Abstract
Purpose
To evaluate the clinical manifestations of intraocular inflammation associated with Bartonella infection and describe the assessment and management of patients with cat-scratch disease (CSD).
Methods
This is a retrospective review of the clinical records of patients diagnosed with Bartonella henselae and Bartonella quintana intraocular inflammation from 2011 to 2018 in the Department of Ocular Inflammations and Infections of the University Eye Clinic of Ioannina (Greece). An analysis of the current literature concerning Bartonella-related intraocular infections was also carried out.
Results
This is a retrospective study of 13 patients (7 males and 6 females) with a mean age of 39.2 years that were diagnosed with unilateral intraocular inflammation, except one case with bilateral affection, attributed to Bartonella (either henselae or quintana). Twelve (12) patients (92.3%) had a positive history of traumatic cat contact. The main ocular clinical findings with regard to the type of uveitis included neuroretinitis in 5 eyes (38.5%), vasculitis in 3 eyes (23.1%), iridocyclitis in 2 eyes (15.4%), intermediate uveitis in 2 eyes (15.4%), posterior uveitis in 1 eye (7.7%), panuveitis in 2 eyes (15.4%), retinochoroiditis in 2 eyes (15.4%), vitritis in 1 eye (7.7%), peripheral choroidal granuloma in 1 eye (7.7%). Immunoglobulin (Ig) G was positive in all cases. All patients were treated with antibiotics (mainly rifampicin, doxycycline and azithromycin). The visual acuity was noted to be improved in all patients after treatment, but some of them experienced disturbing complications.
Conclusion
CSD may manifest with various ocular pathological findings. Taking into consideration the increasing frequency of infections by B. henselae and B. quintana, clinicians should always incorporate CSD in the differential diagnosis of such presentations of uveitis. Educating vulnerable groups (children, immunosuppressed, etc.) and also general population, the appropriate preventing measures can contribute in limiting the risk of infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The eye is often involved in disseminated cat-scratch disease (CSD) caused by Bartonella spp. [1]. CSD is defined as a benign, self-limiting systemic condition that typically presents with fever and lymphadenopathy. In most cases, the microorganism is transmitted to human after a cat scratch or bite as cats consist its natural reservoir. Approximately 95% of patients mention a history of cat contact, and about 73% of patients have had a cat scratch [2]. The cat flea (Ctenocephalides felis) has been recently recognized as an arthropod vector of the disease [3, 4]. Various Bartonella species have been associated with human diseases, but it appears that Bartonella henselae is most commonly implicated in intraocular inflammations. In this article, which comes in continuity with our previous work on Bartonella species [5], we similarly focus on intraocular inflammations caused by Bartonella henselae and Bartonella quintana.
The aim of this retrospective study is to evaluate the wide spectrum of clinical features associated with ocular involvement of CSD and analyze the management and follow-up of this clinical entity and its potential complications.
Clinical presentation
Lymphoid CSD has been described as the most common clinical manifestation. The infection is a result of a cat scratch or bite and is usually followed by the development of non-tender erythematous pustules or papules at the site of primary cutaneous inoculation. Within the next one to two weeks, patients develop flu-like systemic symptoms (e.g., fever and fatigue), as well as regional lymphadenopathy (LAP). This particular stage of CSD is self-limited and usually resolves within a few weeks. LAP is typically unilateral affecting a single lymph node (50%), multiple lymph nodes (20%) or even multiple lymph node regions (30%). In some cases, LAP can be painful and suppurative. Symptoms of headache, nausea, vomiting, anorexia and sore throat have also been described. Additionally, some individuals may present non-specific maculopapular rash or erythema nodosum [6, 7].
In some rare cases, approximately 5–14% [8], CSD can manifest as a disseminated disease. The eye is considered as the most commonly involved organ in the disseminated course of CSD. Ocular bartonellosis presents in approximately 5–10% of patients with CSD [9]. The clinical features of eye involvement include Parinaud oculoglandular syndrome, neuroretinitis, intermediate uveitis, anterior uveitis, choroiditis, retinal infiltrate, branch retinal vessel occlusion, choroidal mass, serous retinal detachment and acute endophthalmitis [10, 11]. Apart from the ocular involvement, hepatosplenic disease (i.e., granulomatous hepatitis, splenomegaly or splenic abscess), pneumonia, endocarditis, encephalitis, osteomyelitis and paronychia have also been described [6, 7, 12]. The ocular manifestations have been thoroughly described in our previous study [5] and therefore are summarized in Table 1.
