Abstract
Background
The pivotal role of oxidative stress and inflammation in the pathophysiology of type 2 diabetes mellitus (T2DM) has been firmly established. However, the evidence concerning hypoglycaemic medicinal plants' antioxidant and anti-inflammatory effects remains inconclusive due to inconsistencies in prior studies. To address this gap, our study aims to perform a comprehensive systematic review and meta-analysis of randomized controlled trials (RCTs) to consolidate previous research findings in this field.
Methods
We conducted a comprehensive search in the PubMed, Web of Science, Embase, Cochrane Library, and Scopus databases to identify relevant English randomized controlled trials (RCTs). Our study adhered to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines. All eligible studies that evaluated concurrently the antioxidative and anti-inflammatory effects of hypoglycaemic plant-derived supplements on type 2 diabetes mellitus (T2DM) were included in the meta-analysis. The meta-analysis itself was carried out using both fixed and random effects models to synthesize the findings from the selected studies.
Results
Our study included 47 trials with a total of 2636 participants, both male and female, aged between 20 and 79 years, diagnosed with prediabetes, type 2 diabetes mellitus (T2DM), or metabolic syndrome. The meta-analysis revealed that plant-derived treatments, compared to placebos or other medicines, significantly improved oxidative stress (SMD = − 0.36, 95% CI − 0.64 to − 0.09), inflammation (SMD = − 0.47, 95% CI − 0.63 to − 0.31), total antioxidant capacity (SMD = 0.46, 95% CI 0.16–0.75), and antioxidant enzyme activity (SMD = 1.80, 95% CI 1.26–2.33). The meta-regression analysis showed that treatment duration exceeding 8 weeks significantly impacted the heterogeneity of the oxidative stress data.
Conclusions
Several hypoglycaemic plant-based treatments appear to positively affect T2DM patients by concurrently lowering oxidative stress and inflammatory indicators and boosting antioxidant enzyme activity.
Clinical Trail Registry
PROSPERO ID: CRD42021226147.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most common chronic diseases, with a prevalence estimated to exceed 536.6 million patients and expected to reach 783.2 million patients by 2045 (Sun et al. 2022). The increasing prevalence of T2DM is attributed to an aging population, unhealthy lifestyle behaviors, and the increased prevalence rate of T2DM risk factors, particularly obesity (Chan et al. 2020). Diabetes is a multifactorial disease caused by the interaction of genetic and environmental factors. It is associated with high morbidity and mortality worldwide (Vassalle and Gaggini 2022). T2DM is characterized by chronic hyperglycemia due to insulin resistance, β-cell dysfunction, or both (Giacco and Brownlee 2010). Under hyperglycemic conditions, increased glucose uptake leads to elevated oxidative stress markers. Consequently, this rise in oxidative stress markers can trigger the activation of inflammatory markers like interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). The interplay between inflammatory and oxidative stress pathways may contribute to T2DM dysfunction and complications (Vassalle and Gaggini 2022). It has been suggested that reducing oxidative stress and inflammation may be a target for developing novel antidiabetic medications (Vassalle and Gaggini 2022).
Current medications for T2DM are often costly and associated with various side effects, including severe hypoglycemia (Vassalle and Gaggini 2022). As an alternative, herbal medicines offer a promising avenue for developing new treatments due to their widespread availability, affordability, and relatively safe profile (Thomford et al. 2018; Soltani et al. 2022). An excellent example is metformin, the first-line drug for T2DM, which originated from Galega officinalis, a herbal medicine utilized in Europe to manage diabetes (Bailey 2017). Current evidence suggests that various hypoglycaemic herbal medicines can significantly reduce inflammation and oxidative stress biomarkers in diabetes (Atkin et al. 2016; Ebrahimpour koujan et al. 2015; Ghafouri et al. 2020). Hence, plants offer a valuable resource for developing novel antidiabetic drugs due to their antioxidant and anti-inflammatory effects. Since there are inconsistent results regarding the use of herbal medicines with anti-inflammatory and antioxidant properties on T2DM (Adab et al. 2019; Alnajjar et al. 2020; Azimi et al. 2014; Basu et al. 2013), the current systematic review and meta-analysis were conducted to critically assess all the trials that simultaneously evaluated the antioxidative and anti-inflammatory activities of hypoglycaemic herbal medicinal on T2DM.
