Abstract
Purpose
To evaluate and compare the accuracy of six different formulas (Emmetropia Verifying Optical version 2.0, Kane, SRK/T, Barrett Universal II, Haigis and Olsen) in intraocular lens (IOL) power calculation for extremely long eyes.
Methods
Retrospective case-series. Seventy-three eyes with axial length (AL) ≥ 29.0 mm and underwent phacoemulsification cataract surgery with Rayner (Hove, UK) 920H IOL implantation from January 2018 to March 2020 were included. Prediction errors (PE) were calculated and compared between different formulas to evaluate the accuracy of formulas. Multiple regression analysis was performed to investigate factors associated with the PE.
Results
The Kane formula had mean prediction error close to zero (– 0.01 ± 0.51 D, P = 0.841), whereas the EVO 2.0, SRK/T, Barrett Universal II, Haigis and Olsen formulas produced hyperopic outcomes (all P < 0.001). The median absolute error [inter-quartile range] produced by the EVO 2.0, Kane, Barrett Universal II and Olsen formulas showed no significant difference (0.33 D [0.48], 0.30 D [0.44], 0.34 D [0.39], 0.29 D [0.37], respectively, pairwise comparison P > 0.05), but was significantly lower than that of the SRK/T and Haigis formulas (0.85 D [0.66], 0.80 D [0.54], respectively, pairwise comparison P < 0.001). The AL and the PE produced by the SRK/T formula were significantly positively correlated in extremely myopic eyes (β = 0.248, P < 0.001), whereas the trend was not demonstrated in other formulas.
Conclusions
For cataract patients with axial length greater than 29.0 mm, the accuracy of the EVO 2.0, Kane, Barrett Universal II and Olsen formulas is comparable and significantly better than that of the SRK/T and Haigis formulas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myopia has become a common visual disorder which shows significant increase in its prevalence globally, and one billion people (approximately 10% of the global population) have been estimated to develop high myopia by 2050 [1]. High myopia, which is generally associated with an axial length (AL) longer than 26.0 mm, would lead to vision-threatening complications, including cataract [2]. In Asia, extreme myopia affects a high proportion of highly myopic patients. Many of these patients suffer from unsatisfactory postoperative vision after cataract surgery because of posterior capsular rupture and unpredictable refractive outcomes [3]. The measurement of AL and the intraocular lens (IOL) power calculation formula used might be the main sources of postoperative prediction error for extremely long eyes [4]. Nowadays, modern biometry techniques, such as the partial coherence interferometry (PCI) biometry and swept-source optical coherence tomography (SS-OCT), have reduced AL measurement errors in long eyes with a staphyloma [5]. In order to obtain satisfactory postoperative refraction, two high-resolution optical biometric devices, Zeiss IOL Master 700 and OA 2000, based on the principle of SS-OCT is commonly used for the obtaining multiple measurements of various ocular biometric parameters and calculating IOL power [6].
The different AL behavior of formulas to calculate the IOL power is a crucial problem, since refractive errors inevitably increase as the AL changes. There are various validated IOL formulas applied for various AL, such as the Hoffer Q formula is appropriate for eyes with AL less than 22.0 mm and the SRK/T or Haigis formula might be good choice for eyes with AL of 26.0 mm or more [4, 7]. Therefore, it is important to select suitable formula to reduce the refractive error. In a recent study, the Barrett Universal II (Barrett II), Haigis and Olsen formulas were found to be more accurate than the Holladay 1, Holladay 2, Hoffer Q and SRK/T formulas in eyes with AL longer than 26.0 mm [8]. Furthermore, modern IOL formulas such as the Emmetropia Verifying Optical version 2.0 (EVO 2.0), Kane, Barrett II or Olsen formula have led to remarkable improvements in highly myopic eyes in recent years, and published studies reported excellent outcomes with these formulas [9,10,11]. However, these formulas have rarely been evaluated in extremely myopic eyes with AL of 29.0 mm or more, especially the EVO 2.0 and Kane formulas. Since the SRK/T formula is still a valid option and commonly used clinically, we compared this one with the aforementioned formulas, too [12]. Thus in this study, we investigated the accuracy of six IOL power calculation formulas (EVO 2.0, Kane, SRK/T, Barrett II, Haigis and Olsen) by reviewing eyes with extremely high myopia with AL longer than 29.0 mm.