Materials and methods
The medical records of 13 patients that were diagnosed, treated and followed up from 2011 to 2018 at the Department of Ocular Inflammations and Infections of the University Eye Clinic of Ioannina (Greece) for CSD with ocular involvement were analyzed retrospectively. The study was conducted in accordance with the Declaration of Helsinki (2008). The information recorded includes demographic data, past medical and ocular history, history of cat contact especially scratch/bite, visual acuity, intraocular pressure (IOP), anterior chamber inflammatory activity, anterior and posterior segment findings, laboratory findings, the treatment methods and outcome. The evaluation of anterior chamber and vitreous cells was based on the Standardization of Uveitis Nomenclature criteria [13].
The imaging techniques included fundus photography, fundus fluorescein and optionally indocyanine green angiography, optical coherence tomography (OCT) and echography of the eye. Visual fields examination was performed when needed. Magnetic resonance imaging of the brain and orbit was also performed in patients with intermediate uveitis and optic nerve involvement. Laboratory examination of sera for Bartonella henselae and quintana IgG and IgM was carried out at the Pasteur Institute of Athens. According to the Pasteur Institute instructions, IgG titers > 1:64 were considered positive and titers = 1:64 concerned suspected disease and were interpreted according to positive history of cat’s scratching or even licking, presence of human body lice, preceding illness compatible with CSD and intraocular inflammation not attributed to other causes according to the results of clinical examination of systems and laboratory investigation.
In addition, a follow-up of at least 6 months was considered along with the short- and long-term complications description and management.
Results
Thirteen patients (7 males and 6 females) with unilateral intraocular inflammation (except one with bilateral affection) attributed to Bartonella (either henselae or quintana) were included in this retrospective study. The mean age at presentation was 39.2 (10–77) years. In 12 patients (92.3%), there was a positive history of traumatic cat contact (scratching) and the causative organism was B. henselae. Only one case was positive for B. quintana; this patient was a worker in the fur industry, indicating a possible tick or flea bite. Table 2 shows the profile of patients with intraocular inflammation considered to be of Bartonella origin. The ocular clinical findings included neuroretinitis (Figs. 1 and 2) in 5 eyes (38.5%), vasculitis (Figs. 3 and 4) in 3 eyes (23.1%), iridocyclitis in 2 eyes (15.4%), intermediate uveitis (Fig. 4) in 2 eyes (15.4%), posterior uveitis in 1 eye (7.7%), panuveitis in 1 eye (7.7%), retinochoroiditis in 2 eyes (15.4%), vitritis in 1 eye (7.7%) and peripheral choroidal granuloma (Fig. 4) in 1 eye (7.7%). Therefore, neuroretinitis was the most common clinical feature and in two of these cases was accompanied by either posterior uveitis (Fig. 1) or panuveitis (Fig. 2). On the other hand, retinal vasculitis (occlusive or non-occlusive) was not an uncommon manifestation of intraocular inflammation induced by Bartonella.
