Abstract
Purpose
To evaluate the efficacy and safety of adalimumab (ADA, Humira®) for treatment of non-infectious uveitis (NIU) refractory to conventional medications.
Methods
Anti-tumor necrosis factor-α naive patients with NIU unresponsive to conventional immunosuppressive treatment were treated with ADA. Most cases with NIU were related to ocular Behçet syndrome. Adult cases used 80 mg ADA subcutaneously on day 0, 40 mg in the first week, and then 40 mg every 2-week, while this was 20 mg in children. Evaluations were performed pre-treatment and at weeks 2, 8, and 24. The study endpoints were best-corrected visual acuity (BCVA, LogMAR) improvement, anterior chamber (AC) cell grade, vitreous cell and haze grades, decrease in macular thickness and edema, prednisolone dose, immunosuppressive dose, and adverse reactions.
Results
Thirty-eight eyes (19 right, 19 left) of 24 patients (14 female, 10 male) with (ocular Behçet syndrome) OBS (n = 27 eyes/18 patients) and NIU (n = 11 eyes/6 patients) were included. Mean age was 29.0 ± 14.1 years (range, 5–49) and follow-up time was 24 weeks. After ADA, BCVA increased (p < 0.001), and improvements in AC cell grade (p < 0.001), vitreous cell grade (p < 0.001), and vitreal haze grade (p < 0.001) were achieved at the final visit. Mean macular thickness decreased from 243.5 to 235.5 µm (p < 0.001). Such a rapid control of both anterior and posterior uveitis was observed in all eyes as early as the second week without relapses during follow-up. No ocular or systemic complications emerged during treatment.
Conclusions
ADA is effective and well-tolerated in pediatric and adolescent patients with NIU including OBS refractory to traditional medications and demonstrated corticosteroid- and immunosuppressive-sparing effects with no major side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-infectious uveitis (NIU) is a relapsing–remitting autoimmune disease that is characterized by intraocular inflammation, which includes ocular Behçet syndrome (OBS) and spondyloarthropathies such as juvenile idiopathic arthritis (JIA) and ankylosing spondylitis (AS). They represent 20% of legal blindness in developed countries after unpredictable outbreaks of ocular inflammation with recurrent acute hypopyon iridocyclitis, vitritis, retinitis, and occlusive vasculitis [1]. The highest prevalence of OBS is in Turkey and Japan (1/1000–1/10,000 per year), but 1/300,000 in the USA [1]. In Europe, NIU accounts for two-thirds of uveitis, whereas OBS itself accounts for one-fourth in Turkey, which affects predominantly men in their third decade of life.
Although we can control ocular inflammation and reduce the number of relapses with a combination of corticosteroids and immunosuppressants in NIU [2], their definitive therapy is still unsatisfactory with various drug-related ocular and systemic side effects. Therefore, it is urgent to find rapidly acting, effective, and safer management modalities to obtain full remission and vision protection. Tumor necrosis factor (TNF)-α, a pro-inflammatory cytokine, induces inflammation in NIU and OBS and its inhibition may be an effective strategy for uveitis remission that reduces exposure to corticosteroids, preventing permanent loss of vision [1, 2]. Indeed, TNF-α is increased in patients with OBS [3], and sustained remission can be obtained with infliximab (IFX, an anti-TNF-α agent) in uveitis refractory to conventional combined therapies [4]. Adalimumab (ADA, Humira®), a human monoclonal anti-TNF-α agent, may be more effective than IFX [5], and at present, Turkey, like the USA, European countries, and Japan, approved its use for the treatment of NIU and OBS.
In the present prospective research, we investigated the effect of ADA in terms of the degree of clinically detectable anterior and posterior ocular inflammation and macular edema in patients with NIU including OBS who were recalcitrant to conventional immunosuppressants.
Materials and methods
Pediatric and adult patients with active NIU who were unresponsive to the combination of prednisolone plus immunosuppressants (azathioprine, cyclosporine, interferon) for at least 8 weeks were treated with ADA at the Department of Ophthalmology, Division of Uvea-Behçet Unit, (Erciyes University Medical Faculty, Kayseri, Türkiye). The study conformed to the Declaration of Helsinki. The research was reviewed and approved by the department’s academic board and the ethics committee of (Erciyes University) (No: 2020/76), and written informed consent was obtained from all patients after possible side effects of ADA were explained.