Materials and methods
The protocol of this retrospective study was approved by the Ethics Committee for Human Medical Research at the Joint Shantou International Eye Center of Shantou University and the Chinese University of Hong Kong (No. 58, Shanfu Section [2020]). All procedures were in accordance with the tenets of the Declaration of Helsinki. In addition, since the study was done during COVID-19, all the population coming into the hospital, including doctors and patients, should wear face mask, measure body temperature, do nucleic acid testing (NAT) and hand disinfection. Patients with ALs longer than 29.0 mm and underwent phacoemulsification cataract surgery from January 2018 to March 2020 were enrolled. The inclusion criteria were: (1) eyes with postoperative corrected distance visual acuity (CDVA) of 6/20 or more within the 1- to 4-month; (2) IOL implantation using 920H IOL model (Rayner Intraocular Lenses Ltd.); and (3) cases with complete medical records. Patients with amblyopia, squint, complicated cataract surgery, traumatic cataract, previous ocular surgery, acquired retinal diseases and pathology affecting the accuracy of biometry calculations (including severe corneal or vitreous opacity, corneal degeneration, keratoconus, pterygium, secondary glaucoma and retinal detachment) were excluded. We only included one operated eye of each patient. The eye with better CDVA was selected if the patient underwent bilateral cataract surgery.
To investigate whether a correlation exists between AL and postoperative refraction, the studied eyes were divided into two subgroups: 29.0 ≤ AL < 31.0 mm (Extreme Myopia 1, EM1), AL ≥ 31.0 mm (Extreme Myopia 2, EM2). Standard phacoemulsification cataract operation procedure was performed by different experienced surgeons. A 2.8 mm clear corneal incision was created after topical anesthesia, and then phacoemulsification was performed. Next, 920H IOL model (Rayner Intraocular Lenses Ltd.) with appropriate refraction was implanted in the bag. No sutures were used for closure the operated eye. The operation usually lasted 15 min and all subjects were prescribed the same postoperative medications. Ocular biometric parameters including AL, keratometry (K), anterior chamber depth (ACD), lens thickness (LT), central corneal thickness (CCT) and white-to-white (WTW) were measured by OA 2000 (Tomey Corporation, Japan) and IOL Master 700 (Carl Zeiss Meditec, Jena, Germany) preoperatively.
For each eye, the IOL power was calculated using the Emmetropia Verifying Optical version 2.0 (EVO 2.0),A Kane,B Sanders-Retzlaff-Kraff trial (SRK/T),C Barrett Universal II (Barrett II),D Haigis optimized (Haigis),E and Olsen formulas,F respectively. The User Group for Laser Interference Biometry (ULIB, http://www.ocusoft.de/ulib/cl.html)G lens constants were used for all formulas. The refractive prediction error (PE) was calculated by subtracting the formula-predicted postoperative refraction from the actual postoperative refraction. Negative PE indicated overcorrection with a tendency of myopic outcomes, whereas positive PE indicated undercorrection with a tendency of hyperopic outcomes. The mean absolute error (MAE), median absolute error (MedAE), and the percentages of eyes with PE within ± 0.25, ± 0.50 and ± 1.00 D of the targeted refraction were calculated for each formula.
Statistical analysis
All statistical analyses were conducted using the commercially available software (IBM SPSS Statistics 21; SPSS Inc., Chicago, IL). Kolmogorov–Smirnov test was used to check the data distribution for normality. One-sample T-test was used to assess whether the ME for each formula was significantly different from zero. The nonparametric Friedman test was performed to assess the differences in the absolute errors among formulas. The post hoc analysis using the Wilcoxon signed-rank test with Bonferroni correction was conducted if a significant difference was detected between formulas. The Fisher’s exact test was conducted to compare percentage of eyes within ± 0.25, ± 0.50 and ± 1.00D of the targeted refraction. Multiple regression analysis was performed to investigate the relationship between the PE for each formula and associated factors. Mean (mean ± standard deviation) and median absolute (median with the inter-quartile range) values were presented. P < 0.05 was considered statistically significant.