Methods
This systematic review and meta-analysis study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Page et al. 2021). It was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the ID: CRD42021226147. In addition, the ethics committee of the Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences has authorized this study (IR.TUMS.EMRI.REC.1397.046).
Search strategy
A comprehensive and systematic search was conducted through PubMed, Web of Science, Embase, Cochrane Library, and Scopus databases. The following keywords were used: (“herbal medicine”, “diabetes”, “inflammation”, “antioxidant”, the name of each oxidative or inflammatory biomarker, antioxidative enzyme, and their equivalents) to retrieve all clinical trials published in English from their inception to April 2022. In addition, the reference lists of all relevant articles were screened to find any eligible studies that might have been missed. The search strategy is available in Table S1.
Study selection and eligibility criteria
All clinical trials conducted in English that met the following criteria were included in this study: (1) evaluated the effect of extract or powder of hypoglycaemic herbal medicine simultaneously on oxidative and inflammatory biomarkers; (2) conducted on patients with prediabetes, T2DM, or metabolic syndrome; (3) compared the effectiveness of herbal medicines to no treatment, placebo, or conventional pharmacological treatments. Clinical trials on type 1 diabetes mellitus, preclinical studies, review articles, letters, thesis, and unpublished data were excluded. At least two independent researchers evaluated all studies according to inclusion/exclusion criteria during the screening phase. After removing duplicates, the publications’ titles, abstracts, and full text were evaluated to see whether they met the inclusion criteria. Any discrepancies at each screening step were discussed and resolved in consultation with the corresponding author.
Quality assessment of included studies
Methodological quality assessment of each included study was independently performed by at least two researchers using the modified Jadad checklist (Oremus et al. 2001). Clinical trials with a score < 3 were rated as poor quality. In addition, the risk of bias for each study was assessed using the Cochrane Risk of Bias tool (Higgins et al. 2011), where the domains were judged as “low-risk, high-risk, or unclear”.
Data extraction
From the included studies, the following data were extracted: (1) Study characteristics, such as author name, year of publication, study design, and duration of intervention; (2) Patient characteristics, such as age, gender, number of patients in each group, and underlying disease; (3) Specific information regarding the interventions, such as herbal medicine name, intervention modality, dosage, and duration of treatment, as well as type of intervention for the control group, including placebo, standard pharmacological treatments, or no treatment; and (4) Primary outcomes (measurements of antioxidative and anti-inflammatory biomarkers) and secondary outcomes (total antioxidant capacity and antioxidant enzymes activity).
Statistical analysis
All data were extracted from the included studies using mean difference (MD) and standard deviation (SD) for both the intervention and control groups. When the standard error (SE) or confidence interval (CI) was reported, it was transformed into SD. Using standard statistical formulas, the SD of change was calculated based on the baseline and final SDs (Higgins et al. 2019). Clinical and methodological heterogeneity was statistically evaluated using the chi-square and I2 tests, and P < 0.1 was considered statistically significant (Ioannidis 2008). To determine the pooled effect of the herbal medicine, random- or fixed-effect methods were employed to conduct the meta-analysis based on the level of heterogeneity between studies.
Furthermore, a random-effect meta-regression analysis was performed to evaluate the impact of influencing factors (Higgins et al. 2011). Forest plots were used to show the findings of the meta-analysis schematically. STATA software, version 14.0 (StataCorp, College Station, TX, USA), was used for all analyses.
