Abstract
Purpose
To evaluate the lamina cribrosa, retinal nerve fiber layer (RNFL), and macula in patients with primary open-angle glaucoma (POAG) and pseudoexfoliation glaucoma (PEXG) and healthy individuals using enhanced depth imaging (EDI) of spectral-domain optical coherence tomography (SD-OCT).
Methods
A total of 158 eyes were included in the study, comprising 58 eyes of 29 patients with POAG, 50 eyes of 25 patients with PEXG, and 50 eyes of 25 healthy individuals. The lamina cribrosa thickness (LCT) (at three locations), lamina cribrosa depth (LCD), RNFL thickness, and the macular thickness were measured using the EDI mode of the SD-OCT. The results were compared among the three groups.
Results
In both POAG and PEXG groups, the LCT was significantly thinner in the center, mid-superior, and mid-inferior areas in both eyes than in the control group (p < 0.001). However, no statistically significant difference was observed between the POAG and PEXG groups in terms of LCT at all three measurement locations in both eyes (p > 0.05). The LCD was significantly lower in the control group compared to the POAG and PEXG groups (p < 0.05), but there was no significant difference between the POAG and PEXG groups (p > 0.05). The RNFL thickness was significantly lower in both the POAG and PEXG groups compared to the control group in both eyes (p < 0.05).
Conclusion
The LCT and LCD of patients with POAG and PEXG were thinner than those of healthy individuals, but there was no significant difference between the patients with POAG and PEXG.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glaucoma is an optic neuropathy characterized by the progressive loss of retinal ganglion cells and their axons, excavation of the optic nerve head (ONH), and visual field defects [1]. The most common type of glaucoma is primary open-angle glaucoma (POAG), which is distinguished by an increase in drainage resistance in the trabecular meshwork [2]. Pseudoexfoliation syndrome (PEX) is an age-related systemic disease, and its ocular presentations consist of whitish-gray protein accumulation on the lens, iris, ciliary epithelium, corneal endothelium, and the trabecular network [3]. Pseudoexfoliation glaucoma (PEXG), an important complication of PEX, is the most common cause of secondary open-angle glaucoma [4]. Since PEXG has a higher intraocular pressure (IOP) than POAG and responds poorly to medication, it has a worse prognosis than POAG [3]. As a result, PEXG causes optic nerve damage more quickly and leads to more severe and rapidly progressing visual field defects [5]. As the damage in glaucoma is irreversible, it is important to recognize glaucomatous optic disc changes before detectable loss of visual fields occurs. Glaucoma is diagnosed by recognizing characteristic structural changes to the ONH and retinal nerve fiber layer (RNFL). ONH abnormalities can be a useful indicator of early-stage glaucoma. However, ONH examination is subjective, and even among the findings of experienced researchers, significant differences can be observed. Moreover, the appearance and dimensions of the optic disc vary even in the healthy population. As a result, objective methods to evaluate ONH are required for definitive and early diagnosis. On the other hand, some studies have suggested that RNFL changes will lead to optic disc and cup changes in eyes with normal visual field but high IOP, and therefore abnormality in RNFL examination can be guiding [6,7,8,9,10].
Optical coherence tomography (OCT), which produces high-resolution tomographic cross-sectional images of the retina and optic nerve in a non-contact, non-invasive manner, has enabled in vivo visualization of the eye structures. Lamina cribrosa (LC), which is anatomically localized in the posterior sclera and helps maintain the pressure gradient between the intraocular and extraocular spaces, has been identified as the main site of retinal ganglion cell axonal injury in glaucoma [11,12,13]. Recent histological studies have shown that morphological changes begin in the LC even in the early stages of glaucoma [13]. Furthermore, it has been reported that eyes with pseudoexfoliation have abnormal elastic tissue in the LC and reduced LC stiffness [14]. For this reason, it is suggested that the LC in PEXG may show structural changes due to irregular elastosis in addition to the mechanical damage caused by high IOP in glaucoma [15]. However, the capability to assess these changes in vivo in deep structures was previously limited because older-generation OCTs required light to penetrate tissues to provide imaging. With advances in OCT devices, deep structures such as the LC can be currently easily assessed, especially using enhanced depth imaging (EDI) available in spectral-domain (SD) OCT [16].