Results
Patients' demographics
A total of 73 eyes from 73 patients with a mean age of 58.86 ± 10.95 years were included in the current study. There were 24 males and 49 females, as well as 40 right eyes and 33 left eyes. The mean AL was 31.17 ± 1.34 mm. Table 1 shows the demographic and biometric data of the study population.
Comparison of IOL power calculation formulas in the whole population
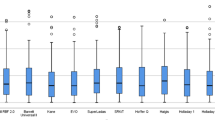
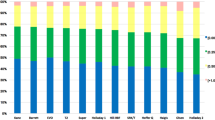
The Kane formula had mean prediction error (-0.01 D) close to zero, which was not significantly different from zero (P = 0.841). The EVO 2.0, SRK/T, Barrett II, Haigis and Olsen formulas produced hyperopic outcomes (all P < 0.001, Table 2 and Fig. 1). The median absolute error [inter-quartile range] predicted by the EVO 2.0, Kane, Barrett Universal II and Olsen formulas showed no significant difference (0.33 D [0.48], 0.30 D [0.44], 0.34 D [0.39], 0.29 D [0.37], respectively, P > 0.05), but were significantly lower than that of the SRK/T and Haigis formulas (0.85 D [0.66], 0.80 D [0.54], respectively, and all the pairwise comparisons were P < 0.001, Fig. 2). Figure 3 shows the percentages of eyes with PE within ± 0.25 D, ± 0.50 D and ± 1.00 D of the targeted refraction for all formulas. For the percentage of eyes within ± 1.00 D of the targeted refraction error, the EVO 2.0, Kane, Barrett II and Olsen formulas had the same value 94.52% (69/73), and was higher than the 60.27% (44/73) of SRK/T and 61.64% (45/73) of Haigis formulas (all P < 0.001). The same trend could be seen in the differences between formulas in the percentage of eyes within ± 0.25 D and ± 0.50 D of the targeted refraction. The Olsen formulas had the highest percentage of eyes within ± 0.50 D compared with the EVO 2.0, Kane and Barrett II formulas but without significance (78.08%, 57/73 vs 64.38%, 47/73, 67.12%, 49/73 and 71.23%, 52/73, all P > 0.05).
The distribution of prediction errors for four formulas in each group. The tops of the bars represent the mean prediction errors, and the whiskers represent the standard deviations. P values were calculated using the one-sample T-test, values < 0.05 were considered statistically significant. *: P < 0.05. **: P < 0.01. ***: P < 0.001. Note that hyperopic prediction errors are indicated by positive values, and myopic prediction errors are indicated by negative values
Box plots of absolute refractive error produced by each formula in each group. The boxes represent the upper and lower quartiles, and the dash inside the box is the median. The whiskers represent the minimum and maximum values. P values were calculated using the Wilcoxon signed-rank test with Bonferroni correction, values < 0.05 were considered statistically significant. *: P < 0.05. **: P < 0.01. ***: P < 0.001
Comparison of IOL power calculation formulas in the subgroups
To investigate whether there is a correlation between the PE and the AL, eyes were divided into two groups (EM1 and EM2) based on AL value. There were 37 eyes in EM1 and 36 eyes in EM2 group, respectively. No significant between-group differences were shown in baseline demographic and biometric data except in LT. All the evaluated formulas produced significant hyperopic surprise in EM1 group, and the mean prediction error ranged from 0.18 D (Kane) to 0.91 D (Haigis). The Olsen formula had mean prediction error (0.14 D) that showed no significant difference from zero in EM2 group (P = 0.089). It is worth noting that the Kane formula produced significant myopic outcome (– 0.21 D) when eyes with AL greater than 31.0 mm (P = 0.017). In terms of the mean absolute error, the EVO 2.0, Kane, Barrett II and Olsen formulas had the equal accuracy (all within ± 0.50 D, P > 0.05) in both groups. The post hoc analysis showed that the four mentioned formulas had a much lower MedAE than the SRK/T and Haigis formulas (all P < 0.001, Table 2 and Fig. 2). The proportion of predictive refraction within ± 1.00 D was 62.16% (23/37, Haigis) to 97.30% (36/37, Olsen), 50.00% (18/36, SRK/T) to 97.22% (35/36, Barrett II) in EM1 and EM2 group, respectively. However, there was no significant difference in proportion of eyes within ± 0.25 D, ± 0.50 D and ± 1.00 D of intended between the two subgroups.