All uveitis patients were evaluated at baseline for full (purified) protein derivative (PPD) tests, complete blood count (CBC), biochemical analyses, pulmonary radiology, and functions of the kidney and liver. Anti-tuberculosis prophylaxis was performed if latent tuberculosis was present or if the PPD test was over 10 mm. Exclusion criteria were patients with active HBV, HCV, HIV, fungal infections, liver/kidney insufficiency, or malignancy.
According to the Standardization of Uveitis Nomenclature (SUN) Working Group and adapted National Eye Institute criteria [6, 7], ocular inflammation was classified as “active” if the patient had at least one active “anterior chamber” (AC) inflammatory cell grade 1 + or higher, vitreous cell or haze grade 1 + or higher, or chorioretinal vascular lesions while the patients were under treatment with topical or systemic corticosteroids plus immunosuppressants; or “inactive”, which corresponds to eyes without the abovementioned active inflammatory status in addition to an AC cell grade and vitreous cell and haze grades of 0.5 + or less. Inactive patients were also included in the study if they demonstrated frequent relapses with abovesaid traditional management. Indeed, some inactive eyes had central macular thickening without macular edema as a result of previous panuveitic attacks. Therefore, eyes with (central macular thickness) CMT over 300 µm and/or cystic changes were accepted as CME. Patients who had cataract surgery or retinal diseases that could affect visual acuity during the last 6 months were excluded. Children were followed-up by a pediatrician at regular intervals during the ADA treatment. During the follow-up period, 2 + or more increases in AC and vitreous cell grades or transition from grade 3 + to 4 + were accepted as disease activity. Similarly, the development of new retinitis, vascular occlusion, retinal hemorrhage, macular edema, or papillitis was also accepted as posterior segment activation. Active inflammation of the eye after 8 weeks from ADA initiation was accepted as treatment failure.
The patients were evaluated at baseline and at second week, 8th week, and 24th week after the ADA treatment. At each visit, best-corrected visual acuity (BCVA, LogMAR) was obtained using a Snellen chart. Anterior segment and vitreal evaluations were performed by slit-lamp biomicroscopy, intraocular pressure was measured by applanation tonometry, and dilated fundoscopy was performed with a 90-D fundus lens. CMTs were calculated in the subfoveal section by (spectral-domain optic-coherence tomography) SD-OCT (Heidelberg Spectralis, Germany). Ultra-widefield fundus fluorescein angiography (FFA) images were obtained with an Optos device (Optos Advance, Optos, USA) to detect retinal vasculitis on the first visit. Patients were questioned regarding the side effects of ADA during the management period, and CBC and serum tests were repeated at 8-week interval. Complications such as cataracts, glaucoma, epiretinal membrane, and retinoschisis were noted, if present, in all adult and pediatric patients after ADA treatment.
Patients with NIU unresponsive to conventional immunosuppressives were treated with subcutaneous injections of 80 mg ADA on day 0, 40 mg at the first week, and then 40 mg every 2-week (20 mg in children < 30 kg). Prednisolone was gradually lowered and stopped within 2–4 weeks. Immunosuppressives were gradually reduced at 4–6 weeks but not stopped completely to prevent the formation of anti-ADA antibodies. The study endpoints were improvement in BCVA, decrease in CMT, decrease in vitreous haze grade, AC and vitreous cell grades, prednisolone, and immunosuppressive doses. Possible adverse reactions were noted if present.
Statistical analyses were performed using SPSS for Windows 21.0 (IBM statistics, NYC, USA). Compatibility with normal distribution was evaluated by Shapiro–Wilk’s test. Descriptive statistics exhibiting normal distribution were expressed as mean ± standard deviation, while those not exhibiting normal distribution were expressed as median, maximum, and minimum values. The Friedman test was applied for non-normally distributed dependent groups and the Wilcoxon test was used as indicated. Statistical graphs were created using GraphPad Prism Version 8 (GraphPad Coorp, San Diego, USA). P values < 0.05 were regarded as significant.