This study aimed to investigate the differences among groups by measuring LC structural parameters, RNFL thickness, and macular thickness in patients with POAG and PEXG and in healthy individuals using the EDI mode of SD-OCT. Thus, examining the features of LC in both POAG and PEXG eyes and comparing the results with those from healthy individuals may reveal structural differences between these two types of glaucoma and shed more light on this issue.
Materials and methods
Patients
The study was conducted in the ophthalmology department of Kayseri Education and Research Hospital, Kayseri, Türkiye in accordance with the principles of the Helsinki Declaration. The approval was obtained from the Education Planning Board of Kayseri Education and Research Hospital and the Ethics Committee of Erciyes University (registration number: 592) for the study. A total of 158 eyes, consisting of 58 eyes of 29 patients with POAG, 50 eyes of 25 patients with PEXG, and 50 eyes of 25 healthy individuals, were included in the study. Patients with angle closure glaucoma or narrow-angle view on gonioscopic examination, nystagmus, spherical refractive errors greater than ± 5 Diopters (D), cylindrical refractive errors greater than ± 3 D, history of eye trauma or ocular surgery, presence of pregnancy, and those with any cranial pathology were excluded from the study. Additionally, patients with ocular conditions such as cataracts, vitreous opacities, corneal opacity that might reduce image quality and an eye disease such as keratoconus, diabetic retinopathy, hypertensive retinopathy, chorioretinitis, and central serous chorioretinopathy that might affect the measurements were also excluded.
Examination and measurements
A detailed ocular examination including the best-corrected distance visual acuity measurement with the Snellen chart, autorefractometer, biomicroscopic examination, angle examination, a visual field test (Humphrey Field Analyzer 24–2 test; Carl Zeiss Meditec, Dublin, CA), and fundus examination was performed in all patients. The iridocorneal angle was evaluated on four quadrants using a three-mirror Goldmann lens. The central corneal thickness was measured using a Scheimpflug imaging system (Pentacam, Oculus GmbH, Wetzlar, Germany). The fundus examination was performed with a 90-diopter (D) aspheric lens after pupillary dilation with 1% tropicamide (Tropamide, Bilim İlaç, Türkiye). Primary open-angle glaucoma was defined as the presence of IOP > 21 mmHg, typical glaucomatous ONH changes (cup/disc [C/D] ratio above 0.5 or the difference in [C/D] ratio between the two eyes greater than 0.2 and thinning in neuroretinal rim [NRR]), typical glaucomatous visual field defects (localized defect, paracentral scotoma, Bjerrum scotoma, nasal step, temporal sector defect or diffuse defect), and open anterior chamber angle (Schaffer classification grade 3–4) and normal structure in gonioscopic examination. PEX was accepted as the appearance of typical PEX material at the pupillary border or on the anterior lens capsule during routine examination or after mydriasis. PEXG was defined as IOP elevation, glaucomatous optic neuropathy, and glaucomatous visual field changes accompanying anterior segment findings of PEX. For IOP measurement, 0.5% proparacaine hydrochloride (Alcain, Alcon) was instilled in the patients’ eyes before measurement. The IOP measurement was made using Goldman applanation tonometry before 12 o'clock.
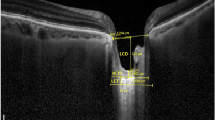
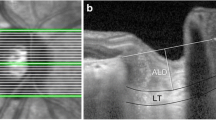
LC assessment was performed using the EDI mode of SD-OCT (Spectralis, Heidelberg, Germany) [17]. Six high-resolution radial B-scans were generated by the same experienced technician using a radial scanning pattern that visually focused on the entire ONH. From these images, 3 frames passing through the ONH (center, mid-superior, and mid-inferior) were selected for measurements [15]. LC was observed in B-scan images as a highly reflective plate-like structure below the optic cup, with its anterior and posterior borders (Fig. 1). The distance between the line connecting both ends of Bruch's membrane (yellow line) and the anterior border of the LC (green line) was defined as the lamina cribrosa depth (LCD). Lamina cribrosa thickness (LCT) was defined as the distance between the anterior (green line) and posterior surfaces (blue line) of the LC. LC parameters were measured using the manual caliper tool of Adobe Photoshop software (version 6.0, Adobe Systems, Inc., San Jose, CA). Two independent examiners measured both LCT and LCD twice, and the mean values were used. RNFL thickness was measured by scanning a peripapillary circle with a diameter of approximately 3.45 mm with the standard protocol while the eye tracking system was activated. The global and regional (temporal, superotemporal, superonasal, nasal, inferonasal, and inferotemporal sectors) RNFL thicknesses were obtained from the analysis, and the global measurement value was used for the mean RNFL thickness. Macular thickness was obtained from the macular thickness map examination procedure of the EDI mode of SD-OCT.