Factors influencing prediction errors of the formulas
Age, gender and the ocular biometric parameters, such as AL, K, ACD, LT and WTW, were assessed in the multiple regression analysis to identify the factors that influenced PE. The results showed that the AL was significantly associated with the PE produced by the SRK/T formula (β = 0.248, P < 0.001, Table 3). The highest coefficient of determination was demonstrated in the regression equation for the SRK/T formula (PESRK/T = – 10.384 + 0.248*AL, R2 = 0.270, P < 0.001). In general, a longer AL was always associated with a hyperopic surprise and a shorter AL was always associated with a myopic surprise for the SRK/T formula. However, there was no significant associated factor detected in the regression equation for the EVO 2.0, Barrett II, Haigis and Olsen formulas. Interestingly, a slightly significant relationship between the PE and the AL was found in the Kane formula (β = – 0.099, P = 0.023), but the regression equation showed no significance (P = 0.059).
Discussion
Hyperopic refractive errors can result from the incorrect calculation of the IOL power required, especially in extremely high myopia [13, 14]. The current study mainly focused on the accuracy comparison of well validated IOL calculation formulas in a cohort of eyes with AL longer than 29.0 mm who underwent cataract operation implanted with the 920H IOL model. Our results showed that in the whole sample, the EVO 2.0, Kane, Barrett II and Olsen formulas exhibited a significantly higher proportion of eyes within ± 0.25 D, ± 0.50 D and ± 1.00 D of the targeted refraction and lower MedAE and MAE, compared to the earlier generation formulas: SRK/T and Haigis.
The Olsen formula had the lowest MAE and MedAE as well as the highest proportion of spherical equivalent (SE) refraction within ± 0.25 D and ± 0.50 D in both two subgroups, although there was no significant difference compared to the EVO 2.0, Kane and Barrett II formulas. Our investigation in extremely high myopia was in agreement with a previous study which showed no significant difference between these four IOL formulas across all AL subgroups [15]. Whereas Connell et al. [16] found that the Kane formula had a significantly lower MAE (0.329 D) than the Olsen formula in 846 eyes of the entire AL range. The newer formulas, EVO 2.0 and Kane, have rarely been investigated in eyes with AL long than 29.00 mm, meaning that we are unable to compare our results to other published papers critically regarding this specific topic.
The EVO 2.0 is a new thick-lens formula based on the theory of emmetropization [17]. Its original version was not as accurate as the Kane, Olsen and Barrett II formulas in the short and long AL eyes. In accordance with the previous results, our study showed that the EVO 2.0 formula ranked at the fourth accuracy, suggesting that the emmetropization concept might not suitable at the extreme situation of the AL [18]. The Kane formula is another newer method which composes regression and elements of artificial intelligence based on theoretical optics [19]. As has been reported in recent studies, the Kane formula outperformed other existing formulas regardless of the AL and ACD values [16, 18, 20]. However, this formula was inferior to the Olsen formula and ranked as the second accurate one in our study, although without statistical significance. Since the subjects of those previous studies mainly were European ancestry, the authors thought the differences in results could be due to the different ethnicity of our patients, or the relatively small sample size of our study [21, 22]. Further evaluations including more Chinese patients of extremely high myopia are required for confirmation. However, although the structure of the Kane formula has not been published, it seems that it looks promising and deserves more attention.
As for the Barrett II formula, it uses LT and WTW values as determined by paraxial ray tracing method in its prediction and the lens factor of the formula may provide precise estimation of the effective lens position [23]. Therefore, before the introduction of the Kane formula, it exhibits as the most accurate formula for eyes with long, medium and short AL [8, 24]. Again, in another study including 106 eyes with an AL longer than 26.00 mm, the Barrett II formula had the lowest prediction error within 0.28 ± 0.19 D (0.26 D) [25]. These findings are comparable to ours for extremely long eyes in which excellent performance was reported with this formula.