Results
General results
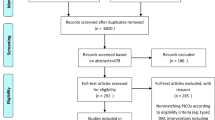
Out of 6209 studies, 47 clinical trials were eligible for inclusion in the meta-analysis (Fig. 1). A total of 2636 participants, including men and women aged between 20 and 79 years, were included in these studies. Forty-four studies were conducted on patients with T2DM, two on metabolic syndrome patients, and one on patients with prediabetes. The design of most studies was a double-blind, randomized controlled trial (DBRCT) in which the use of a sole plant was compared to a placebo. Some of the herbal medicines used include turmeric, bilberry, M. officinalis, garlic, cardamom, cinnamon, ginger, saffron, pomegranate, Camellia sinensis, Cichorium intybus, Crataegus laevigata, silymarin, Rheum ribes, Nigella sativa, Anethum graveolens, grape, or blueberry. Details of the included studies and characteristics of the participants are shown in Table 1. The quality score of most studies was ≥ 3. The quality assessment results based on the modified Jadad checklist and the Cochrane risk of bias assessment are presented in Tables 1 and 2, respectively.
Meta-analyses of the main outcomes
Total change in oxidative stress biomarkers, including malondialdehyde (MDA), nitric oxide (NO), and F2-isoprostane, was assessed in 30 studies (68 data), which pooled data showed a significant reduction with a standardized mean difference (SMD) of − 0.36 and 95% CI − 0.64 to − 0.09 (P < 0.001) in a random-effect meta-analysis (I2: 91.9%), Fig. 2.
Total change in inflammatory biomarkers, including high-sensitivity C-reactive protein (hs-CRP), TNF-α, IL-6, and CRP, was measured in 45 studies (98 data) and showed a significant reduction (P < 0.001) with an SMD = − 0.47 and 95% CI − 0.63 to − 0.31 in the random-effect meta-analysis (I2: 85.4%), Fig. 3.
Meta-analyses of the secondary outcomes
Total antioxidant capacity (TAC) was measured in 27 studies (28 data). It was significantly (P = 0.002) improved with an SMD = 0.46 and 95% CI 0.16–0.75 in a random-effect meta-analysis (I2: 86.9%), Fig. S1.
The activity of antioxidant enzymes, including catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GPX), was assessed in 14 studies (37 data). The pooled results showed a significant (P < 0.001) increase in total activity (SMD = 1.80, 95% CI 1.26–2.33), in the random-effect meta-analysis (I2: 94.9%), Fig. S2.
Subgroup analyses
In subgroup analyses, a significant change was observed in oxidative stress biomarkers, including MDA and NO (SMDs = − 0.96 and 1.01, 95% CIs − 1.30 to − 0.63 and 0.63 to 1.40, respectively). However, the change was not significant (P ≥ 0.05) for F2-isoprostane (SMD = − 0.67 95% CI − 1.42 to 0.09). Regarding the inflammatory biomarkers, subgroup analyses showed significant changes for hs-CRP and TNF-α (SMDs = − 0.64 and − 0.71, 95% CIs − 0.84 to − 0.44, and − 1.18 to − 0.24, respectively). On the other hand, the change for IL-6 and CRP were not significant (P ≥ 0.05) (SMDs = − 0.14 and 0.25, 95% CIs − 0.28 to 0.005 and − 0.01 to 0.52, respectively).
Except for CAT (SMD = 0.24, 95% CI − 0.19 to 0.67), the subgroup analysis antioxidant enzyme revealed a significant increase in SOD (SMD = 2.29, 95% CI 0.97–3.62) and a significant decrease in GSH (SMD = 1.44, 95% CI 0.79–2.10) and GPX (SMD = 3.29, 95% CI 0.79–5.79).
Additional subgroup analyses were performed to assess the effect of the herbal medicines on primary and secondary outcomes compared to placebo/non-placebo treatment in the control group and also based on the duration of intervention; ≤ 8 weeks vs. > 8 weeks. The details of the analyses are shown in Table S2.
Meta-regression test
A meta-regression analysis was performed to determine the sources of the heterogeneity. The results of this analysis demonstrated that the type of treatment in the control groups (placebo/non-placebo) and the duration of the intervention (≤ 8 weeks vs. > 8 weeks) were not the sources of heterogeneity observed in the results of primary and secondary outcomes. However, biomarkers of oxidative stress were significantly affected by the duration of the intervention (coefficient = 1.31 for > 8 weeks, P = 0.027).
