Abstract
Purpose
To evaluate the diagnostic accuracy of OCT angiography (OCT-A) detecting or predicting choroidal neovascularization (CNV), by ophthalmologists of disparate degrees of skills in retinal diseases, using spectral domain optical coherence tomography (SD-OCT) and fluorescein angiography (FA) as a standard reference.
Methods
Retrospective observational case series. Patient presenting maculopathy and complete imaging were included. FA, SD-OCT, OCT-A and FA coupled to SD-OCT images were graded independently for presence or absence of CNV by ophthalmologists with varying expertise levels.
Results
Overall sensitivity of OCT-A was 85.62% (95% CI 79.04–90.76%) and specificity was 81.51% (95% CI 73.36–88.03). Sensitivity of FA was 74.51% (95% CI 66.84–81.20), and specificity was 82.35% (95% CI 74.30–88.73). Sensitivity of FA + SD-OCT was 92.72% (95% CI 87.34–96.30), and specificity was 90.91% (95% CI 84.31–95.37).
Conclusion
OCT-A has good sensitivity and specificity for the detection of CNV in all expertise level groups. OCT-A may soon become a routine tool for CNV diagnosis and follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Choroidal neovascularization (CNV) is the hallmark of exudative age-related macular degeneration (wAMD) and may complicate other diseases (uveitis, pathological myopia, etc.). In wAMD, CNV accounts for over 80% of severe visual loss cases [1]. CNV detection methods, other than fundus exam, consist of fluorescein angiography (FA), indocyanine green angiography (ICGA) and optical coherence tomography (OCT). Leakage on late phase of the FA is the hallmark of neovascularization, but leakage may be hard to interpret if lesion components overlap and/or if the lesion is in regression or not exudative, as may be the case in lesions partially responding to therapy or quiescent CNV [2]. Confounding factors may be noted: Certain types of hyperfluorescence patterns may be mistaken for leakage, often in central serous chorioretinopathy (CSCR), in vitelliform lesions or in fibrosis. Nevertheless, the gold standard in CNV detection imaging remains FA because of its availability and relatively quick performance time. However, FA is considered an invasive exam, with potential of risk of anaphylaxis [3].
OCT has become a major protagonist for management of CNV, in part because it can be repeated in minimal time, a perfect tool for monitoring CNV treatment response. Regression of lesion size, small areas of serous retinal detachment indicating neovascular activity and consolidation of fibrotic tissue are more easily assessed with OCT than with FA. CNV by itself is not clearly visualized; diagnosis of CNV is only based on indirect signs, such as pigment epithelial detachment or hyperreflectivity above the RPE [4] coupled with exudative features.
Spectral domain OCT (SD-OCT) has improved quality of images when compared to earlier generation OCT technologies; interpretation has become more accessible. Validation of SD-OCT in wAMD was recently published by Wilde et al. [5]. They conclude that while SD-OCT is highly sensitive, it cannot replace FA. Chhablani et al. [6] demonstrated that sensitivity of SD-OCT was superior to that of FA; FA had a better reproducibility than SD-OCT, but agreement between methods was poor.
Optical coherence tomographic angiography (OCT-A) is a novel and noninvasive imaging tool, that allows visualization of retinal microvasculature by detecting intravascular blood flow, based on the split-spectrum amplitude-decorrelation (SSADA) angiography and/or an “en face” OCT-derived technique [7] without any dye injection. It allows imaging of the dynamic motion of erythrocytes using sequential OCT cross-sectional scans which are repeated at the same location on the retina. This new technique may be used in daily clinical ophthalmology practice and may, in the future, replace invasive techniques [8].
With improvement in image decorrelation technology, OCT angiography (OCT-A) has improved largely, making it a valuable alternative for imaging of the posterior pole. Interpretation of images acquired by this technology remains challenging.
In light of these issues, the purpose of this study was to determine sensitivity and specificity of OCT-A for detection of CNV, compared to FA and also comparing it to FA plus SD-OCT by ophthalmologist holding varying degrees of expertise in retinal disease.
Patients/materials and methods
Population
In this retrospective case series review, files of all patients seen at the macular clinic in the University Eye Clinic of Creteil, France, from November 17 to November 21, 2014, regardless of disease etiology, were evaluated. This study was approved by the Institutional Review Board of the Federation France Macula. The research adhered to the tenants of the declaration of Helsinki.