Interestingly, the poor performance of the SRK/T and Haigis formulas enabled us to have less than 50% of eyes within ± 0.50 D of PE refraction in our extremely high myopia cohort. The proportion is much lower than the corresponding values of prior studies which showed a high accuracy in long eyes [4, 26]. Our multivariate regression analysis with regression equation detected a significant effect of AL on the refractive errors of the SRK/T formula which relied on AL and central corneal power to provide prediction of the postoperative IOL position. But the assumption that long eyes would have deeper ACD adopted in the SRK/T formula might explain why it was less accurate in this study, since importance of preoperative ACD measurement was always highlighted. Bang et al. [4] found that the Haigis formula was the best at predicting postoperative refraction in all eyes longer than 27.00 mm, even in the subgroups of eyes with AL longer than 29.07 mm. Contrary to the SRK/T formula, the Haigis uses actual measurement of the preoperative ACD in its calculation, and the predictive error of this formula was not significantly affected by eye AL [27]. It was surprising that the Haigis formula ranked near the bottom in our study. Perhaps because we included more modern formulas and excluded more confounding factors, such as only one IOL model was used and eyes with previous retinal detachment surgery were abandoned, compared to theirs. Formulas whose accuracy are not affected by AL should be preferred, especially in extreme situations.
Our study has strengths and limitations. One of the strengths is that the current study mainly focused on the accuracy of well validated IOL calculation formulas in extremely myopic eyes with AL of 29.0 mm or more, which has rarely been evaluated previously, especially the accuracy of EVO 2.0 and Kane formulas. Another strength is that all the examination and cataract procedures were performed at one research center using identical protocols, thus increasing the homogeneity across subjects and the reasonability of the outcomes. The main limitation of the current study was the relatively small sample size. A minimum of 71 eyes might be required to conduct a multiple comparison among the six evaluated formulas for a test power of 0.8 and a significance level of 0.05 based on PASS sample size calculation software (version 15.0.5; NCSS, LLC). Although the sample size included in our study meet the threshold, whether the same results can be found requires more extremely long eyes to further investigate. Second, multiple surgeons that were included in the study might result in bias from their operational style. However, the aforementioned limitation could be clinically negligible because of the variations of surgeons might not significantly affect the postoperative refraction after cataract surgery in a single eye center [28]. Because all the surgeons have been trained through the center’s unified cataract surgery training program. And this might make the study more approach to real-life practice. Third, two different measurements, IOL Master 700 and OA 2000, were used in the study, although this is unlikely to affect the refractive outcome, since the IOL Master 700 and OA 2000 have been reported to have good agreement in biometric measurements [29].
Overall, for cataract patients with axial length greater than 29.0 mm, the EVO 2.0, Kane, Barrett Universal II and Olsen formulas provide equally excellent IOL power prediction and are significantly better than that of the SRK/T and Haigis formulas. Any of these four formulas is a reasonable choice in extremely myopic eyes. Furthermore, the older-generation formulas, such as the SRK/T and Haigis formulas, are not recommended in this kind of patients.
Data availability
Data are available upon request.
