Abstract
Purpose
The purpose of the present study was to investigate the effect of alpha-lipoic acid (ALA) on the thicknesses of various retinal layers and on the numbers of retinal ganglion cells and vascular endothelial growth factor levels in experimental diabetic mouse retinas.
Methods
Twenty-one male BALB/C mice were made diabetic by the intraperitoneal administration of streptozotocin (200 mg/kg). One week after the induction of diabetes, the mice were divided randomly into three groups: control group (non-diabetic mice treated with alpha-lipoic acid, n = 7), diabetic group (diabetic mice without treatment, n = 7), and alpha-lipoic acid treatment group (diabetic mice with alpha-lipoic acid treatment, n = 7). At the end of the 8th week, the thicknesses of the inner nuclear layer (INL), outer nuclear layer (ONL), and full-length retina were measured; also retinal ganglion cells and VEGF expressions were counted on the histological sections of the mouse retinas and compared with each other.
Results
The thicknesses of the full-length retina, ONL, and INL were significantly reduced in the diabetic group compared to the control and ALA treatment groups (p = 0.001), whereas the thicknesses of these layers did not show a significant difference between ALA treatment and control groups. The number of ganglion cells in the diabetic group was significantly lower than those in the control and ALA treatment groups (p = 0.001). The VEGF expression was significantly higher in the diabetic group and mostly observed in the ganglion cell and inner nuclear layers compared to the control and ALA treatment groups (p = 0.001). Therefore, the number of ganglion cells and VEGF levels did not show significant differences between the ALA treatment and control groups (p = 0.7).
Conclusions
Our results show that alpha-lipoic acid treatment may have an impact on reducing VEGF levels, protecting ganglion cells, and preserving the thicknesses of the inner and outer layers in diabetic mouse retinas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a good model of chronic oxidative damage and it is a particularly suitable disease for antioxidant supplementation. Oxidative stress caused by the formation of free radicals and reactive oxygen species (ROS) leads to retinal vasculature damage by stimulating the release of proinflammatory cytokines such as interleukin 1β (IL-1β) and tumor necrosis factor (TNF-α) and hence it plays an important role in the pathogenesis of DR [1]. Reactive oxygen species (ROS) products have been shown to stimulate protein kinase C (PKC) which is responsible for the upregulation of retinal vascular endothelial growth factor (VEGF) proteins [2]. Vascular endothelial growth factor, a key angiogenic growth factor, plays an important role in the angiogenic process and pathogenesis of diabetic retinopathy [3]. Since VEGF production is increased by hypoxia [4] and oxidative stress, its upregulation has been documented in the early course of diabetes in rat and mouse models [5, 6].
A significant correlation between increased blood sugar levels and depletion of antioxidants is demonstrated, which eventually leads to pathological and biochemical changes in DR [7]. One of the best characterized antioxidants is alpha-lipoic acid (ALA), a disulfide derivative of octanoic acid, which can alter the redox status of cells and interact with thiols and other antioxidants such as glutathione (GSH), vitamin C, and vitamin E, for reducing or slowing down ROS damage. It reduces oxidative stress by scavenging a number of free radicals in both membrane and aqueous domains, by chelating transition metals in biological systems and by preventing membrane lipid peroxidation [8]. ALA is contained in all cells rich in mitochondria where the energy demand is high, thus leading to the production of oxidant activity. A mitochondrial dysfunction, observed during DR, inevitably results in the apoptosis of cells with a high energy demand, such as the retinal ganglion cells and photoreceptors.
Administration of ALA to diabetic rats has been shown to inhibit the parameters of oxidative stress in various organs of diabetic rats, including kidney, nerve, and retina [9, 10].
In the present study, we aimed to evaluate the impact of ALA on the levels of VEGF and retinal ganglion cells (RGCs) and on the thicknesses of the inner and outer neuronal layers in an experimental diabetic mice model.