Results
All uveitis patients attended regular follow-ups. Five of the 24 patients were pediatric cases, and 19 were adults. Five of 24 patients had unilateral total vision loss as a result of previous aggressive uveitis attacks and complications even though they received traditional anti-inflammatory drugs, and another 5 patients had unilateral involvement. Therefore, 14 of 24 patients (14 females, 10 males) had bilateral and 10 had unilateral involvement. The systemic diagnosis was OBS (n = 18, 75.0%), JIA (n = 3, 12.5%), and idiopathic (n = 3, 12.5%). Therefore, a total of 38 eyes (19 right, 19 left) with OBS-related uveitis (n = 27 eyes of 18 patients) and NIU (n = 11 eyes of 6 patients) were included. Demographic characteristics of adult and pediatric patients with non-infectious uveitis are given in Table 1.
The mean age was 29.0 ± 14.1 years (range, 5–49), and the follow-up time was 6 months. All uveitis patients were classified based on the International Uveitis Study Group criteria. Fourteen of 24 patients (58.3%) had active uveitis before ADA therapy and the remaining 10 (41.7%) were in the inactive period but had frequent ocular relapses (≥ 4 recurrences in the previous year), which resulted in deterioration of vision with every attack. Of 38 eyes, panuveitis was present in 27 (71.0%), retinal vasculitis in six (15.7%), papillitis in two (5.3%), and intermediate uveitis with vitritis in one (2.7%). Two eyes (5.3%) had panuveitis with papillitis. All active uveitis cases were using oral methylprednisolone (1 mg/kg/d) and topical steroid drops for at least 2 weeks. In addition, five of the 24 patients were using systemic IFX and the remaining 19 patients were under treatment with systemic immunosuppressive drugs (azathioprine and cyclosporine). The patients were only on ADA therapy after its initiation and all prior non-effective drugs including systemic corticosteroids were gradually discontinued within 2–4 weeks, except topical drops in cases with anterior uveitis signs.
Baseline ocular findings of the patients and the progressions of improvements are shown in Table 2. The median value before ADA treatment for BCVA (LogMAR) was 1.00 (0.28–1.5), AC cell score was 0.50 (0.0–1.0), posterior segment cell score was 0.47 (0.0–1.0), and vitreal haze degree was 0.82 (0.00–1.25). The outcomes of ADA treatment were analyzed based on the last visit. Complete and rapid control of both anterior and posterior uveitis was observed in all eyes without relapses during the follow-up. After ADA usage, BCVA significantly improved at the last visit (p < 0.001). Such an increase started as early as the second week (p = 0.012), significantly increased at the 8th week (p = 0.003), but remained stable afterward until 24 weeks (Table 2). Both AC and vitreous cell grades and vitreal haze grade started to decrease within 2 weeks and improvement was observed until the last visit at 24 weeks (for each, p < 0.001). Similarly, the mean CMT was 243.5 µm at baseline, which decreased to 234.5 µm at the second week (p = 0.001). CMT was stable after the 2nd week until post-treatment 24 weeks (p < 0.001). No ocular complications emerged during the management period. Baseline CMT status and the effectiveness of ADA treatment in three cases included in the study are shown in Figs. 1, 2, and 3.
Case 1, an 8-year-old girl is being followed up for juvenile idiopathic arthritis-related uveitis. A Horizontal spectral-domain optic-coherence tomography (SD-OCT) image of the macula region (left eye). B Before adalimumab treatment, the presence of cystoid (macular) edema is observed in the central macula. C 8 weeks after adalimumab treatment, SD-OCT image shows resolution of cystoid macular edema. D SD-OCT image at 24 weeks after adalimumab treatment
Case 2, a 19-year-old men is being followed up for Behçet uveitis. A Horizontal spectral-domain optic-coherence tomography (SD-OCT) image of the macula region (right eye). It was observed that severe macular edema before adalimumab treatment (B) resolved at 8 weeks after treatment (C) and remained stable at 24 weeks control (D)
Case 3, a 21-year-old men is being followed up for Behçet uveitis. A Horizontal spectral-domain optic-coherence tomography (SD-OCT) image of the macula region (right eye). It is observed that cystoid macular edema was present before adalimumab treatment (B), the cysts significantly reduced at 8 weeks after treatment (C) and remained stable at 24 weeks control (D)
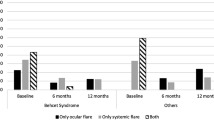
Comparative subgroup analyses of pediatric and adult patients are given in Figs. 4 and 5. While a partial improvement was observed in BCVA, vitreous haze grade, and CMT in the pediatric group compared to pre-treatment, the results obtained in the adult group were that there was an improvement in all parameters.