References
Holden BA, Fricke TR, Wilson DA et al (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123(5):1036–1042. https://doi.org/10.1016/j.ophtha.2016.01.006
Saw SM, Gazzard G, Shih-Yen EC et al (2005) Myopia and associated pathological complications. Ophthalmic Physiol Opt 25(5):381–391. https://doi.org/10.1111/j.1475-1313.2005.00298.x
Zuberbuhler B, Seyedian M, Tuft S (2009) Phacoemulsification in eyes with extreme axial myopia. J Cataract Refract Surg 35(2):335–340. https://doi.org/10.1016/j.jcrs.2008.10.044
Bang S, Edell E, Yu Q et al (2011) Accuracy of intraocular lens calculations using the IOLMaster in eyes with long axial length and a comparison of various formulas. Ophthalmology 118(3):503–506. https://doi.org/10.1016/j.ophtha.2010.07.008
Savini G, Hoffer KJ, Shammas HJ et al (2017) Accuracy of a new swept-source optical coherence tomography biometer for IOL power calculation and comparison to IOL master. J Refract Surg 33(10):690–695. https://doi.org/10.3928/1081597X-20170721-05
Cho YJ, Lim TH, Choi KY et al (2018) Comparison of ocular biometry using new swept-source optical coherence tomography-based optical biometer with other devices. Korean J Ophthalmol 32(4):257–264. https://doi.org/10.3341/kjo.2017.0091
Doshi D, Limdi P, Parekh N et al (2017) A comparative study to assess the predictability of different IOL power calculation formulas in eyes of short and long axial length. J Clin Diagn Res 11(1):C1–C4. https://doi.org/10.7860/JCDR/2017/22095.9136
Melles RB, Holladay JT, Chang WJ (2018) Accuracy of intraocular lens calculation formulas. Ophthalmology 125(2):169–178. https://doi.org/10.1016/j.ophtha.2017.08.027
Darcy K, Gunn D, Tavassoli S et al (2020) Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10 930 eyes from the UK national health service. J Cataract Refract Surg 46(1):2–7. https://doi.org/10.1016/j.jcrs.2019.08.014
Liu J, Wang L, Chai F et al (2019) Comparison of intraocular lens power calculation formulas in Chinese eyes with axial myopia. J Cataract Refract Surg 45(6):725–731. https://doi.org/10.1016/j.jcrs.2019.01.018
Cooke DL, Cooke TL (2016) Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg 42(8):1157–1164. https://doi.org/10.1016/j.jcrs.2016.06.029
Kapamajian MA, Miller KM (2008) Efficacy and safety of cataract extraction with negative power intraocular lens implantation. Open Ophthalmol J 2:15–19. https://doi.org/10.2174/1874364100802010015
Kane JX, Melles RB (2020) Intraocular lens formula comparison in axial hyperopia with a high-power intraocular lens of 30 or more diopters. J Cataract Refract Surg 46(9):1236–1239. https://doi.org/10.1097/j.jcrs.0000000000000235
Wan KH, Lam T, Yu M et al (2019) Accuracy and precision of intraocular lens calculations using the new hill-RBF version 2.0 in eyes with high axial myopia. Am J Ophthalmol 205:66–73. https://doi.org/10.1016/j.ajo.2019.04.019
Cheng H, Kane JX, Liu L et al (2020) Refractive predictability using the IOLMaster 700 and artificial intelligence-based IOL power formulas compared to standard formulas. J Refract Surg 36(7):466–472. https://doi.org/10.3928/1081597X-20200514-02
Connell BJ, Kane JX (2019) Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol 4(1):e251. https://doi.org/10.1136/bmjophth-2018-000251
Zhao J, Liu LP, Cheng HH et al (2020) Accuracy of eight intraocular lens power calculation formulas for segmented multifocal intraocular lens. Int J Ophthalmol 13(9):1378–1384. https://doi.org/10.18240/ijo.2020.09.07
Melles RB, Kane JX, Olsen T et al (2019) Update on intraocular lens calculation formulas. Ophthalmology 126(9):1334–1335. https://doi.org/10.1016/j.ophtha.2019.04.011
Szalai E, Toth N, Kolkedi Z et al (2020) Comparison of various intraocular lens formulas using a new high-resolution swept-source optical coherence tomographer. J Cataract Refract Surg 46(8):1138–1141. https://doi.org/10.1097/j.jcrs.0000000000000329
Hipolito-Fernandes D, Luis ME, Serras-Pereira R et al (2020) Anterior chamber depth, lens thickness and intraocular lens calculation formula accuracy: nine formulas comparison. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2020-317822
Reitblat O, Gali HE, Chou L et al (2020) Intraocular lens power calculation in the elderly population using the Kane formula in comparison with existing methods. J Cataract Refract Surg 46(11):1501–1507. https://doi.org/10.1097/j.jcrs.0000000000000308