Materials and methods
Animals and induction of diabetes
All experiments were performed in accordance with the Guidelines for Animal Experiments in Karadeniz and Technical University adopted by the Committee on the Care and Use of Laboratory animals of the University and tenets of the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. Twenty-one male BALB/C mice aged 8 weeks were maintained in an animal room with controlled temperature (21–25 °C) and humidity (45–65%) under a 12-h light/dark cycle (light on 06:00 to 18:00). Mice had free access to standard food and water. After 7 days of acclimation, the mice were made diabetic by the intraperitoneal administration of streptozotocin (STZ) (200 mg/kg) (Streptozotocin, Sigma, Deisenhofen, Germany) dissolved in 0.1 mol/l citrate buffer (pH 4.5). One week after STZ injection, the blood samples were collected from the tail vein and all animals with plasma glucose level > 300 mg/dl were considered as diabetic and included in the study. Plasma glucose levels were determined with a commercially available Medisense Optium Xceed kit (Abbott, USA).
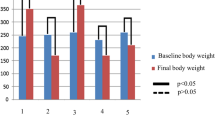
We evaluated the initial and final body weights and blood glucose levels among groups. The final body weights and blood concentrations are shown in Table 1.
Grouping and experimental procedure
One week after the induction of diabetes, the mice were divided randomly into 3 groups: control group (non-diabetic mice treated with ALA, n = 7), diabetic group (diabetic mice without treatment, n = 7), and alpha-lipoic acid treatment group (diabetic mice with ALA treatment, n = 7). Three weeks after the induction of diabetes, ALA (Sigma-Aldrich, St. Louis, MO, USA) (100 mg/kg) was dissolved in ethanol and applied intraperitoneally weekly for 5 weeks. At the end of the 8th week, all animals were euthanatized by an overdose of carbon dioxide asphyxiation followed by cervical dislocation. After cervical dislocation, fourteen eyeballs were collected from each experimental group and fixed in 10% neutral buffered formalin solution for 24 h.
Histological analysis
The samples were embedded in paraffin blocks. Then 4-μm-thick sections from each block were cut tangentially through the pupil and optic nerve, passing through the temporal ora serrata, the optic nerve, and the nasal ora serrata, which included a full length of retina. Cryostat sections were stained with hematoxylin and eosin (H&E) for ganglion cell counting and histopathology. Ganglion cells were counted in central and peripheral retina (both peripheral sides, nasal and temporal), and the average ganglion cell number was counted per 100 mm length of the retina from each group. The thicknesses of the full-length retina and the outer and inner nuclear layers were measured in the central and peripheral retina (both peripheral sides, nasal and temporal). The respective measurements were then averaged to obtain the values. All measurements were performed under a light microscope (Olympus-BX53F, Tokyo/Japan) with an attached digital camera (Digital Camera; DP72 Olympus, Tokyo/Japan, Software; DP2-BSW Ver.2.1, Olympus Corporation, Tokyo/Japan). Immunohistochemical staining was performed by the automated immunohistochemical device (Ventana BenchMark XT, Inc. Tucson, Arizona/USA) using an anti-VEGF antibody (ABCAM Inc. USA, ab46154). VEGF expression was counted in central and peripheral retina (both peripheral sides, nasal and temporal), and the average VEGF number was counted per 100 mm length of the retina from each group. We utilized ImageJ (imagej.nih.gov/ij) program to count both VEGF and RGCs. All of the histopathological and immunohistochemical examinations were conducted by a single blind pathologist.
Statistical analysis
Statistical analyses were undertaken using SPSS (version 13.0; SPSS Inc., Chicago, IL). The results were expressed as median (min–max) and mean ± SD. Kruskal–Wallis variance analysis was used to compare the groups. Then post hoc Mann–Whitney u test was used to determine which of the two groups leads to these significant differences. A post hoc p value less than 0.016 was considered statistically significant (Bonferroni corrections = 0.05/number of comparisons).