The distance between the line connecting both ends of Bruch’s membrane (yellow line) and the anterior border of the LC (green line) was defined as the lamina cribrosa depth (LCD). Lamina cribrasa thickness (LCT) was defined as the distance between the anterior (green line) and posterior surfaces (blue line) of the LC
Statistical analysis
Statistical Package for Social Science (SPSS) (version 22.0; SPSS Inc. Chicago IL, USA) was used in the analyses. Continuous variables were defined as mean ± SD. The chi-square test was used to evaluate gender differences between groups. To compare data among different groups, the Kruskal–Wallis test was used. To assess whether there was a statistically significant difference among the groups, the One-Way ANOVA was performed. A p-value less than 0.05 was considered statistically significant.
Results
Patient characteristics are shown in Table 1. The mean age was 65.1 ± 10.7 years in the POAG group, 64.4 ± 11.0 years in the PEXG group, and 63.0 ± 9.6 years in the control group. There was no statistically significant difference in terms of age among the three groups (p = 0.91). There were 27 females and 27 males in the POAG and PEXG groups, whereas the control group had 13 females and 12 males, with no significant difference (p = 0.57 and 0.71, respectively). All cases in the study groups had a bilateral clinical presentation.
LCT, LCD, IOP, and mean deviation (MD) values of the study groups are presented in Table 2. Remarkably, in both POAG and PEXG groups, LCT was significantly thinner in the center, mid-superior, and mid-inferior areas in both eyes than in the control group (p < 0.001). However, no statistically significant difference was detected between the POAG and PEXG groups in terms of LCT in all three measurement locations in both eyes (p > 0.05). Similarly, LCD was significantly lower in the control group compared to the POAG and PEXG groups (p < 0.05), but there was no significant difference in LCD between the POAG and PEXG groups (p = 0.978 and 0.599, respectively). Additionally, the mean MD value in the visual field test was significantly lower in the control group than in the POAG and PEXG groups (p < 0.001), but there was no significant difference between the POAG and PEXG groups (p = 0.933 and 0.887, respectively).
Although there was no significant difference in the mean IOP value of both eyes between the POAG and PEXG groups (p = 0.895 and 0.123, respectively), a significant difference was detected between the POAG group and the control group in both eyes (p = 0.012 and 0.005, respectively). On the other hand, while there was a significant difference in the mean IOP value between the PEXG group and the control group in the right eye, no significant difference was detected in the left eye (p = 0.050 and 0.432, respectively).
The comparison of RNFL thickness and macular thickness among groups is presented in Table 3. The mean RNFL thickness was significantly lower in both the POAG group and the PEXG group compared to the control group in both eyes (p < 0.05). When the POAG and PEXG groups were compared, the mean RNFL thickness of the POAG group was significantly lower than that of the PEXG group in both eyes (p = 0.04 and 0.016, respectively). The mean macular thickness was significantly lower in the POAG and PEXG groups than in the control group in both eyes (p < 0.05). When comparing the PEXG and POAG groups, there was no significant difference in the mean macular thickness in both eyes (p = 1.00 and 0.998, respectively).
Discussion
Glaucoma is a progressive disease that affects millions of people worldwide and causes severe vision loss in many cases. Since the damage caused by glaucoma is irreversible, early diagnosis of the disease and monitoring of its progression are critical. Considering that in addition to functional vision loss that may occur during the course of glaucoma and that serious losses may also arise in the retinal nerve fiber layer (RNFL), it is obvious that the use of visual field testing alone will be insufficient in the diagnosis and follow-up of the disease. Therefore, objective and quantitative evaluation of ONH and RNFL is of great importance. To achieve this, modern diagnostic devices, including the enhanced depth imaging (EDI) technique, which is available in SD-OCT, have been developed [16].