Discussion
The present meta-analysis demonstrated a simultaneous and significant reduction in oxidative stress and inflammatory biomarkers, along with a noteworthy increase in TAC and antioxidant enzyme activity.
Recognizing the escalating global prevalence of T2DM and the limited accessibility of conventional antidiabetic medications, the World Health Organization (WHO 2013) advocates for the utilization of accessible and indigenous natural products. Consequently, the use of herbal medicines is on the rise in both developed and developing countries (WHO 2013), underscoring the need for a scientific evaluation of their efficacy and safety (WHO 2013). It is essential to note that meta-analysis studies rank as the highest level of evidence-based medicine, contributing valuable scientific insights (Howick et al. 2022). Our meta-analysis revealed that when a placebo was administered to the control group, the effect size was more significant, suggesting that a standard trial design yields a more reliable effect. A standard trial design eliminates the effect of chance on the trial outcomes through randomization, blinding, and placebo (Ahmad et al. 2021).
Curcuma longa L. root (turmeric), the rhizome of Zingiber officinale Roscoe (ginger), stigmas of Crocus sativus L. (saffron), Vaccinium spp. fruits (bilberry), fruit extract of Punica granatum L. (pomegranate extract), Nigella sativa L. oil extract, and Elettaria cardamomum (L.) Maton (cardamom) were the extensively studied herbal medications in this study. Remarkably, these medicinal plants are commonly used as spices or food additives in daily culinary practices, suggesting their safety even over prolonged periods. Utilizing herbal medicines as spices or food additives could pave the way for functional foods, offering a promising approach to harnessing food as a medicine without the risk of adverse effects or non-adherence, which often presents a significant barrier to conventional pharmacological therapies (Nieto 2020).
Plant-derived supplementations contain numerous bioactive compounds responsible for their pharmacological effects. Therefore, it is valuable to identify these bioactive compounds and investigate their pharmacological effects alone or in combination to determine synergistic, additive, or antagonistic effects (Soltani et al. 2022). On the contrary, each active compound within herbal medicine may exhibit its beneficial effects by acting through distinct pathways. This implies that herbal medicines containing multiple active compounds can simultaneously exert therapeutic effects through multiple pathways. This may make the combination of the active compounds of a herb more effective than using a single ingredient (Yuan et al. 2017). Therefore, it is necessary to apply modern pharmacological science in studying the beneficial effects of polyherbal medicine.
Terpenoids, carotenoids, and phenolic compounds such as phenolic acid, flavonoids, and stilbenoids were the primary active compounds detected in these hypoglycaemic plant-derived medicines. These bioactive compounds could exert their pharmacological effects through attenuation of oxidative stress, inhibition of inflammation, protection of pancreatic beta cells, activation of insulin signaling, stimulation of insulin secretion, and beneficial effects on glucose metabolism (Smitha Grace et al. 2019; Sun et al. 2020; Zhang and Tsao 2016).