Subgroup analysis plot of pediatric uveitis within the group. In the subgroup analysis of 8 uveitic eyes, best corrected visual acuity (BCVA, LogMAR), anterior chamber (AC) cell grade, vitreous cell grade, vitreous haze grade, and central macular thickness (CMT, µm) were evaluated. There was an improvement in BCVA at week 24 after adalimumab treatment compared to pre-treatment (p = 0.004), and no statistically significant differences were found between the other weeks (ns: nonsignificant). There was no difference in AC cell grade and vitreous cell grade changes. It was observed that vitreous haze grade improved at 8 weeks (p = 0.042) and 24 weeks (p = 0.049) after treatment compared to before adalimumab treatment. On the other hand, while there was a significant decrease in CMT compared to the baseline in the 2nd week (p = 0.049) and the 8th week (p = 0.02), there was no significant change in the other weeks. Statistically significant comparisons are indicated by p values with "*" and "**" signs
Subgroup analysis plot of adult uveitis within the group. In the subgroup analysis of 30 uveitic eyes, best corrected visual acuity (BCVA, LogMAR), anterior chamber (AC) cell grade, vitreous cell grade, vitreous haze grade, and central macular thickness (CMT, µm) were evaluated. BCVA showed significant improvement at week 8 compared to the baseline before adalimumab treatment (p = 0.001). AC cell grade decreased significantly at the 8th week compared to the pre-treatment (p = 0.028), and the decrease in the cell count was significant at the 8th week compared to the 2nd week (p = 0.046). Vitreous cell grade decreased significantly at the 8th week, similarly (p = 0.028). A decrease in the vitreous haze is observed at the 24th week (p = 0.001). A significant decrease was observed in CMT in all weeks when compared to baseline. Statistically significant comparisons are indicated by p values with "*", "**" and "***" signs
Taken together, rapid remission was observed within two to four weeks in all evaluations. However, recurrence was encountered in four eyes as panuveitis at the fourth week, CME in three eyes at the eighth week, and anterior uveitis with vitritis in one eye at the sixth month after cessation of corticoids. Two patients with previous IFX treatment had panuveitis with vasculitic attacks at the fourth week, one had + 1 vitreal haze with anterior segment inflammation at the sixth month, and three had CME at the eighth week. Table 3 summarizes the recurrence status of the patients. Systemic prednisolone (1 mg/kg/d) was again started for all these patients and ADA therapy was maintained. With these exceptions, the remaining eyes did not develop attacks during the course of ADA treatment. ADA was well-tolerated by the majority of patients, though local pain at the injection site, rash, and itching were commonly encountered.
Discussion
Recent treatment modalities using anti-TNF antibodies (IFX, ADA) are promising for NIU recalcitrant to traditional drugs, which decrease the number of uveitis attacks with their immediate onset of action that is considered to be critical in sight-threatening NIU [8]. In a multicenter study, the efficacy of IFX and ADA treatments was compared in refractory uveitis cases due to Behçet disease [9]. According to the 1-year follow-up results, the improvement and efficacy of anterior and posterior segment inflammations were similar in both groups, but the improvement results in the ADA group were better than the IFX group. In this study, uveitis attacks could not be controlled in 5 patients (3 with OBS, 2 with JIA) despite the use of IFX before ADA. However, with ADA, both anterior and posterior inflammation findings and visual prognosis were improved until the 24th week. Although systemic corticoids plus traditional immunosuppressive agents are the first management modality for OBS patients with severe posterior segment uveitis, effective alternative approaches are mandatory due to the adverse effects of long-term corticoid use. Prompt and aggressive use of biologicals in patients with recurrent and resistant ocular complications improves visual prognosis [10]. Although ADA is approved for the treatment of uveitis, the knowledge of its efficacy and safety for the management of patients with NIU and OBS is limited. Our study included both children and adults and we tried to identify the efficacy of ADA by evaluating intraocular inflammation (AC and vitreous cell grades, vitreal haze), macular thickness, and BCVA. Therefore, the present prospective study demonstrated that ADA was found to be effective in improving visual acuity, decreasing ocular inflammatory findings, and reducing macular edema in OBS and NIU refractory to conventional drugs with both corticoids- and immunosuppressive-sparing effects. No major side effects were encountered.