Inclusion criteria were patient files documenting complete ophthalmic examination (visual acuity, slit lamp examination, fundus exam), image workup including fundus photography (Canon CRX), FA, indocyanine green angiography (ICGA), SD-OCT (Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany) and OCT-A (RTVue100× Avanti, Optovue, Fremon, CA, USA) performed on the same day. Final diagnostic information had to be confirmed by clinical presentation (signs, symptoms, evolution, response to treatment), imaging and concordance between two of the authors (VC and RBG). The overall gold standard for determining the presence of CNV used by the authors was FA coupled to SD-OCT images.
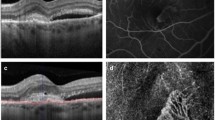
Because our purpose was to identify CNV, we included all types of CNV in the CNV population and all types of macular disease in others. In Fig. 1 is an example of imaging obtained for CSCR (no CNV) and in Fig. 2 an example of images presented for a patient with type 2 CNV.
Patient#1 presenting a typical case of CSCR on FA and SD-OCT Imaging. Yellow arrow serous detachment of the neurosensory retina in the macula area in FA. White arrow appearance of a single dot of fluorescein with leakage over the course of the angiogram. Star subretinal hyporeflective area on SD-OCT imaging: retina serous detachment. Yellow bracket thickened choroid
Patient#5 presenting a typical case of type 2 CNV with subretinal hemorrhage complicating nAMD. Yellow arrow fluorescein leakage expanding over the course of the angiography. White arrow subretinal hemorrhage, masking effect. White star dome-shaped hyperreflective subretinal area on SD-OCT imaging corresponding subretinal hemorrhage. Yellow star exudative signs on SD-OCT: intraretinal cyst, retina serous detachment, subretinal hyperreflective exudation, thickened retina
Imaging
In our center, 30 or 50 degree FA images are obtained for each eye at each of the following phases: very early (0–40 s), early (1–2 min), middle (2–3 min) and late (4–5 min). Very late phase images are obtained if suspicion of pooling or polyps arises. SD-OCT imaging in our center follows a protocol: high-resolution map of the posterior pole (19 horizontal scans in a 20 by 20 degree area) coupled with horizontal and a vertical scans of 30 degrees passing through the fovea.
OCT angiography images were done with AngioVue Imaging System (Optovue, Inc., Freemont, CA). This instrument has an A-scan rate of 70,000 scans per second, using a light source centered on 840 nm and a bandwidth of 50 nm. Each OCT-A volume contains 304 × 304 A-scans with two consecutive B-scans captured at each fixed position before proceeding to the next sampling location. The scan area was 3 × 3 mm. Split-spectrum amplitude-decorrelation angiography was used to extract the OCT angiography information. Two orthogonal OCT-A volumes were acquired in order to perform motion correction. Angiography information displayed is the average of the decorrelation values when viewed perpendicularly through the thickness being evaluated. Automatic retinal segmentation was performed by integrated software.
No ICGA or color fundus images were used for the specificity/sensitivity part of the study. ICGA and color fundus images were used in case of diagnostic uncertainty.
Image presentation
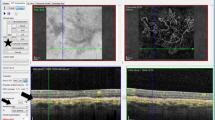
Images were shown in three stages. The first stage consisted of presentation of OCT-A images (four image frames per patient: superficial plexiform vascular layer, deep plexiform vascular layer, outer retinal layer and choriocapillaris; Fig. 3 shows examples of images of choriocapillaris or outerretinal layer slabs to be interpreted by study participants). 1 week later, FA images were presented [3–4 frames: very early phase, early phase, late phase and very late phase (when available) per case].
Highlight of the most informative layer in OCT-A imaging for two of the cases. a Patient #8 presenting a typical case of type 1 CNV in AMD. White star: larger trunk. Yelllow star: branching pattern and anastomosis. b Patient #13 presenting vascular dilatation. Yellow arrows: perimacular vascular dilatation. White arrow: perifoveal microvasculature rupture
Finally, another week later, FA images plus one SD-OCT scan per case were presented (the most representative according to two of the authors (VS and RBG)).
Images were shown individually to 17 clinicians who voluntarily agreed to participate in the study. Anonymized images were presented randomly on a computer screen, in electronic JPG format. We asked: « Is there or not a CNV? » . Only yes/no answers were allowed and no participant was allowed incomplete responses. The participating clinicians were then distributed in three groups according to level of expertise: confirmed experts (Professors), knowledgeable specialists (Retina Fellows and ophthalmologists with less than 5 years in practice) and beginners (ophthalmology residents). Images were then further analyzed by the authors in order to characterize the lesions. Clinicians were blinded to each other’s reads and also to reading results from previous imaging modalities at each phase.