Rong X, He W, Zhu Q et al (2019) Intraocular lens power calculation in eyes with extreme myopia: comparison of Barrett universal II, Haigis, and Olsen formulas. J Cataract Refract Surg 45(6):732–737. https://doi.org/10.1016/j.jcrs.2018.12.025
Iijima K, Kamiya K, Iida Y et al (2020) Comparison of Predictability Using Barrett Universal II and SRK/T Formulas according to Keratometry. J Ophthalmol 2020:7625725. https://doi.org/10.1155/2020/7625725
Kane JX, Van Heerden A, Atik A et al (2017) Accuracy of 3 new methods for intraocular lens power selection. J Cataract Refract Surg 43(3):333–339. https://doi.org/10.1016/j.jcrs.2016.12.021
Abulafia A, Barrett GD, Rotenberg M et al (2015) Intraocular lens power calculation for eyes with an axial length greater than 26.0 mm: comparison of formulas and methods. J Cataract Refract Surg. 41(3):548–556. https://doi.org/10.1016/j.jcrs.2014.06.033
Zhang JQ, Zou XY, Zheng DY et al (2019) Effect of lens constants optimization on the accuracy of intraocular lens power calculation formulas for highly myopic eyes. Int J Ophthalmol 12(6):943–948. https://doi.org/10.18240/ijo.2019.06.10
Olsen T (2006) Prediction of the effective postoperative (intraocular lens) anterior chamber depth. J Cataract Refract Surg 32(3):419–424. https://doi.org/10.1016/j.jcrs.2005.12.139
Arstodemou P, Knox CN, Sparrow JM et al (2011) Intraocular lens formula constant optimization and partial coherence interferometry biometry: refractive outcomes in 8108 eyes after cataract surgery. J Cataract Refract Surg 37(1):50–62. https://doi.org/10.1016/j.jcrs.2010.07.037
Liao X, Peng Y, Liu B et al (2020) Agreement of ocular biometric measurements in young healthy eyes between IOLMaster 700 and OA-2000. Sci Rep 10(1):3134. https://doi.org/10.1038/s41598-020-59919-y
Acknowledgements
The authors would like to express their deepest gratitude to all the participants in this study. This work was supported by the Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program (No. 58, Shanfu Section [2020]), China
Funding
This work was supported by the Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program (No. 58, Shanfu Section [2020]), China.
Author information
Authors and Affiliations
Contributions
Guo CY was responsible for writing the manuscript, original draft preparation, conducting the search, investigation and visualization. Yin SJ was responsible for conceptualization, methodology, funding acquisition, and reviewing the manuscript. Qiu KL was responsible for conceptualization, methodology, and reviewing the manuscript. Zhang MZ was responsible for the supervision, project administration, and reviewing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee for Human Medical Research at the Joint Shantou International Eye Center of Shantou University and the Chinese University of Hong Kong (No. 58, Shanfu Section [2020]).
Consent to participate
Individual consent for this retrospective analysis was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Other cited material
-
A.
Tun Kuan Yeo. Emmetropia Verifying Optical Formula Version 2.0. Available at: http://www.evoiolcalculator.com.
-
B.
Jack X Kane. Kane Formula. http://www.iolformula.com.
-
C.
Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg 1990;16:333–40.
-
D.
Barrett GD. Barrett Universal II Formula. Singapore, Asia–Pacific Association of Cataract and Refractive Surgeons. Available at: http://www.apacrs.org/barrett_universal2/.
-
E.
Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol 2000;238:765–73.
-
F.
Olsen T, inventor; IOL Innovations ApS, assignee. System and method for determining and predicting IOL power insitu. US patent 8 657 445, February 25, 2014. Available at: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=8,657,445.PN.&OS=PN/8,657,445&RS=PN/8,657,445.
-
G.
User Group for Laser Interference Biometry. Optimized IOL constants for the Zeiss IOLMaster calculated from patient data on file. Available at: http://www.ocusoft.de/ulib/cl.html.
Rights and permissions
About this article
Cite this article
Guo, C., Yin, S., Qiu, K. et al. Comparison of accuracy of intraocular lens power calculation for eyes with an axial length greater than 29.0 mm. Int Ophthalmol 42, 2029–2038 (2022). https://doi.org/10.1007/s10792-021-02194-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-02194-1