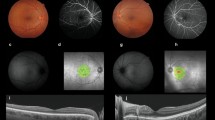
a–d Patient (no 4), woman, 77 years old with posterior uveitis (apparent vitritis and optic disk edema in B-mode echography) with vitreous haze 2 + (photograph of the fundus) and neuroretinitis along with cystoid macular edema (fundus autofluorescence and OCT). (IgG titers for Bartonella henselae, 1: 1024)
a–e Panuveitis (including findings of neuroretinitis) in a 10-year-old girl (case no 3) with Bartonella henselae infection (IgG titers, 1: 1024) along with a juxtapapillary chorioretinal granuloma (a, b), complicated by a full thickness macular hole (c) closed after prolonged therapy (d, e) [note the presence of epiretinal membrane and shrinking at the nasal macular area (c) and the absence of this feature after treatment (e)]. The optic disk pallor is a late consequence of neuroretinitis
a, b Focal retinochoroiditis and vasculitis with an ipsilateral retinal ischemia in a young man (no 5), 18 years old (titers IgG for Bartonella henselae, 1: 256). In fluorescein angiography, an apparent branch retinal artery occlusion is noted due to the inflammatory process. c Improvement of the inflammatory lesion with absorption of hemorrhage and restoration of the retinal circulation (absence of ischemic edema). The bright white spot inferiorly to the optic disk consists an artifact due to the fundus reflection while acquiring the fundus photograph
a–e Optic nerve head involvement in a 22-year-old woman (case no 2) (IgG titer for bartonella henselae, 1: 64 (a) along with peripheral choroidal granuloma (b) due to intermediate uveitis (c). There is a leakage from peripheral neovascularization (at the area of granuloma) (d and e). The main reasons of neovascularization in patients with intermediate uveitis are the inflammation itself and a precedent occlusive retinal vasculitis often associated with the intermediate uveitis
Eight out of 13 cases (61.5%) did not present a systemic or another ocular illness prior to the intraocular inflammatory manifestations (Table 2). Treatment with antibiotics was efficient in all cases. The main antibiotics administered in our cases were rifampicin, doxycycline and azithromycin. Ciprofloxacin and ceftriaxone were also used in cases with treatment failure, allergy and early side effects. However, per os methylprednisolone (initial dose depended on age and uveitis severity) with tapering was administered in 6 patients in an effort to control the inflammation and its sequences (Table 3, cases number 1, 2,3,4,6 and 12). On the other hand, peripheral retinal neovascularization was treated using laser photocoagulation (Tables 2 and 3, second case). Table 3 summarizes the visual acuities before and after treatment, the antibiotics used and the complications recorded during the period of active inflammation and follow-up.
In one patient (Table 2, second patient), very high rates of serum IgM and IgG titers for Bartonella henselae were noted 3 years after the initial diagnosis, suggesting a current disease (reinfection or relapse of a chronic indolent bartonellosis). Investigation for Q fever, chlamydia and rickettsiae (as a possibility of cross reaction) was negative.
Finally, the majority of patients, 8 out of 13 (61.5%) experienced complications, seven of them ocular (2 cases with an irreversible complication—those with macular scar) and one systemic (Table 3).
Discussion
CSD is a zoonotic disease with no gender or race predilection. Although it may insult individuals of any age, the vast majority of recorded cases involve children and adolescents [1]. In our study, 12 out of the 13 patients were adults, whereas only one child (7.7%) was diagnosed with intraocular infection attributed to Bartonella.
Interestingly, it has been demonstrated that 90–95% of patients with CSD report a history of cat contact. However, it has been also reported that ocular CSD may present in patients without a history of cat contact [14]. In the current study, 12 out of 13 patients had a cat contact, but none of them had been previously diagnosed with ocular or systemic CSD.
Neuroretinitis, defined by sudden and painless vision loss, consists the most typical and common sign of ocular CSD but cannot be considered as pathognomonic. Despite the fact that Bartonella henselae is the etiologic factor in approximately two-thirds of neuroretinitis cases, it is always important to exclude other causes such as toxoplasmosis, Adamantiades-Behçet’s disease, tuberculous uveitis or even spirochetal diseases [15,16,17]. Although neuroretinitis is mainly unilateral, bilateral cases have also been described [18]. Visual acuity in the affected eye at presentation can vary from perception of light to 1.0, while vision may impair suddenly within a few days. Many patients are found to have relative afferent pupillary defect, distortion in color vision, and central, cecocentral or arcuate visual field defects. Chi et al. [19] reported 53 cases of cat-scratch optic neuropathy. To our knowledge, Bartonella-related optic neuropathy with or without other ocular findings (i.e., macular star, panuveitis, posterior uveitis, anterior and intermediate uveitis, and retinal vasculitis) is the 30, 43% of our cases (including those reported in our previous published work) [5]. In our center, we recently revealed B. henselae as a cause of papillitis without other ocular findings, but this case was not incorporated in the current case series. Older studies have described neuroretinitis accompanied by macular star [20] and/or isolated foci of retinitis or choroiditis [21] the most common clinical findings. Macular star may be detected a few days after the onset of vision loss and becomes more distinct within the next 2–3 weeks [15, 16, 18]. In this cohort of patients, 3 patients (23.1%) were diagnosed with isolated unilateral neuroretinitis and 2 patients (15.4%) with unilateral neuroretinitis and uveitis (1 with posterior uveitis and 1 with panuveitis). Isolated optic neuritis has also been described in a previous study [22], underlying the importance of excluding infectious agents like Bartonella henselae before commencing treatment with pulse methylprednisolone, especially in children. Interestingly, in our previous study intermediate uveitis was the most prominent clinical entity (64.2%) [5]. Ocular CSD can also present with retinal artery occlusion, retinal infiltrates resembling cotton-wool exudates, or endophthalmitis [1]. The pathogenetic mechanism of retinal infiltrates is probably associated with ischemia secondary to retinal arteriole occlusion [19]. It is critical for the ophthalmologist to differentiate the superficial retinal infiltrates in ocular CSD from retinitis or retinal infiltrates observed in patients with sarcoidosis, Adamantiades-Behçet’s disease, and toxoplasmosis or rickettsia infection [22]. Ophthalmic vascular occlusions related to CSD have been reported in the literature in several case reports and series [5, 21, 23,24,25,26,27,28]. Interestingly, the alterations and distortion in visual acuity are correlated with the location of the affected artery or vein [22].
