Abstract
Purpose
We aimed to reveal whether static and dynamic pupillary responses can be used for the detection of autonomic nervous system (ANS) dysfunction in patients with obstructive sleep apnea syndrome (OSAS).
Methods
We included in this study patients with OSAS, who were divided into three groups according to the apnea–hypopnea index (AHI) (group 1, mild [n = 20]; group 2, moderate [n = 20]; and group 3, severe [n = 20]), and healthy controls (group 4, n = 20). Pupillary responses were measured using a pupillometry system.
Results
Static (mesopic PD, P = 0.0019; low photopic PD, P = 0.001) and dynamic pupil responses (resting diameter, P = 0.004; amplitude of pupil contraction, P < 0.001; duration of pupil contraction, P = 0.022; velocity of pupil contraction, P = 0.001; and velocity of pupil dilation, P = 0.012) were affected in patients with different OSAS severities. Also, AHI was negatively correlated with mesopic PD (P = 0.008), low photopic PD (P = 0.003), resting diameter (P = 0.001), amplitude of pupil contraction (P < 0.001), duration of pupil contraction (P = 0.011), velocity of pupil contraction (P < 0.001), and velocity of pupil dilation (P = 0.001).
Conclusion
We detected pupil responses innervated by the ANS were affected in the OSAS patients. This effect was more significant in the severe OSAS patients. Therefore, the pupillometry system can be an easily applicable, noninvasive method to detect ANS dysfunction in the OSA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) can be defined as a syndrome that is characterized by recurrent full or partial obstructed attacks of the upper airway during sleep, causing nighttime snoring and daytime sleepiness [1, 2]. It has been reported that OSAS, which is characterized by decreased blood oxygen saturation, is associated with cardiovascular diseases and diabetes mellitus and can cause serious morbidity and mortality [3,4,5,6]. The diagnosis of OSAS is made with the apnea–hypopnea index (AHI) > 5/h in the polysomnography record, which is a test based on the simultaneous and continuous recording of neurophysiological, cardiac, respiratory, physiological, and other physical parameters overnight [7]. The apnea–hypopnea index (AHI) measures the number of times that an abnormally low breathing rate or complete cessation of breathing occurs every hour [8]. According to AHI, patients can be classified as simple snoring (AHI < 5/h), mild OSAS (5/h ≤ AHI ≤ 15/h), moderate OSAS (15 < AHI < 30/h), and severe OSAS (AHI ≥ 30/h) [9]. The sympathetic nervous system (SNS) activity increases with the stimulation of peripheral and central chemoreceptors caused by chronic hypoxia, hypercarbia, and respiratory acidosis in patients with OSAS [10, 11]. Similarly, because of the autonomic nervous system (ANS), dysfunction developing in these patients, the sympathetic tone increased during daytime [12].
The pupillary light reflex innervated by the ANS is defined as the pupil's response to light. While the circular muscles of the iris cause the pupil to constriction, the radial muscles (dilator pupillae) cause dilatation. The circular muscles of the iris, whose functions are regulated by the parasympathetic nervous system (PNS), cause the pupil to constriction, while the radial muscles (dilator pupillae), whose functions are regulated by the sympathetic nervous system (SNS), cause dilatation [13]. In PNS dysfunction, pupil constriction is delayed in light exposure, while in SNS dysfunction, the dilation of the pupil in the dark is delayed. In addition, the pupil response may vary depending on many diseases affecting the neuronal pathways of the visual system [14,15,16,17]. Measurement of pupillary responses can be done noninvasively and objectively in different light conditions with automatic pupillometry, which enables obtaining pupillary response with infrared pupillography [18, 19].
ANS dysfunction is considered to play an important role in OSAS-related morbidity. ANS evaluation can be done using invasive and labor-intensive methods such as heart rate variability (HRV), blood pressure (BP), and BP variability (BPV) [20, 21]. Therefore, in this study, it was aimed to reveal whether pupillary responses can be used in the detection of autonomic dysfunction in the OSAS patients.
Methods
Study design
Patients who were diagnosed with OSAS (group 1, mild [n = 20]; group 2, moderate [n = 20]; and group 3, severe [n = 20]), after polysomnography (PSG) taken in the sleep disorder department of the chest diseases clinic of Dicle University Hospital and referred to the Department of Ophthalmology for eye examination and healthy controls (group 4, n = 20), were included in this study. We obtained approval from the Dicle University School of Medicine ethics committee for the study. Our study was conducted according to the Declaration of Helsinki, and written informed consent was obtained from all patients before the measurement.