Results
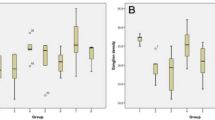
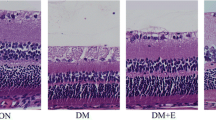
The thickness of the full-length retina was 79 μm (60–95), 105 μm (99–135), and 117 μm (106–171) in diabetic, ALA treatment, and control mouse retinas, respectively. The thickness of ONL was 19 μm (14–21), 27 μm (22–35), and 31 μm (23–45) in the diabetic, ALA treatment, and control mouse retinas, respectively. The thickness of INL was 10 μm (7–12), 14 μm (7–12), and 19 μm (17–25) in the diabetic, ALA treatment, and control mouse retinas, respectively. Diabetic mouse retinas showed a significant decrease in the thickness of the full-length retina, ONL, and INL compared to the control and ALA-treated mouse retinas (p = 0.001). The thicknesses of each layer among groups are shown in Table 2 per mouse. However, the thicknesses of these layers did not show a significant difference between the ALA treatment and control mouse retinas (p = 0.07) (Table 3). The numbers of ganglion cells were counted as 94 (80–100), 180 (160–192), and 185 (120–204) in the diabetic, ALA treatment, and control mouse retinas, respectively. H&E stained retinal sections showed a significant loss of ganglion cells in diabetic mouse retinas compared to the control and ALA-treated mouse retinas (p = 0.001). The number of ganglion cells did not show a significant difference between the control and ALA-treated mouse retinas (p = 0.7) (Fig. 1). The numbers of VEGF expressions were 40 (33–48), 22 (16–29), and 20 (18–26) in the diabetic, ALA treatment, and control mouse retinas respectively. VEGF expression was highest in the diabetic group compared to the other groups (p = 0.001) and the expression was mostly observed in the ganglion cell and inner nuclear layers. VEGF expression did not show a significant difference between the ALA-treated and control mouse retinas (p = 0.09) (Table 4). The VEGF staining in groups is illustrated in Fig. 2.
An illustration of the retinal sections of mouse retinas using H&E staining among the groups: a control mouse retina, b diabetic mouse retina, and c alpha-lipoic acid-treated mouse retina. Magnification ×400. GCL ganglion cell layer, IPL inner plexiform layer, INL inner nuclear layer, OPL outer plexiform layer, ONL outer nuclear layer, IS inner segment, OS outer segment, RPE retinal pigment epithelium, Chr choroid, L lens, arrow RGC
An illustration of immunohistochemical analysis of the retinas using anti-VEGF antibody among diabetic groups: a diabetic mouse retina, b control mouse retina, and c alpha-lipoic acid-treated mouse retina. Magnification ×400. GCL ganglion cell layer, IPL inner plexiform layer, INL inner nuclear layer, OPL outer plexiform layer, ONL outer nuclear layer, IS inner segment, OS outer segment, arrow VEGF staining in the GCL
Mice with STZ-induced diabetes and ALA treatment showed a significant decrease in body weight and a significant increase in blood glucose compared with non-diabetic controls (p < 0.05) (Table 1). No statistically significant difference was found between the final body weights and blood glucose concentrations in diabetic mice and diabetic mice treated with ALA. Treatment with ALA did not significantly change these metabolic variables in the diabetic mice (p > 0.05).
Discussion
In the present study, we immunohistochemically analyzed the retinas of diabetic, control, and ALA-treated mice by measuring the thicknesses of various retinal layers and by counting the RGCs in ganglion cell layer. Besides, we counted the VEGF expressions in the groups and compared with each other. Retinal ganglion cell counts were significantly reduced in the retinas of diabetic group compared to those of the control and ALA treatment groups. We also found statistically higher levels of VEGF in diabetic mouse retinas compared to the ALA-treated and control mouse retinas.
Chronic hyperglycemia causes different events such as the activation of aldose reductase metabolic pathway, activation of the diacylglycerol–protein kinase C pathway, and non-enzymatic glycation of proteins with the formation of advanced glycation/lipoxidation end products (AGE/ALE) with the release of superoxide radicals [11]. These reactive radicals, located near retinal capillaries, are responsible for the apoptosis of the retinal capillary cells (pericytes and endothelial cells), leading to the loss of pericytes and the formation of microaneurysm by mitochondrial DNA modifications [12]. Asnaghi et al. examined TUNEL-positive cells in diabetic rat retinas and found a four-fold increase in the number of apoptotic neurons [13]. These characteristic changes occur before the appearance of histopathological lesions of diabetic retinopathy despite good glycemic control [14].
Because there is a strong understanding that oxidative stress may be the trigger of all other pathologies implicated in the pathogenesis of diabetic retinopathy, the use of appropriate antioxidants may have therapeutic potential effects on the metabolic and functional abnormalities found in diabetic retinopathy [15].