The LC, which is located in the scleral foramen, is a porous, mesh-like structure consisting of retinal blood vessels, retinal ganglion cell axons, and collagen fiber bundles from the inner layers of the sclera [18]. The LC, on the other hand, is also the primary site of retinal ganglion cell axonal damage in glaucoma. [19]. High IOP causes mechanical stress and strain on the posterior structural elements of the eye, particularly on the LC and surrounding tissues. The optic nerve fibers, which penetrate the sclera at the LC level and exit the eye, are extremely sensitive to increased pressure. High IOP-induced stress and tension cause axonal damage and disrupt axonal transport, resulting in compression and deformation of the LC [6, 7]. Histological studies on animals and cadaveric or enucleated eyes revealed that morphological changes in the LC occur in the early stages of the disease [20]. Furthermore, structural differences in the LC may play an important role in glaucoma pathogenesis, explaining why increased IOP does not cause equal damage in each eye. These changes are assumed to be caused by a variety of underlying mechanisms. General structural changes in the LC, such as thinning and back displacement, are thought to occur as a result of applying a mechanical force of high IOP to the LC [8]. However, due to local differences such as laminar density in the LC architecture, this mechanical stress does not disperse homogeneously [8]. Conversely, chronic exposure to increased IOP causes localized changes in addition to widespread modifications, especially in areas of large pores and weak connective tissue on the upper and lower periphery of the LC [1, 8].
In the study of Kim et al. examining how IOP affects the LC position in patients with POAG, it was found that the LC position was significantly dislocated posteriorly in eyes with POAG and high IOP compared to eyes with POAG and low IOP [10]. In Won et al.’s study, the curvature of the LC was compared in PEXG and POAG, and it was determined that PEXG eyes had a steeper curved LC than POAG eyes with a similar level of glaucoma severity [9]. Kim YW et al. evaluated the LC curvature with swept-source OCT in POAG patients, and it was determined that the LC posterior bowing increased in POAG eyes, which was significantly associated with structural ONH changes [10]. Ersöz et al. examined the LC curvature of 70 patients with PEXG and 68 age- and gender-matched healthy subjects with SD-OCT and found that the increase in the curvature was associated with the severity of PEXG [11]. Yang et al. measured the LC insertion into the peripapillary sclera and optic nerve pia in normal and early experimental glaucoma monkey eyes and found that migration of the LC is a sign of early cupping in monkey experimental glaucoma [13]. In the study of Braunsmann et al., which included three donor eyes with PEX and three age-matched control eyes, the stiffness of the LC and peripapillary sclera was examined by atomic force microscopy [14]. The authors found that the stiffness of the LC was significantly reduced in eyes with PEX and concluded that the ONH was more vulnerable to glaucomatous damage in these PEX eyes. Wang et al. examined the 3-dimensional microarchitecture of the LC with swept-source OCT and showed that the beam thickness to pore diameter ratio and pore diameter standard deviation were higher in patients with glaucoma [21]. All these mentioned studies show that the LC is vulnerable to damage in many ways in glaucoma and is the primary location of glaucomatous damage.
In their study on mild, moderate, and advanced glaucoma patients using electron microscopy, Quigley et al. found significant thinning in advanced glaucoma patients [1]. Furlanetto et al. reported that central and mid-peripheral LC were located more posteriorly in glaucomatous eyes than in the control group [22]. According to our results, although there was no statistically significant difference between the POAG and PEXG groups in the LCT measurement made in the center, mid-superior, and mid-inferior areas of both eyes, LCT in both the POAG and PEXG groups was lower than the control group. Similarly, we found that the LCD was significantly lower in the control group compared to the POAG and PEXG groups, but there was no significant difference in LC depth between the POAG and PEXG groups. Kim S et al. evaluated the LC characteristics in patients with PEXG (n = 21) and POAG (n = 35) using EDI-OCT and found that the LCT was thinner in eyes with PEXG (121.3 ± 13.0 µm) compared to POAG eyes (133.4 ± 14.5 µm) at similar levels of glaucoma severity [15]. However, similar to our results, the authors reported that the depth of the anterior LC was not different between the POAG and PEXG groups. Li et al. analyzed the morphological changes in the LC of patients with POAG using EDI-OCT and compared 52 POAG eyes with 50 healthy eyes [23]. The authors found that the LCT was thinner in eyes with POAG than in healthy eyes. Similarly, in our study, LCT in the POAG group was significantly thinner in the central, mid-superior, and mid-inferior areas of both eyes compared to the control group. This shows that LCT is obviously thinner in glaucoma patients, and our findings are consistent with the results of previous studies.