Statistical analysis
Statistical analysis for sensitivity and specificity was performed using MedCalc software version 15.2.2 (MedCalc Software bvba, Belgium; available at: www.medcalc.org).
Results
A total of 24 patients had complete patient files and imaging, but 8 presented inferior quality imaging (rendering interpretation impossible) or atypical clinical presentations and were excluded. In total 16 eyes of 16 patients were included. Of these 16 eyes, 9 presented with CNV (all nAMD). The other cases were one re-perfused central retinal artery obstruction, one adult onset pseudovitelliform lesion, one drusenoid pigment epithelium detachment, two CSCRs, one macroaneurysm and one reticular dystrophy. Final diagnosis was the result of collegiate agreement.
A total of 17 clinicians analyzed the OCT-A, FA and FA + SD OCT images of sixteen eyes of sixteen patients on three occasions (OCT-A images, FA images, FA + SD OCT images; 867 instances in total analyzed).
Overall, the sensitivity in CNV detection for OCT-A was 85.62% and the specificity was 81.51%.
In the resident group (n = 6), fellow group (n = 5) and confirmed expert group (n = 6), the sensitivity of OCT-A was, respectively, 90.74, 82.22 and 83.33%, the specificity was, respectively, 80.95, 82.86 and 82.86% (See details in Table 1).
Sensitivity of FA for all participants combined was 74.51%, and specificity was 82.35%.
In the resident group, fellow group and confirmed expert group, the sensitivity of FA was, respectively, 64.81, 73.33 and 85.19% and the specificity was, respectively, 71.43, 82.86 and 92.86% (See details in Table 2).
Finally, for all participants combined, the sensitivity of FA + SD-OCT was 92.72% and the specificity was 90.91%. In the resident group, fellow group and confirmed expert group, the sensitivity of FA + SD-OCT was, respectively, 96.30, 91.11 and 90.38%; and the specificity was, respectively, 90.48, 97.14 and 86.36% (Table 3).
Upon analyzing OCT-A images, we found that in cases presenting a high rate of true positives (defined as 15 out of 17 or more correct answers), 4 were cases where CNV presented itself as a hypereflective structure giving the appearance of anastomoses and terminal loops and two cases had a distinctive branching pattern (Fig. 4).
For cases presenting with a relatively high rate of false negatives (over seven false negative responses), the authors could not conclude on a distinctive CNV pattern. Two cases of patients presenting with central serous chorioretinopathy without CNV on multimodal imaging presented a high rate of false positives, both of these cases presented choroidal no flow signals.
Agreement coefficient was determined for each case (Table 4).
Discussion
Here, we investigated the sensitivity and specificity of OCT-A compared to FA and FA plus SD-OCT in groups of varying ophthalmological expertise.
Overall, sensitivity and specificity were acceptable in all groups (over 80%). In our study, OCT-A was more sensitive than FA. However, multimodal imaging consisting of FA combined with SD-OCT remained more sensitive and specific than OCT-A alone.
Our residents had better results with OCT-A than with FA. We hypothesize that for residents, the learning curve for OCT-A interpretation was easier than that for FA. We could also argue that residents were the group that performed the best in OCT-A interpretation due to the fact that they had vast hands on experience performing OCT-A imaging.
In the expert group, FA was the most sensitive and specific imaging method with almost 10% better results than OCT-A. This was probably because they were more experienced in CNV semiology in FA, and they may have had preconceived ideas of what CNV in OCT-A should look like (based on previous knowledge derived from classic FA), which could explain worse performance in OCT-A interpretation.
FA imaging requires just some degree of patient cooperation: Leakage can be evidenced in two to three good-quality frames. On the contrary, OCT-A needs ample patient cooperation: Patients should not move at all for 3–4 s, as artifacts are obtained and images renderer uninterpretable. Some of these artifacts are accounted for and are calculated out in newly developed algorithms [9].
Other artifacts could limit the interpretation of images, such as projection artifacts. These could result in misinterpretation of the localization of vascularized tissue [10]. The “remove artifacts” function available in the software can subtract projection artifacts, resulting in a “softened” signal. The quantity of data collected and analyzed in order to obtain OCT-A imaging facilitates the appearance of artifacts and requires the physician to have interactions in interpretation [10, 11]. Understanding of artifacts’ origins and development of new algorithms are leading to a more acute interpretation of OCT-A imaging. [7, 8, 12, 13].
