increased during cycling, the stress and strain will increase due to rapid diffusion of lithium ions between the cathode electrode and the electrolyte, which can lead to damage of the electrode, such as structural changes or chemical decomposition/dissolution of the metal oxide. Specifically, the damage will focus on the surface of the powder because it is contacting and reacting with the electrolyte. The vulnerable surface of the electrode could be protected by a nanoparticle Co3(PO4)2 coating. Co3(PO4)2 nanoparticles were reported to have completely diffused into the surface of the cathode material and reacted with lithium of the cathode material or a surface impurity, such as Li2CO3 [10]. As such, it is expected that the surface of Co3(PO4)2-coated LiNi0.8Co0.15Al0.05O2 powder would be covered with LixCoPO4 phase [12, 24]. It is obvious that the LixCoPO4 phase can effectively prevent structural changes or chemical decomposition/dissolution of the metal oxide [12], thus maintaining low impedance during high current damage testing. Moreover, the covalent-bond nature of (PO4)3− with its cation contributed to a strong resistance toward reaction with the electroltyte [8]. However, a new small semicircle was formed in the very low frequency range after the damage test, which may be associated with a new impedance factor originating from a surface reaction between the surface coating layer and the electrolyte during the high current cycling process. As shown in Figs. 2 and 3, the capacity and charge retention of the Co3(PO4)2-coated electrode was deteriorated after the damage test, although it was less serious than the deterioration of the bare electrode. The new semicircle can explain the degradation of the electrochemical properties of the Co3(PO4)2-coated electrode after the damage test.
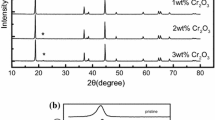
The phases of bare and Co3(PO4)2-coated LiNi0.8Co0.16Al0.04O2 powders were investigated by XRD analysis, as shown in Fig. 5. The XRD patterns of both samples after damage testing are identical to those of samples before damage testing. Even though, specifically in the case of bare LiNi0.8Co0.16Al0.04O2 powder, it is possible that there could be formation of a surface layer, structural phase transformation, or dissolution of cation ions, they were not detected by XRD analysis. The damage of LiNi0.8Co0.16Al0.04O2 powder during high current cycling must occur only on the surface of the electrode, such that it is not enough to detect by XRD analysis. Herein, a TEM analysis was introduced to investigate the surface morphology of LiNi0.8Co0.16Al0.04O2 powder in detail after the damage test. Figure 6 displays the bright field TEM images of LiNi0.8Co0.16Al0.04O2 primary particles after the high current damage test. Some bare particles showed unstable features containing microcracks and other extended defects on the surface. Figure 6a shows highly strained particles in which microcracks are clearly visible (indicated by arrows). Approximately 20–30% of particles appeared to have some sign of damage, which must have originated due to strain from rapid lattice expansion/extraction during high current cycling. In contrast, most of the Co3(PO4)2-coated particles displayed clear surfaces and unchanged features during high current cycling. Even though some particles had extended defects on their surface, it was a much smaller amount when compared with that of bare particles. The TEM analysis indicates that the Co3(PO4)2 treatment is a very effective method to decrease surface damage developed during damage testing, thus it can reduce capacity loss during high current cycling.
4 Conclusions
Co3(PO4)2 coating on LiNi0.8Co0.15Al0.05O2 powder successfully suppressed damage due to high current cycling. Damage to electrodes, including degradation of capacity and rate performance, following high current cycling were improved by a Co3(PO4)2 coating. Based on impedance and TEM analysis, it can be concluded that Co3(PO4)2 coatings effectively prevent an increase in impedance and reduce the generation of cracks and other extended defects during high current damage testing, thereby resulting in less degradation of the electrochemical properties of Co3(PO4)2-coated electrodes.
References
Cho J, Kim YJ, Kim TJ, Park B (2001) Angew Chem Int Ed Engl 40:3367
Cho J, Kim YJ, Park B (2000) Chem Mater 12:3788
Cho J, Lee JG, Kim B, Park B (2003) Chem Mater 15:3190
Cho J, Kim YJ, Park B (2001) J Electrochem Soc 148:A1110
Zhang ZR, Liu HS, Gong ZL, Yang Y (2004) J Electrochem Soc 151:A599
Cho J (2003) Electrochem Commun 5:146
Kim YJ, Kim H, Kim B et al (2003) Chem Mater 15:1505
Cho J, Kim H, Park B (2004) J Electrochem Soc 151:A1707
Amine K, Yasuda H, Yamachi M (2000) Electrochem Solid State Lett 3:178
Lee H, Kim MG, Cho J (2007) Electrochem Commun 9:149
Gao G (2004) Nanostructures and nanomaterials. World Science Publishing Co. Ltd., Singapore
Kim Y, Cho J (2007) J Electrochem Soc 154:A495
Saadoune I, Delmas C (1998) J Solid State Chem 136:8
Li W, Reimers JN, Dahn JR (1993) Solid State Ion 67:123
Nishida Y, Nakane K, Satoh T (1997) J Power Sources 68:561
Omanda H, Brousse T, Marhic C, Schleich DM (2004) J Electrochem Soc 151:A922
Belharouak I, Lu W, Vissers D, Amine K (2006) Electrochem Commun 8:329
Shaju KM, SubbaRao GV, Chowdari BVR (2004) J Electrochem Soc 151:A1324
Kim J, Hong Y, Ryu KS, Kim MG, Cho J (2006) Electrochem Solid State Lett 9:A19
Liu HS, Zhang ZR, Gong ZL, Yang Y (2004) Electrochem Solid State Lett 7:A190
Matsumoto K, Kuzuo R, Takeya K, Yamanaka A (1999) J Power Sources 81–82:558
Liang HY, Qiu XP, Chen HL, He ZQ, Zhu WT, Chen LQ (2004) Electrochem Commun 6:789
Abraham KM, Pasquariello DM, Willstaedt EM (1998) J Electrochem Soc 145:482
Ryu KS, Lee SH, Kwak D, Kim J, Cho J, Park YJ (2007) Materials Chemistry and Physics submitted
Troltzsch U, Kanoun O, Trankler H (2006) Electrochim Acta 51:1664
Zhou W, Bao S, He B, Liang Y, Li H (2006) Electrochimica Acta 51:4701
Mohamedi M, Takahashi D, Itoh T, Umeda M, Uchida I (2002) J Electrochem Soc 149:A19
Vetter J, Novak P, Wagner MR et al (2005) J Power Sources 147:269
Acknowledgements
This work was supported by the Division of advanced batteries in NGE program (Project No. 10016454).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ryu, K.S., Lee, S.H., Koo, B.K. et al. Effects of Co3(PO4)2 coatings on LiNi0.8Co0.16Al0.04O2 cathodes during application of high current. J Appl Electrochem 38, 1385–1390 (2008). https://doi.org/10.1007/s10800-008-9576-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9576-5