In some cases of secondary epiretinal membrane (ERM) (including those of uveitic origin), a spontaneous release of the ERM is observed. It has been suggested that this phenomenon is facilitated by the treatment [29,30,31]. The release of ERM leads to reducing or releasing the tractional forces on the macula and therefore permits the closing of the macular hole. On the other hand, in cases with permanent tractional epiretinal membrane (as a complication of uveitis) with or without macular hole development, the appropriate management is pars plana vitrectomy and ERM peeling.
CSD is expected to have a more severe systemic impact in immunocompromised or immunosuppressed patients. Characteristically, it has been reported to cause bacillary angiomatosis in HIV-positive patients [32]. In our current and previous [5] case series, none of the patients were immunosuppressed and none was found with signs of angiomatosis.
The diagnosis of CSD is based on systemic and/or ophthalmologic symptoms (Table 4) [5, 19, 21, 22, 33,34,35] and clinical findings, whereas serologic tests can be used to confirm the final diagnosis. Indirect fluorescent antibody assay (IFA) for anti-B. henselae IgG is considered as the gold standard, due to its high specificity [36]. Elevated B. henselae or quintana IgM titer is indicative of a recent infection, and values become normal again within a 3-month period. However, the sensitivity of IFA for anti-B. henselae IgM (IgM-IFA) is low [37]. B. henselae or quintana IgG titer can increase over the course of time and remain positive for up to 2 years. A positive B. henselae IgM and mainly an elevated B. henselae IgG titer can establish a diagnosis for CSD [38]. In the current study, all patients were found to be positive for B. henselae or B. quintana IgG (Table 2).
Doxycycline and rifampicin and alternatively one of them combined with azithromycin are the antibiotics of choice in individuals without systemic diseases, such as diabetes, or immune deficiency [5]. Trimethoprim–sulfamethoxazole, quinolones or intravenous aminoglycosides have also been reported as effective alternatives [1]. In our case series, the most commonly administered antibiotic was doxycycline, and in the majority of patients, it was administered in combination with rifampicin, macrolide and/or quinolone. There is a wide spectrum of opinions regarding the duration of treatment with antibiotics. An overall treatment between 10 days to 3 weeks has been shown to be adequate for cases with ocular involvement. However, it has been suggested that immunosuppressed patients (e.g., HIV-positive) should continue their treatment for an overall of 2–4 months [6]. The efficacy of systemic steroids remains debatable, but in cases with severe inflammatory findings administration of systemic steroids could be beneficial.
Taking into account that CSD is a zoonotic infection, it is crucial to establish and maintain preventive measures [39]. It has been recommended that strict ectoparasite control can limit the risk of Bartonella infection from arthropod vectors to domestic animals and pets and therefore prevent the transmission of pathogen from animals to humans. Hygiene education after contact with cats, especially of children and immunosuppressed individuals, could also contribute to reducing the risk of infection [1].
According to our results, Bartonella uveitis is more commonly induced by Bartonella henselae and rarely by quintana (the later observed in a worker if furs elaboration industry) and this intraocular inflammation mimics that caused by other diseases.