Moult et al. [14] investigated ultrahigh-speed swept-source OCT-A to visualize vascular changes in patients with wAMD. In a retrospective observational study, De Carlo et al. [15] demonstrated that OCT-A had sensitivity and specificity of CNV detection of 50 and 91%, respectively.
Coscas et al. [16] compared OCT-A versus multimodal imaging in patients with wet AMD. They demonstrated an excellent level of correlation in treatment decision based on OCT-A.
A commonly shared fear expressed by OCT-A detractors is that CNV activity might not be well assessed with OCT-A, as minimal or incomplete regression of CNV is not always present in inactive lesions, so that modification of CNV size, extension or even rearrangement of the CNV component might not always translate into clinical response or even correlate with visual acuity. Coscas et al. [17] suggest that OCT-A can detect decreased visibility and size of CNV after anti-VEGF treatment and that CNV monitoring and follow-up can be managed by OCT-A.
Lumbroso et al. [18] observed a cyclic response to anti-VEGF in CNV, followed by flow shutdown on OCT-A, which could guide treatment timing [19]. De Carlo et al. [15] describe a reduction in size of CNV after anti-VEGF therapy.
Kuehlewein et al. [20] also describe a reduction of the vascular lesion size after anti-VEGF injection. For these authors, CNV regression can be quantified using OCT-A and could thus enhance its potential use. In a recent study by Spaide et al. [21], he eloquently concludes upon the nonexistence of “normalization” of neovascular structures after anti-VEGF injections, but rather a pruning of newly developing vessels. In any case, there is visible modification in the structure of abnormal vessels in CNV after anti-VEGF therapy, which could potentially lead to further developing algorithms for response evaluation. Evidence has recently been published by McClintic et al. [22] concerning the fact that phase variance OCT-A imaging could noninvasively detect PED vascularization prior to the onset of symptomatic exudation and by extension, prior to visible signs in traditional imaging. This could potentially improve treatment outcomes..
As a consequence of the popularization of OCT-A, a whole new semiology is being described [23] for type 1 [24], type 2 [25] and type 3 [26] CNVs; for polypoidal vasculopathy [27]; and neovascular network inside subretinal fibrosis [28]. Another advantage of OCT-A versus traditional imaging is its ability to study non-exudative areas [29, 30].
Some limitations of our study should be acknowledged: We must consider our results knowing that a small number of cases were presented for analysis, but given the repeated number of analysis made by each participant, more cases would have probably only contributed to fatigue induced error. On the other hand, it would have been quite interesting to analyze a more varied array of non-CNV pathologies, and to perform a more profound diagnostic challenge on participants. Also, unfortunately, we examined the performance results in a cohort of individuals who interpreted images exclusively from only one of the OCT-A machines on the market.
In conclusion, this study shows that OCT-A has good sensitivity and specificity in CNV detection in different expertise levels. This is in our knowledge the first study evaluating OCT-A image interpretation in groups of varying degrees of expertise. The ability of OCT-A in CNV detection may lead to a decrease in use of invasive imaging techniques such as FA, all the more so in view of the fact that this new technology still has considerable potential to further improve its efficiency. We conclude that OCT angiography imaging should be considered as a serious role player in the management of CNV. Further studies comparing a wider spectrum of OCT-A devices in a larger patient cohort with more varied pathologies could provide more insight into the matter.