Subjects and measurements
We performed a complete ophthalmologic examination for all subjects included in this study. Patients who did not have any systemic disease, who did not have iris pupil pathology, who did not have pseudo exfoliation, who did not have glaucoma, who did not have previous intraocular surgery or inflammatory disease, who did not use eye drops with the potential to affect pupillary responses, and who did not use systemic anticholinergic drugs were included in the study.
A single experienced clinician made all pupillometry measurements (MonPack One; Metrovision, Pérenchies, France). Pupillary responses (both static [scotopic, mesopic, low photopic, and high photopic] and dynamic [resting PD, contraction amplitude, latency, duration, velocity of contraction, dilation latency, duration, and velocity at rest]) were detected using a pupillometry system in each subject included in the study. The average of three consecutive measurements was taken. Measurements were made at a similar time to reduce the effect of circadian changes.
According to the international 10–20 electrode system, with 44-channel digital video polysomnography; electroencephalography (EEG), electrooculography (EOG), chin and both pretibial electromyography (EMG), electrocardiography (ECG), oximeter, pulse transit time (PTT), thoraco-abdominal breathing effort, oro-nasal airflow, snoring sound, pulse and body position were recorded. A sleep technician was present during the entire recording.
Statistical analysis
SPSS 26.0 version (Chicago, IL, USA) was used for all statistical analyzes. Descriptive statistics were used for demographic analysis of the groups. One-way ANOVA analysis was used for comparison between different groups. Tukey's test was used in post hoc analysis to determine whether there was a significant difference in paired comparisons of the groups. We used Pearson correlation analysis to analyze the correlation between AHI and static and dynamic pupillary responses in patients with OSAS.
Results
The mean ages of the study participants were 47.25 ± 6.86, 51.25 ± 11.11, 48.10 ± 8.40, and 48.35 ± 11.05 years in groups 1, 2, 3, and 4, respectively. Mean disease durations of OSAS in groups 1, 2, and 3 were 3.60 ± 1.90, 7.35 ± 1.81, and 8.55 ± 1.79 years, respectively. There were 42 (52.5%) female subjects and 38 (47.5%) male subjects in this study. (Table 1).
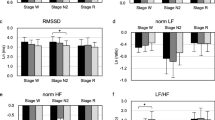
According to the one-way variance analysis, we found that mesopic PD and low photopic PD were significantly different in the groups (F[3,76] = 3.500; P = 0.0019 and (F[3,76] = 6.531; P = 0.001, respectively). When we made the paired comparison of static pupillometry measurements, mesopic PD was significantly detected lower in the group 3 than the group 1 and 4 (P = 0.041, and P = 0.025, respectively). In addition, low photopic PD in the groups 1, 2, and 3 was significantly lower than that in the group 4 (P = 0.037, P = 0.004, and P = 0.001) (Table 2).
According to the one-way ANOVA analysis, among the groups included in the study, we determined the resting diameter (F[3,76] = 4.794; P = 0.004), the amplitude of pupil contraction (F[3,76] = 6.891; P < 0.001), the duration of pupil contraction (F[3,76] = 3.414; P = 0.022), the velocity of pupil contraction (F[3,76] = 6.512; P = 0.001), and velocity of pupil dilation (F[3,76] = 4.86; P = 0.012) to be significantly different. When we made the paired comparison of the static pupillometry measurements, the resting diameter was significantly detected lower in the group 3 than that in the groups 2 and 4 (P = 0.052 and P = 0.002). Similarly, the amplitude of pupil contraction was significantly lower in the group 3 than that in the groups 1, 2, and 3 (P = 0.027, P = 0.001, and P = 0.001, respectively). In addition, the duration of pupil contraction was significantly detected lower in the group 3 than that in the group 4 (P = 0.011). Although the velocity of pupil contraction was significantly lower in the group 3 than that in the groups 1 and 4 (P = 0.001 and P = 0.005, respectively), this value was significantly lower in the group 2 than that in the group 1 (P = 0.076). In addition, the velocity of pupil dilation was significantly lower in group 3 than that in groups 2 and 4 (P = 0.048 and P = 0.012) (Table 3).
We detected a negative correlation between AHI and mesopic PD (r = − 0.293, P = 0.008), low photopic PD (r = − 0.323, P = 0.003) (Fig. 1), resting diameter (r = − 0.352, P = 0.001), amplitude of pupil contraction (r = − 0.384, P < 0.001), duration of pupil contraction (r = − 0.283, P = 0.011), velocity of pupil contraction (r = − 0.408, P < 0.001), and velocity of pupil dilation (r = − 0.353, P = 0.001) (Fig. 2).