Conclusion
Intraocular inflammation caused by B. henselae and B. quintana is being diagnosed with increasing frequency worldwide. Although neuroretinitis and optic neuritis remain the most common manifestations, ocular CSD can present with a wide spectrum of clinical findings suggesting that bartonellosis is a new emerging mimicker. The history of cat contact can be the key for setting a clinical diagnosis, and Bartonella serologic investigations are substantial in the differential diagnosis between other diseases with similar clinical features. Preceding systemic symptoms must also be taken into consideration. Detailed recording and observation of the epidemiologic features can be helpful in establishing preventive measures especially in high-risk regions and populations. Raising awareness among clinicians of both ocular and systemic findings can contribute setting a prompt and early diagnosis, leading to a more efficient management of the disease. Well-designed randomized control trial studies are essential to define the most favorable treatment especially for sight-threatening cases.
References
Amer R, Tugal-Tutkun I (2017) Ophthalmic manifestations of Bartonella infection. Curr Opin Ophthalmol 28(6):607–612
Jones DB (1996) Cat-scratch disease. In: Pepose JS, Holland GN, Wilhelmus KR (eds) Ocular infection and immunity. Mosby-Year Book, St. Louis, pp 1389–1396
Koehler JE, Glaser CA, Tappero JW (1994) Rochalimaea henselae infection: a new zoonosis with the domestic cat as reservoir. JAMA 16(271):531–535
Chomel BB, Kasten RW, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield AN, Abbott RC, Pedersen NC, Koehler JE (1996) Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol 34:1952–1956
Kalogeropoulos C, Koumpoulis I, Mentis A, Pappa C, Zafeiropoulos P, Aspiotis M (2011) Bartonella and intraocular inflammation: a series of cases and review of literature. Clin Ophthalmol (Auckland, NZ) 5:817
Spach DH, Koehler JE (1998) Bartonella-associated infections. Infect Dis Clin North Am 12:137–155
Midani S, Ayoub EM, Anderson B (1996) Cat-scratch disease. Adv Pediatr 43:397–422
Angelakis E, Raoult D (2014) Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents 44:16–25
Biancardi AL, Curi ALL (2014) Cat scratch disease. Ocul Immunol Inflamm 22:148–154
Ormerod LD, Dailey JP (1999) Ocular manifestations of cat-scratch disease. Curr Opin Ophthalmol 10:209–216
Saatci AO, Oner FH, Kargi A, Kavukcu S (2002) Unilateral neuroretinitis and peripapillary serous detachment in Cat- scratch disease. Korean J Ophthalmol 16:43–46
Windsor JJ (2001) Cat-scratch disease: epidemiology, aetiology and treatment. Br J Biomed Sci 58:101–110
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of Uveitis Nomenclature (SUN) Working Group. In: Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop, Am J Ophthalmol, vol 140, pp 509–516
Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, Plikaytis BD, Hadler JL, Cartter ML, Wenger JD (1993) Cat-scratch disease in Connecticut: epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med 329:8–13
Dreyer RF, Hopen G, Gass JDM, Smith JL (1984) Leber’s idiopathic stellate neuroretinitis. Arch Ophthalmol 102:1140–1145
Weiss AH, Beck RW (1989) Neuroretinitis in childhood. J Pediatr Ophthalmol Strabismus 26:198–203
Abdelhakim A, Rasool N (2018) Neuroretinitis: a review. Curr Opin Ophthalmol 29(6):514–519
Reed JB, Scales KD, Wong MT, Lattuada CP, Dolan MJ, Schwab IR (1998) Bartonella henselae neuroretinitis in cat scratch disease: diagnosis, management, and sequelae. Ophthalmology 105:459–466
Chi SL, Stinnett S, Eggenberger E, Foroozan R, Golnik K, Lee MS, Bhatti MT (2012) Clinical characteristics in 53 patients with cat scratch optic neuropathy. Ophthalmology 119(1):183–187
Ormerod LD, Skolnick KA, Menosky MM, Pavan PR, Pon DM (1998) Retinal and choroidal manifestations of cat-scratch disease. Ophthalmology 105(6):1024–1031
Solley WA, Martin DF, Newman NJ, King R, Callanan DG, Zacchei T, Wallace RT, Parks DJ, Bridges W, Sternberg P Jr (1999) Cat scratch disease: posterior segment manifestations. Ophthalmology 106(8):1546–1553