References
Grossniklaus HE, Green WR (2004) Choroidal neovascularization. Am J Ophthalmolog 137(3):496–503
Querques G, Srour M, Massamba N et al (2013) Functional characterization and multimodal imaging of treatment-naive “quiescent” choroidal neovascularization. Invest Ophthalmol Vis Sci 54(10):6886–6892
Yannuzzi LA, Rohrer KT, Tindel LJ et al (1986) Fluorescein angiography complication survey. Ophthalmolog 93(5):611–617
Ores R, Puche N, Querques G et al (2014) Gray hyper-reflective subretinal exudative lesions in exudative age-related macular degeneration. Am J Ophthalmol 158(2):354–361
Wilde C, Patel M, Lakshmanan A et al (2015) The diagnostic accuracy of spectral-domain optical coherence tomography for neovascular age-related macular degeneration: a comparison with fundus fluorescein angiography. Eye 29:602–610
Chhablani J, Deepa MJ, Tyagi M et al (2015) Fluorescein angiography and optical coherence tomography in myopic choroidal neovascularization. Eye 29:519–524
Lozzi A, Agrawal A, Boretsky A et al (2015) Image quality metrics for optical coherence angiography. Biomed Opt Express 6(7):2435–2447
Zhang A, Zhang Q, Chen CL, Wang RK (2015) Methods and algorithms for optical coherence tomography-based angiography: a review and comparison. J Biomed Opt 20(10):100901
Liu X, Kirby M, Zhao F (2014) Motion analysis and removal in intensity variation based OCT angiography. Biomed Opt Express 5(11):3833–3847
Spaide RF, Fujimoto JG, Waheed NK (2015) Image artifacts in optical coherence tomography angiography. Retina. 35(11):2163–2180
Cheng Y, Guo L, Pan C et al (2015) Statistical analysis of motion contrast in optical coherence tomography angiography. J Biomed Opt 20(11):116004
Zhang A, Zhang Q, Wang RK (2015) Minimizing projection artifacts for accurate presentation of choroidal neovascularization in OCT micro-angiography. Biomed Opt Express 6(10):4130–4143
Liu L, Gao SS, Bailey ST et al (2015) Automated choroidal neovascularization detection algorithm for optical coherence tomography angiography. Biomed Opt Express 6(9):3564–3576
Moult E, Choi W, Waheed NK et al (2014) Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina 45(6):496–505
De Carlo TE, Bonini Filho MA, Chin AT et al (2015) Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmolog 122(6):1228–1238
Coscas GJ, Lupidi M, Coscas F et al (2015) Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina 35(11):2219–2228
Coscas G, Lupidi M, Coscas F et al (2015) Optical coherence tomography angiography during follow-up: qualitative and quantitative analysis of mixed type I and II choroidal neovascularization after vascular endothelial growth factor trap therapy. Ophthalmic Res 54(2):57–63
Lumbroso B, Rispoli M, Savastano MC (2015) Longitudinal optical coherence tomography-angiography study of type 2 naive choroidal neovascularization early response after treatment. Retina 35(11):2242–2251
Huang D, Jia Y, Rispoli M et al (2015) Optical coherence tomography angiography of time course of choroidal neovascularization in response to anti-angiogenic treatment. Retina 35(11):2260–2264
Kuehlewein L, Sadda SR, Sarraf D (2015) OCT Angiography and sequential quantitative analysis of type 2 neovascularisation after ranibizumab therapy. Eye (Lond) 29(7):932–935
Spaide RF (2015) Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am J Ophthalmol 160:6–16
Palejwala NV, Jia Y, Gao SS et al (2015) Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina 35(11):2204–2211
Mastropasqua R, Di Antonio L, Di Staso S et al (2015) Optical coherence tomography angiography in retinal vascular diseases and choroidal neovascularization. J Ophthalmol 2015:343515
Kuehlewein L, Bansal M, Lenis TL et al (2015) Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Am J Ophthalmol 160(4):739–748
El Ameen A, Cohen SY, Semoun O et al (2015) Type 2 neovascularization secondary to age-related macular degeneration imaged by optical coherence tomography angiography. Retina 35(11):2212–2218
Miere A, Querques G, Semoun O et al (2015) Optical coherence tomography angiography in early type 3 neovascularization. Retina 35(11):2236–2241
Inoue M, Balaratnasingam C, Freund KB (2015) Optical coherence tomography angiography of polypoidal choroidal vasculopathy and polypoidal choroidal neovascularization. Retina 35(11):2265–2274
Miere A, Semoun O, Cohen SY et al (2015) Optical coherence tomography angiography features of subretinal fibrosis in age-related macular degeneration. Retina 35(11):2275–2284
Choi W, Moult EM, Waheed NK et al (2015) Ultrahigh-speed, swept-source optical coherence tomography angiography in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmolog 122(12):2532–2544
McClintic SM, Kim DY, Fingler J et al (2015) Detection of pigment epithelial detachment vascularization in age-related macular degeneration using phase-variance OCT angiography. Clin Ophthalmol 15(9):1299–1305
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare not having received any grant support or research funding for the presser article. They also declare not having any proprietary interest in the materials described in the article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Souedan, V., Souied, E.H., Caillaux, V. et al. Sensitivity and specificity of optical coherence tomography angiography (OCT-A) for detection of choroidal neovascularization in real-life practice and varying retinal expertise level. Int Ophthalmol 38, 1051–1060 (2018). https://doi.org/10.1007/s10792-017-0559-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-017-0559-6