Alpha-lipoic acid and its reduced form, dihydrolipoic acid, reduce oxidative stress by scavenging a number of free radicals such as superoxide, hydroxyl radicals, and singlet oxygen, by preventing membrane lipid peroxidation and protein damage through the redox regeneration of other antioxidants such as vitamins C and E. Additionally, Kawabata and Suzuki found the inhibitory effects of ALA on glycation process, which is one of the major pathways involved in the pathogenesis of diabetic retinopathy by non-covalent binding to albumin [16, 17]. ALA has also been shown to exhibit a protective effect against apoptosis. The anti-apoptotic effect of ALA has been shown in a study in which ALA decreased the number of apoptotic capillary cells and acellular capillaries in the retina of diabetic rats [18].
Retinal ganglion cells serve as an ideal model to investigate cell death and improve the novel treatments for retinal degeneration, particularly in diabetes [19, 20]. Rapid decreases in RGC counts in diabetic animals have been reported in various studies. For example, Zheng et al. observed a 20% loss of cells in the ganglion cell layer 4 months after the induction of diabetes [21]. In another study, STZ-induced diabetes resulted in an extensive loss of RGCs, with 20–25% fewer cells in the GCL compared with age-matched control mice after 14 weeks of diabetes [22]. Although we evaluated RGC decrease earlier (8 weeks after the induction of diabetes), our findings are consistent with the previous observations with reduced numbers of RGCs in diabetic retinas. This result shows that the loss of RGCs may occur by 8 weeks after the onset of diabetes. Moreover, in the present study the number of RGCs was found to be statistically higher in ALA-treated mouse retinas compared to the diabetic mouse retinas, supporting the idea that ALA treatment might have prevented cell death.
The thicknesses of the layers were also evaluated in diabetic mice. Barber et al. reported significant differences in the thickness of the INL as a consequence of diabetes [23]. Park et al. reported the thinning of INL and the marked thinning of ONL by 24 weeks after the onset of diabetes [24]. In the present study, thinning in both the inner and outer nuclear layers was significantly higher in diabetic mouse retinas compared to the ALA-treated mouse retinas which might turn out the protective effect of ALA against neuronal cell loss.
VEGF, an angiogenesis inducer, plays a pivotal role in diabetic retinopathy and is implicated as the mediator and an initiator of non-proliferative and proliferative diabetic retinopathies. Local hypoxia and the increased levels of inflammatory cytokines, AGEs, and ROS that occur in diabetes can induce VEGF gene expression [25, 26]. As AGEs were shown to play a role in angiogenesis by the induction of VEGF production in diabetic retinopathy, non-enzymatic glycation inhibitors were found to suppress retinal VEGF immunoreactivity in the experimental models [27, 28]. In the present study, we evaluated the effect of ALA on VEGF expression and compared the results between groups. As shown in Fig. 2a, diabetic mouse retinas showed statistically higher levels of VEGF expression compared to the control and ALA-treated mouse retinas, which is mostly localized in the GCL and inner retinal layers. Median VEGF levels were similar between the ALA-treated and control mouse retinas (p = 0.09).
The mechanism by which ALA may inhibit the production of VEGF was elicited in some studies. In an experimental diabetic rat model, after rats had been made diabetic, they were treated with ALA and VEGF levels were measured and compared with the untreated diabetic mouse retinas. Retinal VEGF upregulation occured as early as 6 weeks after diabetes induction and VEGF levels were found to be lower in ALA-treated mouse retinas compared to the diabetic mouse retinas [29]. Lee et al. evaluated the effect of ALA on the formation of ROS and angiogenic factors. In diabetic rats, the administration of ALA reduced the production of ROS. Treatment with ALA suppressed the expression of VEGF, angiopoietin 2, and erythropoietin induced by high glycemic levels [30]. In an another study, ALA was shown to inhibit diabetes-induced activation of two oxidants, such as nitrotyrosine and nuclear transcription factor (NF-kB), which have been postulated to play roles in the pathogenesis of VEGF secretion in diabetic rat retinas [18]. In our study, decreased levels of VEGF expression found in the ALA-treated mouse retinas might be the result of the antioxidative and inhibitory effects of ALA on the glycation process.