ADA caused a reduction in the degree of inflammation in NIU at the 6th month with decreased macular thickness and complete resolution of CME in about two-thirds of eyes, which was found to be proportional to the improved visual gain [10]. Similarly, ADA decreased CME with regression of vasculitis at the end of the 12th month [11] with a lower risk of uveitic flare [12]. Lee et al. [13] demonstrated that AC cells decreased in 100% of eyes with active and inactive NIU at the 6th week, whereas vitreal haze improvement was 50% at the 6th week. In a randomized investigation, AC inflammation was reduced by 30% within the 2nd month, indicating ADA as a good choice in children with chronic anterior uveitis refractory to topical steroids and methotrexate [14]. In our study, the efficacy of ADA on vitreal haze and CMT started as early as the 2nd week, which required 6 months for complete vitreal haze resolution, indicating the efficacy of ADA in improving posterior segment inflammation with an early improvement in BCVA, which is very important, especially for visual rehabilitation in small children.
More than four-fifths of OBS patients responded well to ADA treatment at the 12th week with decreased inflammatory flares between the disease onset and the 12th month, which prevent irreversible sight-threatening complications with no recurrence, suggesting dose optimization after remission with a corticosteroid-sparing effect [15,16,17]. Indeed, retinal vasculitis regressed in all patients, CMT decreased at a higher rate in active eyes than in inactive eyes, and complete resolution occurred in an average of 3.4 weeks [17]. In our study, both AC and vitreous cell and haze grades were found to be decreased at the 2nd week, which gradually continued until the 24th week. The possible reason for early improvement may be due to the fact that about two-thirds of the eyes included in the present article were in the active period.
Silvestri et al. [18] demonstrated that all patients with NIU and OBS achieved complete ocular inflammation control with resolved macular edema in about three-fourths of cases. Yang et al. [19] reported that the improvement in AC inflammation was significantly better and the relapse time was significantly lower in ADA than in the conventional treatment group of OBS patients with poorly controlled retinal vasculitis, suggesting combined ADA plus conventional therapy usage in such cases. Similar to these reports, we found that BCVA increased significantly at each visit after ADA initiation, which was stable after the 8th week. Similarly, ADA caused a significant decrease in CMT at the 2nd week, which was stable afterward. Therefore, such an improvement in BCVA seems to be related to the decrease in intraocular inflammation along with the resolution of CME. Panuveitis improved in most of the eyes, though 8 eyes showed relapse, all of which were brought under control with the addition of corticoids to the regimen.
Although the prospective design of the present study is its strong characteristic, the limitation is that the study did not include a placebo-control group. It was thought that the statistics obtained in the pediatric group were different from the total evaluation due to the small number of samples. In addition, AC cells were manually evaluated using slit-lamp biomicroscopy, not measured by an automated system.
Conclusion
ADA yields prompt control of intraocular inflammatory parameters and significantly lowers the risk of management failure and visual loss by reducing AC cell grade, vitreous cell grade, vitreal haze grade, and CMT in refractory pediatric and adult patients with NIU and OBS. Sustained remission was achieved during the 24-week period and ADA treatment seems to be a safe, well-tolerated, and effective option in preventing irreversible sight-threatening uveitis-related complications and demonstrates both corticoid and immunosuppressive sparing effects even in IFX-resistant eyes with a low side effect profile.