Discussion
We detected that both the static pupillary responses (mesopic and low photopic) and dynamic pupillary responses (the resting diameter, the amplitude of pupil contraction, the duration of pupil contraction, the velocity of pupil contraction, and the velocity of pupil dilation) were affected in patients with OSAS. In these patients, AHI was also negatively correlated with the mesopic PD, the low photopic PD, the resting diameter, the amplitude of pupil contraction, the duration of pupil contraction, the velocity of pupil contraction, and the velocity of pupil dilation.
Information about neural pathways that control pupil responses can be obtained by evaluating pupillary reactions. PNS works basically in pupil contraction, whereas the effect of SNS is minimal. Therefore, the SNS controls PD at rest, whereas PD in light and pupillary function parameters developing in response to light reflect PNS function. However, both PNS and SNS are involved in redilation. In PNS dysfunction, pupil constriction is delayed in light exposure, while, in SNS dysfunction, it can be seen that pupil dilation is delayed in the dark environment. It has been determined that disturbances in the pupil function will be detected before the findings related to ANS dysfunction in the cardiovascular system appear [22,23,24,25,26,27].
It is believed that many systemic diseases affecting patients with OSAS are caused by SNS hyperactivity [28]. In contrast, it has been reported that PNS dysfunction may play a role in ANS dysregulation in patients with OSAS [29]. Therefore, it may be important to evaluate pupil responses for screening ANS, which may be an important pathological reason for morbidity and mortality in patients with OSAS where autonomic neuropathy can be observed [30,31,32].
Changes in the SNS–PNS balance in OSAS have been found using HRV analysis. In addition, increased sympathetic vascular reactivity during wakefulness has been reported in these patients owing to ANS dysfunction [33].
Similar to the results of this study, a study comparing mild OSAS with a control group reported that pupil responses were affected as an indicator of ANS dysfunction [34]. In a study, it was reported that pupil responses were affected as an indicator of ANS dysfunction, similar to the results of current study. But, only 10 patients with mild OSAS were included in this study, and the generalization of this study is limited. However, in our study, patients were diagnosed with OSAS disease in the presence of polysomnography, and patients were grouped according to their OSAS severity. In addition, in our study, the pupillometric results of patients with OSAS of different severity were evaluated. Therefore, our data are stronger and uniquely in the literature.
Unlike the results of this study, it has been reported that there is no difference between primary snorers and children with OSAS in terms of pupillometric measurements. The reason for the different results obtained in this study can be considered as the prominence of ANS dysfunction in children with OSAS over time. However, in this study, levels of plasma norepinephrine were reported to be significantly higher in the severe OSAS patients [35]. Similarly, increased systolic and diastolic BP, ANS dysfunction, and increased catecholamine levels in urine have been reported in patients with OSAS [36].
While the limitations of this study are the relatively small number of participants and the fact that it is a cross-sectional study conducted in a single center, the potentially valuable aspect of this study is that it is the first study to report that impaired pupillary responses, which may be an indicator of ANS dysfunction in patients with OSAS, can be detected with an easily applicable automated pupilometer.
In conclusion, according to the results of this study, it was determined that static and dynamic pupillary responses controlled by the autonomic nervous system were affected in the OSAS patients. This effect was more significant in the severe OSAS patients. Also, a negative correlation was found between AHI and pupillary responses. Therefore, the pupillometry system can be an easily applicable, non-invasive method to detect ANS dysfunction in patients with OSAS. However, studies with larger series and multicenter are needed.