In the present study, since we did not observe any histopathologic changes demonstrating diabetic retinopathy, we assumed our retinas as preretinopathic diabetic retinas. Recently, the effects of ALA on preretinopathic diabetic patients were also evaluated by some authors. In a study, preretinopathic diabetic patients were randomized into two groups: one of which received oral AO treatment with ALA and the other group received a placebo treatment. The concentration of free radicals and AOs in plasma was measured by analytical techniques, and ERG was performed to measure oscillatory potentials (OPs) in patients. Plasma AO levels and ERG OP oscillatory potential values were found to be increased in the group treated with ALA compared to the control group. Results of this study suggested that ALA treatment might have a protective effect on retinal cells, in preretinopathic diabetic subjects, as detected by ERG analysis [31].
Numerous early retinal function tests in diabetic patients and molecular studies in the retinas of experimental diabetic animals suggest that neurons are vulnerable to damage shortly after the onset of diabetes. Alpha-lipoic acid has been used with the aim of preventing the initial damage in experimental diabetic models. In a study aiming to evaluate the early effects of ALA treatment, glutathione (GSH) content, malondialdehyde (MDA) concentration, and glutathione peroxidase (GPx) activity, which are the indicators of oxidative stress, were measured 3 weeks after diabetes induction in mouse retinas. Retinal function of diabetic mice was also studied by electroretinography (ERG), which was used to detect early signs of retinal damage. Early administration of lipoic acid to diabetic mice prevented the statistically significant decrease of GSH content and GPx activity and normalized MDA concentration. The b-wave amplitude was reduced, indicating the onset of early damage in retinal function caused by diabetes. The administration of ALA restored b-wave amplitude by 70% in a statistically significant manner [32].
There are various routes of administration of ALA such as oral and intraperitoneal (i.p.) use in various animal models. Although oral ALA is used, the most common approach for the administration of ALA is intraperitoneal administration in rats and mouse models. Although up to 90% of an orally administered dose is absorbed from the gut, gastrointestinal absorption of ALA may be variable [33]. That is why in the present study we preferred i.p. use of ALA instead of oral intake. In the diabetic neuropathy clinical trials, the oral dose of ALA varied among trials in the range of 100–1800 mg/day and IV dose varied from 600 to 1200 mg/day. In this study, we used a 100 mg/kg dose in mice, which can be translated to approximately 10 mg/kg in humans. This dose is considered safe in human clinical trials [34].
Our result shows that alpha-lipoic acid treatment reduces VEGF levels, protects ganglion cells, and preserves the inner and outer layer thicknesses. To our knowledge, our study is the first to analyze these effects together on mouse retinas. We think that ALA may have a potential therapeutic benefit in both neuroprotection and inhibition of early neovascularization in preretinopathic retinas. Since oxidative stress plays a major role in the pathogenesis of DR, we think that the observed beneficial effects of ALA may make it a candidate as an early adjunct therapeutic supplement to limit the initial damage and slow down or even prevent the onset of diabetic retinopathy. However, further studies are recommended to elucidate its role in diabetes mellitus.