References
Evereklioglu C (2005) Current concepts in the etiology and treatment of Behçet disease. Surv Ophthalmol 50:297–350
Evereklioglu C (2004) Managing the symptoms of Behçet’s disease. Expert Opin Pharmacother 5:317–328
Evereklioglu C, Er H, Türköz Y, Çekmen M (2002) Serum levels of TNF-α, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet’s disease. Mediat Inflamm 11:87–93
Evereklioglu C, Borlu M (2008) Sustained remission after infliximab in a child with systemic vasculitis refractory to conventional immunosuppressive therapy including interferon-α. Br J Ophthalmol 92:1034
Evereklioglu C (2011) Ocular Behçet disease: current therapeutic approaches. Curr Opin Ophthalmol 22:508–516
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. results of the first international workshop. Am J Ophthalmol 140:509–516
Nussenblatt RB, Palestine AG, Chan CC, Roberge F (1985) Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 924:467–471
Evereklioglu C (2023) Twenty years of quiescence after nonstop Remicade® (Infliximab) infusions in a child with ocular Behçet disease presenting as hypopyon-anterior uveitis refractory to immunosuppressants. Case Rep Ophthalmol 14:75–82
Atienza-Mateo B, Martín-Varillas JL, Calvo-Río V, Demetrio-Pablo R, Beltrán E, Sánchez-Bursón J et al (2019) Comparative study of infliximab versus adalimumab in refractory uveitis due to Behçet’s disease: national multicenter study of 177 cases. Arthritis Rheumatol 71:2081–2089
Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, Cordero-Coma M, Ortega G, Ortego N et al (2012) Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology 119:1575–1581
Calvo-Río V, Blanco R, Beltrán E, Sánchez-Bursón J, Mesquida M, Adán A et al (2014) Anti-TNF-α therapy in patients with refractory uveitis due to Behçet’s disease: a 1-year follow-up study of 124 patients. Rheumatology (Oxford) 53:2223–2231
Jaffe GJ, Dick AD, Brézin AP, Quan Dong Nguyen QD, Thorne JE, Kestelyn P et al (2016) Adalimumab in patients with active noninfectious uveitis. N Engl J Med 375:932–943
Lee JT, Yates WB, Rogers S, Wakefield D, McCluskey P, Lim LL (2018) Adalimumab for the treatment of refractory active and inactive non-infectious uveitis. Br J Ophthalmol 102:1672–1678
Quartier P, Baptiste A, Despert V, Allain-Launay E, Koné-Paut I, Belot A (2018) ADJUVITE Study Group. ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis 77:1003–1011
Fabiani C, Vitale A, Emmi G, Vannozzi L, Lopalco G, Guerriero S (2017) Efficacy and safety of adalimumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol 36:183–189
Martín-Varillas JL, Calvo-Río V, Beltrán E, Sánchez-Bursón J, Mesquida M, Adán A (2018) Successful optimization of adalimumab therapy in refractory uveitis due to Behçet’s disease. Ophthalmology 125:1444–1451
Ho M, Chen LJ, Sin HPY, Lu LPL, Brelen M, Ho ACH (2019) Experience of using adalimumab in treating sight-threatening paediatric or adolescent Behcet’s disease-related uveitis. J Ophthalmic Inflamm Infect 9:14
Silvestri E, Bitossi A, Bettiol A, Emmi G, Urban ML, Mattioli I (2020) Adalimumab effectively controls both anterior and posterior noninfectious uveitis associated with systemic inflammatory diseases: focus on Behçet’s syndrome. Inflammopharmacology 28:711–718
Yang S, Huang Z, Liu X, Li H, Xie L, Chen X (2021) Comparative study of adalimumab versus conventional therapy in sight-threatening refractory Behçet’s uveitis with vasculitis. Int Immunopharmacol 93:107430
Funding
The authors declare that no funds, grands, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CE contributed to conceptualization; HKS helped in methodology; DGS and HA performed formal analysis and investigation; CE and OAP contributed to writing—original draft preparation; HŞ and FH helped in writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Consent to participate
Informed consent was obtained from all individual participants, and written informed consent was obtained from the parents of pediatric cases in this study.
Consent for publication
This submission has not been published anywhere previously and it is not simultaneously being considered for any other publication.
Ethics approval
The study was reviewed and approved by the department’s academic board and the ethics committee of Erciyes University, Kayseri, Türkiye (No: 2020/76).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evereklioglu, C., Sonmez, H.K., Sevim, D.G. et al. Adalimumab rapidly controls both anterior and posterior inflammation in patients with ocular Behçet syndrome and non-infectious uveitis refractory to conventional therapy: a prospective, 6-month follow-up investigation. Int Ophthalmol 43, 4461–4472 (2023). https://doi.org/10.1007/s10792-023-02846-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02846-4