Netland et al. examined ONHs from patients with PEXG and POAG and age-matched healthy individuals using electron microscopy and immunogold detection of elastin [24]. They concluded that in eyes with PEXG, elastin synthesis and/or degradation in the ONH was abnormally regulated and that these eyes were more susceptible to damage. Moghimi et al. compared one eye each of 32 nonglaucomatous PEX cases and 29 healthy individuals to evaluate LCT in patients with PEX [25]. After taking images through the center, mid-superior, and mid-inferior of the ONH, the researchers found that the LCT of PEX cases was thinner than that of healthy individuals in three areas. In our study, LCT in the PEXG group was found to be significantly thinner in the central, mid-superior, and mid-inferior areas of both eyes compared to the control group, similar to previous studies. However, the noteworthy point here is that, according to our results, there is no difference between PEXG and POAG in terms of LCT and LCD. Yet, it has been reported that PEXG manifests itself as high IOP with severe fluctuations despite IOP-lowering treatments and has a worse prognosis than POAG [26]. However, in our study, there was no significant difference in mean IOP pressure level between the PEXG and POAG groups, which may explain the lack of significant difference between the two groups. Moreover, it has been suggested that LC parameters may be affected by various other factors apart from the type of glaucoma, such as glaucoma severity [15]. Additionally, it has been reported that some factors such as the age of onset of glaucoma, its acute or chronic course, duration of the disease, LC structure of the patient, and local blood flow differences may affect the severity of local or widespread changes in the LC [8]. All these factors may explain the lack of significant difference between PEXG and POAG groups in LC parameters in our study as well as in some previous studies [15]. Undoubtedly, multicenter and longitudinal studies analyzing LC changes in glaucoma patients are necessary to prove this.
OCT provides objective and quantitative data to demonstrate the known structural defects of glaucoma. In particular, the measurement of peripapillary RNFL thickness is very critical in the diagnosis and follow-up of glaucoma. In the study of Schuman et al., the mean RNFL thickness was 95.9 ± 10.09 µm in the control group (n = 107), 80.3 ± 18.4 µm in the early glaucoma group (n = 64), and 50.7 ± 13.6 µm in the advanced glaucoma group (n = 18) [27]. The authors found a statistically significant difference in RNFL thickness between healthy and glaucomatous eyes, particularly in the lower quadrant, and a strong correlation between RNFL thickness and visual field parameters. In the study of Korkmaz et al. comparing eyes with glaucoma and healthy individuals, the mean RNFL thickness was significantly lower in the glaucoma group (97.03 ± 13.85 µm) than in the control group (113.48 ± 9.00 µm) [28]. Similarly, in our study, the mean RNFL thickness was significantly lower in both the POAG group and the PEXG group compared to the control group in both eyes. Moreover, when the POAG and PEXG groups were compared, the mean RNFL thickness of the POAG group was significantly lower than that of the PEXG group in both eyes.
Paunescu et al. determined the mean macular thickness in normal eyes as 235 ± 9.8 µm using a time-domain OCT (Stratus, Carl Zeiss Meditec Inc., Dublin, CA), and this value was lower than the mean macular thickness in the control group in our study (288.5 ± 21 µm in the right eye, and 286.7 ± 29 µm in the left eye) [29]. However, in some studies comparing SD-OCT with time-domain OCT, it was reported that subfield thickness was measured 30 to 55 microns lower in time-domain OCT [30, 31]. In their study comparing cases with glaucoma-suspect eyes and glaucoma eyes, Leung et al. reported that the mean macular thickness was significantly less in the glaucoma group, but when the glaucoma identification performance was examined, the measurement of peripapillary RNFL thickness was a more valuable indicator than the total macular thickness [32]. Greenfield et al. discovered that the mean macular thickness in the glaucoma group was significantly lower than in the control group, and it was also significantly associated with the visual field mean defect, pattern standard deviation, and mean RNFL thickness [33]. Parikh et al. observed that outer inferior macular thickness and volume parameters in early glaucoma are significantly different from healthy individuals [34]. According to our findings, similar to the results of other studies, the mean macular thickness was significantly lower in the POAG and PEXG groups than in the control group, but there was no significant difference between the POAG and PEXG groups.