References
Di Leo MA, Ghirlanda G, Gentiloni Silveri N, Giardina B, Franconi F, Santini SA (2003) Potential therapeutic effect of antioxidants in experimental diabetic retina: a comparison between chronic taurine and vitamin E plus selenium supplementations. Free Radic Res 37:323–330
Xu X, Zhu QI, Xia X, Zhang S, Gu Q, Luo D (2004) Blood-retinal barrier breakdown induced by activation of protein kinase C via vascular endothelial growth factor in streptozotocin-induced diabetic rats. Curr Eye Res 28:251–256
Lee P, Wang CC, Adamis AP (1998) Ocular neovascularization: an epidemiologic review. Surv Ophthalmol 43:245–269
Pe’er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E (1995) Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest 72:638–645
Obrosova IG, Minchenko AG, Marinescu V, Fathallah L, Kennedy A, Stockert CM, Frank RN et al (2001) Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia 44:1102–1110
Pierce EA, Avery RL, Foley ED, Aiell LP, Smith LE (1995) Vascular endothelial growth factor/vascular permeability factor expression in mouse model of retinal neovascularization. Proc Natl Acad Sci USA 92:905–909
Funatsu H, Yamashita H (2002) Pathophysiology of diabetic retinopathy. Drug News Perspect 15:633–639
Packer L, Kraemer K, Rimbach G (2001) Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 17:888–895
Santos JM, Kowluru RA (2011) Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Invest Ophthalmol Vis Sci 52:8791–8798
Obrosova IG, Fathallah L, Liu E, Nourooz-Zadeh J (2003) Early oxidative stress in the diabetic kidney: effect of DL-(alpha)-lipoic acid. Free Rad Biol Med 34:186–195
Kowluru RA, Chan PS (2007) Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007:43603
Mohammad G, Kowluru RA (2010) Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab Invest 90:1365–1372
Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M (2003) A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes 52:506–511
Engerman RL, Kern TS (1987) Progression of incipient diabetic retinopathy during good glycemic control. Diabetes 36:808–812
Kowluru RA, Tang J, Kern TS (2001) Abnormalities of retinal metabolism in diabetes and experimental galactosemia VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 50:1938–1942
Kawabata T, Packer L (1994) Alpha-lipoate can protect against glycation of serum albumin, but not low density lipoprotein. Biochem Biophys Res Commun 30:99–104
Suzuki YJ, Tsuchiya M, Packer L (1992) Lipoate prevents glucose-induced protein modifications. Free Radic Res Commun 17:211–217
Kowluru RA, Odenbach S (2004) Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes 53:3233–3238
Kawasaki A, Han MH, Wei JY, Hirata K, Otori Y, Barnstable CJ (2002) Protective effect of arachidonic acid on glutamate neurotoxicity in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 43:1835–1842
Lafuente MP, Villegas-Perez MP, Selles-Navarro I, Mayor- Torroglosa S, Miralles de Imperial J, Vidal-Sanz M (2002) Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience 109:157–168
Zhen SJ, Howel L, Hatala DA, Huang K, Kern TS (2007) Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes 56:337–345
Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB (2004) Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 45:3330–3336
Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW (1998) Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102:783–791
Park SH, Park JW, Park S (2003) Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia 46:1260–1268
Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Caldwell RW (2003) Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 19:442–455
Nakamura N, Hasegawa G, Obayashi H, Yamazaki M, Ogata M, Nakano K, Yoshikawa T et al (2003) Increased concentration of pentosidine, an advanced glycation end product, and interleukin-6 in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Res Clin Pract 61:93–101
Cohen MP, Hud E, Wu VY, Shearman CW (2008) Amelioration of diabetes-associated abnormalities in the vitreous fluid by an inhibitor of albumin glycation. Invest Ophthalmol Vis Sci 49:5089–5093
Frank RN, Amin R, Kennedy A, Hohman T (1997) An aldose reductase inhibitor and aminoguanidine prevent vascular endothelial growth factor expression in rats with long-term galactosemia. Arch Ophthalmol 115:1036–1047
Obrosova IG, Fathallah L, Greene DA (2000) Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: effect of d-l-alpha-lipoic acid. Eur J Pharmol 9:139–146
Lee SG, Lee CG, Yun IH, Hur DY, Yang JW, Kim HW (2012) Effect of lipoic acid on expression of angiogenic factors in diabetic rat retina. Clin Exp Ophthalmol 40:47–57
Nebbioso M, Federici M, Rusciano D, Evangelista M, Pescosolido N (2012) Oxidative stress in preretinopathic diabetes subjects and antioxidants. Diabetes Technol Ther 14:257–263
Johnsen-Soriano S, Garcia-Pous M, Arnal E, Sancho-Tello M, Garcia-Delpech S, Miranda M, Bosch-Morell F et al (2008) Early lipoic acid intake protects retina of diabetic mice. Free Radic Res 42:613–617
Harrison EH, McCormick DB (1974) The metabolism of dl-(1,6-14C)lipoic acid in the rat. Arch Biochem Biophys 160:514–522
Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R et al (2011) Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care 9:2054–2060
Funding
Partial support for this work was provided by Samsun Training and Research Hospital Research Board.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kan, E., Alici, Ö., Kan, E.K. et al. Effects of alpha-lipoic acid on retinal ganglion cells, retinal thicknesses, and VEGF production in an experimental model of diabetes. Int Ophthalmol 37, 1269–1278 (2017). https://doi.org/10.1007/s10792-016-0396-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-016-0396-z