References
Basoglu OK, Vardar R, Tasbakan MS et al (2015) Obstructive sleep apnea syndrome and gastroesophageal reflux disease: the importance of obesity and gender. Sleep Breath 19(2):585–592
Dempsey JA, Veasey SC, Morgan BJ et al (2010) Pathophysiology of sleep apnea. Physiol Rev 90(1):47–112
Bradley TD, Floras JS (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373(9657):82–93
Strausz S, Havulinna AS, Tuomi T et al (2018) Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: A longitudinal population-based study in Finland. BMJ Open 8(10):e022752
Drager LF, McEvoy RD, Barbe F et al (2017) Sleep apnea and cardiovascular disease: Lessons from recent trials and need for team science. Circulation 136(19):1840–1850
Lattimore JDL, Celermajer DS, Wilcox I (2003) Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol 41(9):1429–1437
Mitchell RB (2007) Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope 117(10):1844–1854
Young T, Shahar E, Nieto FJ et al (2002) Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch Intern Med 162(8):893–900
Flemons WW, Buysse D, Redline S et al (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22(5):667–689
Carlson JT, Hedner J, Elam M et al (1993) Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103(6):1763–1768
Somers VK, Dyken ME, Clary MP et al (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96(4):1897–1904
Narkiewicz K, Somers VK (2003) Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 177(3):385–390
Fotiou F, Fountoulakis KN, Goulas A et al (2000) Automated standardized pupillometry with optical method for purposes of clinical practice and research. Clin Physiol 20(5):336–347
Meeker M, Du R, Bacchetti P et al (2005) Pupil examination: validity and clinical utility of an automated pupillometer. J Neurosci Nurs 37(1):34–40
Truong JQ, Ciuffreda KJ (2016) Quantifying pupillary asymmetry through objective binocular pupillometry in the normal and mild traumatic brain injury (mTBI) populations. Brain Inj 30(11):1372–1377
Adhikari P, Zele AJ, Thomas R et al (2016) Quadrant field pupillometry detects melanopsin dysfunction in glaucoma suspects and early glaucoma. Sci Rep 6(1):33373
Kardon R, Anderson SC, Damarjian TG et al (2011) Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology 118(2):376–381
Zafar SF, Suarez JI (2014) Automated pupillometer for monitoring the critically ill patient: a critical appraisal. J Crit Care 29(4):599–603
Hsieh YT, Hu FR (2007) The correlation of pupil size measured by colvard pupillometer and orbscan II. J Refract Surg 23(8):789–795
Montesano M, Miano S, Paolino MC et al (2010) Autonomic cardiovascular tests in children with obstructive sleep apnea syndrome. Sleep 33(10):1349–1355
Freeman R, Chapleau MW (2013) Testing the autonomic nervous system. Handb Clin Neurol 115:115–136
Nakayama M, Nakamura J, Hamada Y et al (2001) Aldose reductase inhibition ameliorates pupillary light reflex and F-wave latency in patients with mild diabetic neuropathy. Diabetes Care 24(6):1093–1098
McNally PG, Lawrence IG, Panerai RB et al (1999) Sudden death in type 1 diabetes. Diabetes Obes Metab 1(3):151–158
Reichard P, Pihl M (1994) Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study. Diabetes 43(2):313–317
Clarke CF, Piesowicz AT, Spathis GS (1989) Pupillary size in children and adolescents with type 1 diabetes. Diabet Med 6(9):780–783
Vinik AI, Erbas T (2006) Cardiovascular autonomic neuropathy: diagnosis and management. Curr Diab Rep 6(6):424–430
Donaghue KC, Pena MM, Fung ATW et al (1995) The prospective assessment of autonomic nerve function by pupillometry in adolescents with type 1 diabetes mellitus. Diabet Med 12(10):868–873
Yun AJ, Lee PY, Bazar KA (2004) Autonomic dysregulation as a basis of cardiovascular, endocrine, and inflammatory disturbances associated with obstructive sleep apnea and other conditions of chronic hypoxia, hypercapnia, and acidosis. Med Hypotheses 62(6):852–856
Guilleminault C, Poyares D, Rosa A et al (2005) Heart rate variability, sympathetic and vagal balance and EEG arousals in upper airway resistance and mild obstructive sleep apnea syndromes. Sleep Med 6(5):451–457
Pittasch D, Lobmann R, Behrens-Baumann W et al (2002) Pupil signs of sympathetic autonomic neuropathy in patients with type 1 diabetes. Diabetes Care 25(9):1545–1550
Katz BSI (1992) Pupillary changes of diabetic eye. Ophthalmol Clin North Am 5:379–388
Smith SA, Smith SE (1983) Evidence for a neuropathic aetiology in the small pupil of diabetes mellitus. Br J Ophthalmol 67(2):89–93
O’Brien LM, Gozal D (2005) Autonomic dysfunction in children with sleep-disordered breathing. Sleep 28(6):747–752
Monaco A, Cattaneo R, Mesin L et al (2014) Evaluation of autonomic nervous system in sleep apnea patients using pupillometry under occlusal stress: a pilot study. Cranio J Craniomandib Pract 32(2):139–147
Philby MF, Aydinoz S, Gozal D et al (2015) Pupillometric findings in children with obstructive sleep apnea. Sleep Med 16(10):1187–1191
Chaidas K, Tsaoussoglou M, Theodorou E et al (2014) Poincaré plot width, morning urine norepinephrine levels, and autonomic imbalance in children with obstructive sleep Apnea. Pediatr Neurol 51(2):246–251
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent–licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Written informed consent was obtained from the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erdem, S., Yilmaz, S., Karahan, M. et al. Can dynamic and static pupillary responses be used as an indicator of autonomic dysfunction in patients with obstructive sleep apnea syndrome?. Int Ophthalmol 41, 2555–2563 (2021). https://doi.org/10.1007/s10792-021-01814-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01814-0