The main limitations of the present study were the small number of patients and a single-center study design. However, unlike previous studies, the inclusion of a control group and the comparison of patients with POAG and PEXG are important advantages of the study.
In conclusion, in this study, we found that the LCT and LCD of patients with POAG and PEXG were thinner than those of healthy individuals, but there was no significant difference between the LCT of patients with POAG and PEXG. We also found that patients with POAG and PEXG had lower RNFL thickness and total macular thickness than healthy individuals. Therefore, we believe that LC measurement plays an important role in the diagnosis and management of glaucoma patients. For more definitive results, additional clinical studies in larger series and patient groups are recommended.
Data availability
No datasets were generated or analysed during the current study.
References
Quigley HA, Addicks EM (1981) Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol 99:137–143
Kwon YH, Fingert JH, Kuehn MH, Alward WL (2009) Primary open-angle glaucoma. N Engl J Med 12(360):1113–1124
Schweitzer C (2018) Syndrome pseudo-exfoliatif et glaucome exfoliatif. J Fr Ophtalmol 41:78–90
Tomczyk-Socha M, Tomczak W, Winkler-Lach W, Turno-Kręcicka A (2023) Pseudoexfoliation syndrome-clinical characteristics of most common cause of secondary glaucoma. J Clin Med 21(12):3580
Rao V, Doctor M, Rao G (2015) Prevalence and prognosis of pseudoexfoliation glaucoma in western India. Asia Pac J Ophthalmol (Phila) 4:121–127
Fechtner RD, Weinreb RN (1994) Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol 39:23–42
Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT (2005) The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 24:39–73
Park SC (2013) In vivo evaluation of lamina cribrosa deformation in glaucoma. J Glaucoma 22:S29-31
Won HJ, Sung KR, Shin JW, Jo YH, Song MK (2021) Comparison of lamina cribrosa curvature in pseudoexfoliation and primary open-angle glaucoma. Am J Ophthalmol 223:1–8
Kim YW, Jeoung JW, Kim DW, Girard MJ, Mari JM, Park KH, Kim DM (2016) Clinical assessment of lamina cribrosa curvature in eyes with primary open-angle glaucoma. PLoS ONE 10(11):0150260
Ersöz MG, Kunak Mart D, Hazar L, Ayıntap E, Botan Güneş İ, Konya HÖ (2018) Evaluation of prelaminar region and lamina cribrosa with enhanced depth imaging optical coherence tomography in pseudoexfoliation glaucoma. Turk J Ophthalmol 48:109–114
Kim YW, Jeoung JW, Girard MJ, Mari JM, Park KH (2016) Positional and curvature difference of lamina cribrosa according to the baseline intraocular pressure in primary open-angle glaucoma: a swept-source optical coherence tomography (SS-OCT) study. PLoS ONE 9(11):e0162182
Yang H, Williams G, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF (2011) Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest Ophthalmol Vis Sci 9(52):7109–7121
Braunsmann C, Hammer CM, Rheinlaender J, Kruse FE, Schäffer TE, Schlötzer-Schrehardt U (2012) Evaluation of lamina cribrosa and peripapillary sclera stiffness in pseudoexfoliation and normal eyes by atomic force microscopy. Invest Ophthalmol Vis Sci 17(53):2960–2967
Kim S, Sung KR, Lee JR, Lee KS (2013) Evaluation of lamina cribrosa in pseudoexfoliation syndrome using spectral-domain optical coherence tomography enhanced depth imaging. Ophthalmology 120:1798–1803
Kagemann L, Ishikawa H, Wollstein G, Brennen PM, Townsend KA, Gabriele ML, Schuman JS (2008) Ultrahigh-resolution spectral domain optical coherence tomography imaging of the lamina cribrosa. Ophthalmic Surg Lasers Imaging 39:S126-131
Lee EJ, Kim TW, Weinreb RN, Park KH, Kim SH, Kim DM (2011) Visualization of the lamina cribrosa using enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 152:87-95.e1
Anderson DR (1969) Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol 82:800–814
Bellezza AJ, Rintalan CJ, Thompson HW, Downs JC, Hart RT, Burgoyne CF (2003) Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Invest Ophthalmol Vis Sci 44:623–637