Oray M, Önal S, Akbay AK, Tutkun İT (2017) Diverse clinical signs of ocular involvement in cat scratch disease. Turk J Ophthalmol 47(1):9
Eiger-Moscovich M, Amer R, Oray M, Tabbara KF, Tugal-Tutkun I, Kramer M (2016) Retinal artery occlusion due to Bartonella henselae infection: a case series. Acta Ophthalmol 94:e367–e370
Cohen SM, Davis JL, Gass DM (1995) Branch retinal arterial occlusions in multifocal retinitis with optic nerve edema. Arch Ophthalmol 113:1271–1276
Batsos G, Kabanarou SA, Fotiou P, Rouvas A, Xirou T (2013) Retinal arterial occlusive disease in a young patient with cat scratch disease. Case Rep Ophthalmol 4:87–92
Gray A, Michels K, Lauer A, Samples J (2004) Bartonella henselae infection associated with neuroretinitis, central retinal artery and vein occlusion, neovascular glaucoma, and severe vision loss. Am J Ophthalmol 137:187–189
Gray AV, Reed JB, Wendel RT, Morse LS (1999) Bartonella henselae infection associated with peripapillary angioma, branch retinal artery occlusion, and severe vision loss. Am J Ophthalmol 127:223–224
Pinna A, Puglia E, Dore S (2011) Unusual retinal manifestations of cat scratch disease. Int Ophthalmol 31:125–128
Schadlu R, Apte RS (2007) Spontaneous resolution of an inflammation-associated epiretinal membrane with previously documented posterior vitreous detachment. Br J Ophthalmol 91(9):1252–1253. https://doi.org/10.1136/bjo.2006.113597
Andreev AN, Bushuev AV, Svetozarskiy SN (2016) A case of secondary epiretinal membrane spontaneous release. Case Rep Ophthalmol Med 2016:4925763. https://doi.org/10.1155/2016/4925763
Ozgonul C, Besirli CG (2017) Macular hole closure following spontaneous release of vitreomacular traction. BMJ Case Rep. pii: bcr2016218547. https://doi.org/10.1136/bcr-2016-218547
Warren K, Goldstein E, Hung VS, Koehler JE, Richardson W (1998) Use of retinal biopsy to diagnose Bartonella (formerly Rochalimaea) henselae retinitis in an HIV-infected patient. Arch Ophthalmol 116:937–940
Curi AL, Machado D, Heringer G, Campos WR, Lamas C, Rozental T, Gutierres A, Orefice F, Lemos E (2010) Cat-scratch disease: ocular manifestations and visual outcome. Int Ophthalmol 30(5):553–558
Tan CL, Fhun LC, Tai EL et al (2017) Clinical profile and visual outcome of ocular bartonellosis in Malaysia. J Trop Med 2017:7946123
Habot-Wilner Z, Trivizki O, Goldstein M, Kesler A, Shulman S, Horowitz J, Amer R, David R, Ben-Arie-Weintrob Y, Bakshi E et al (2018) Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol 96(4):e524–e532
Bergmans AM, Peeters MF, Schellekens JF, Vos MC, Sabbe LJ, Ossewaarde JM, Verbakel H, Hooft HJ, Schouls LM (1997) Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J Clin Microbiol 35(8):1931–1937
Vermeulen MJ, Verbakel H, Notermans DW, Reimerink JH, Peeters MF (2010) Evaluation of sensitivity, specificity and cross-reactivity in Bartonella henselae serology. J Med Microbiol 59(Pt 6):743–745
Gulati A, Yalamanchili S, Golnik KC, Lee AG (2012) Cat scratch neuroretinitis: the role of acute and convalescent titers for diagnosis. J Neuroophthalmol 32(3):243–245
Regier Y, O’Rourke F, Kempf VAJ (2016) Bartonella spp.: a chance to establish One Health concepts in veterinary and human medicine. Parasit Vectors 9:261
Acknowledgements
Special thanks to Dr. Aliki Geka (Department of Ophthalmology, Olympion Private Hospital, Patras, Greece), Dr. Dimitrios Kournetas (Laser & Ophthalmos S.A., Thessaloniki, Greece) and Dr. Neoklis Razis (Razis eye clinic, Limassol, Cyprus).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
The research followed the tenets of the Declaration of Helsinki. The Scientific Committee of the University Hospital of Ioannina (Greece) approved the current study on the 6th of November 2018 (Protocol number 1326).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalogeropoulos, D., Asproudis, I., Stefaniotou, M. et al. Bartonella henselae- and quintana-associated uveitis: a case series and approach of a potentially severe disease with a broad spectrum of ocular manifestations. Int Ophthalmol 39, 2505–2515 (2019). https://doi.org/10.1007/s10792-019-01096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01096-7