Park SC, Ritch R (2011) High resolution in vivo imaging of the lamina cribrosa. Saudi J Ophthalmol 25:363–372
Wang B, Nevins JE, Nadler Z, Wollstein G, Ishikawa H, Bilonick RA, Kagemann L, Sigal IA, Grulkowski I, Liu JJ, Kraus M, Lu CD, Hornegger J, Fujimoto JG, Schuman JS (2013) In vivo lamina cribrosa micro-architecture in healthy and glaucomatous eyes as assessed by optical coherence tomography. Invest Ophthalmol Vis Sci 19(54):8270–8274
Furlanetto RL, Park SC, Damle UJ, Sieminski SF, Kung Y, Siegal N, Liebmann JM, Ritch R (2013) Posterior displacement of the lamina cribrosa in glaucoma: in vivo interindividual and intereye comparisons. Invest Ophthalmol Vis Sci 18(54):4836–4842
Li L, Bian AL, Cheng GW, Zhou Q (2016) Analysis of morphologic changes of lamina cribrosa in primary open angle glaucoma using enhanced depth imaging optical coherence tomography. Zhonghua Yan Ke Za Zhi 11(52):422–428
Netland PA, Ye H, Streeten BW, Hernandez MR (1995) Elastosis of the lamina cribrosa in pseudoexfoliation syndrome with glaucoma. Ophthalmology 102:878–886
Moghimi S, Mazloumi M, Johari M, Abdi P, Fakhraie G, Mohammadi M, Zarei R, Eslami Y, Fard MA, Lin SC (2016) Evaluation of lamina cribrosa and choroid in nonglaucomatous patients with pseudoexfoliation syndrome using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 57:1293–1300
Ritch R, Schlötzer-Schrehardt U, Konstas AG (2003) Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res 22:253–275
Schuman JS, Wollstein G, Farra T, Hertzmark E, Aydin A, Fujimoto JG, Paunescu LA (2003) Comparison of optic nerve head measurements obtained by optical coherence tomography and confocal scanning laser ophthalmoscopy. Am J Ophthalmol 135:504–512
Korkmaz B, Yiğit U, Ağaçhan A, Helvacıoğlu F, Bilen H, Tuğcu B (2010) The evaluation of the relationship between retinal nerve fiber layer and ganglion cell complex in glaucomatous and normal cases with optikal coherence tomography. Turk J Ophthalmol 40:338–342
Paunescu LA, Schuman JS, Price LL, Stark PC, Beaton S, Ishikawa H, Wollstein G, Fujimoto JG (2004) Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci 45:1716–1724
Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT (2008) Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci 49:4290–4296
Patel N, Chowdhury H, Leung R, Sivaprasad S (2013) Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diabetic macular edema. Indian J Ophthalmol 61:208–212
Leung CK, Chan WM, Yung WH, Ng AC, Woo J, Tsang MK, Tse RK (2005) Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology 112:391–400
Greenfield DS, Bagga H, Knighton RW (2003) Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol 121:41–46
Parikh RS, Parikh SR, Thomas R (2010) Diagnostic capability of macular parameters of stratus OCT 3 in detection of early glaucoma. Br J Ophthalmol 94:197–201
Funding
The authors did not receive any financial support from any public or private sources.
Author information
Authors and Affiliations
Contributions
The study conception, design, material preparation, data collection, were performed by BB, MA and analysis, supervision were performed by BB, MA and SD. The first draft of the manuscript was written by BB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Ethical approval
All procedures were performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the local ethics committee (Erciyes University, Faculty of Medicine Ethics Committee, Kayseri, Türkiye). The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Consent to publish
All participants gave written informed consent for their data to be published.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baskan, B., Atas, M. & Demircan, S. Evaluation of lamina cribrosa parameters, nerve fiber thickness, and macular thickness in primary open-angle glaucoma and pseudoexfoliation glaucoma using optical coherence tomography. Int Ophthalmol 44, 378 (2024). https://doi.org/10.1007/s10792-024-03288-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03288-2