A randomized controlled trial (Maithili Karpaga Selvi et al. 2015) investigated the efficacy of turmeric, with curcumin as its main bioactive compound, as an adjuvant to conventional therapy in T2DM patients. This study showed that consuming four 500-mg capsules daily along with metformin had more beneficial effects than taking metformin alone. It effectively reduced oxidative stress and inflammatory biomarkers and increased TAC without any significant effect on antioxidant enzymes, possibly due to its short duration (4 weeks). Turmeric exerts protective anti-inflammatory effects by inhibiting the synthesis of proinflammatory cytokines and mediators such as cyclooxygenase-2, prostaglandins, and leukotrienes (Maithili Karpaga Selvi et al. 2015). The antioxidant effects of curcumin are mediated by scavenging free radicals, inhibiting the production of lipid peroxides, increasing TAC, the expression of antioxidant enzymes, and enhancing Sirtuin 3 (SIRT3) activity (Bankowski et al. 2022). However, several meta-analysis studies have separately reported curcumin's anti-inflammatory and antioxidant effects (Bengmark 2006; Derosa et al. 2016; Gorbi et al. 2021). In another study (Shidfar et al. 2015), in a DBRCT, the effect of daily intake of 3 g of ginger, in which phenolic and terpenoid compounds are its main bioactive compounds, on glycemic control, oxidative stress, and inflammatory biomarkers in a 12-week trial had been investigated. Their results showed a significant reduction in MDA and CRP and a significant increase in TAC. The potential mechanism of the antioxidative effect of ginger is induced via activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. The underlying mechanisms of its anti-inflammatory effects are the inhibition of phosphatidyl inositol-3-kinase (PI3K)/ protein kinase B (Akt) and the nuclear factor kappa light chain-enhancer of activated B cells (NF-κB) signaling pathways (Mao et al. 2019). Some of its antioxidative and anti-inflammatory effects include reducing free radicals, lipid peroxidation, and nitrosative stress levels, accelerating gene expression, increasing the level and activity of antioxidant enzymes, and reducing proinflammatory cytokines. In a recently published meta-analysis, the beneficial effects of ginger supplementation on hs-CRP, IL-6, and TNF-α levels in T2DM have been shown. These results align with our findings (Mohammad et al. 2021).
Based on our knowledge, the current meta-analysis is the first study that assessed the simultaneous antioxidative and anti-inflammatory effects of plant-derived anti-diabetic medicines on T2DM. The main strength of this review is that most of the included studies are randomized, double-blind, controlled trials with low levels of bias. However, we had several limitations. There was high heterogeneity across studies due to differences in plant species, dosage, duration of interventions, and methods of measuring oxidative stress and inflammatory biomarkers. Moreover, there were differences between the sources of each herb, the part of the plant used, their geographical area, and their preparation method (bulk, powder, or extract), which have been found to cause heterogeneity between the studies (Ahmad et al. 2021).
Conclusions
Some hypoglycaemic plant-based treatments have a positive effect on T2DM patients by concurrently reducing the serum level of oxidative stress and inflammatory indicators and boosting antioxidant enzyme activity. Further research is warranted to explore the antioxidative and anti-inflammatory effects of hypoglycaemic plant-derived medicines, which would enhance our understanding of their active ingredients and shed light on their therapeutic and adverse effects. To achieve this, well-designed trials with substantial sample sizes should be conducted, employing gold-standard methods for measuring and reporting outcomes, allowing for a comprehensive assessment of their impact through comparative data analysis.
Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.
Abbreviations
- Akt:
-
Protein kinase B
- CAT:
-
Catalase
- CI:
-
Confidence interval
- DBRCT:
-
Double-blind, randomized controlled trial
- GPx:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- hs-CRP:
-
High-sensitivity C-reactive protein
- IL-6:
-
Interleukin-6
- MD:
-
Mean difference
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor kappa light chain-enhancer of activated B cells
- NO:
-
Nitric oxide
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- PI3K:
-
Phosphatidyl inositol-3-kinase
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- SD:
-
Standard deviation
- SE:
-
Standard error
- SIRT:
-
Sirtuin 3
- SMD:
-
Standardized mean difference
- SOD:
-
Superoxide dismutase
- T2DM:
-
Type 2 diabetes mellitus
- TAC:
-
Total antioxidant capacity
- TNF-α:
-
Tumor necrosis factor-α
- WHO:
-
World Health Organization
References
Adab Z, Eghtesadi S, Vafa MR et al (2019) Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytother Res 33(4):1173–1181
Ahmad R, AlLehaibi LH, AlSuwaidan HN et al (2021) Evaluation of clinical trials for natural products used in diabetes: an evidence-based systemic literature review. Medicine 100(16):e25641
Alnajjar M, Kumar Barik S, Bestwick C et al (2020) Anthocyanin-enriched bilberry extract attenuates glycaemic response in overweight volunteers without changes in insulin. J Funct Foods 64:103597
Asadi A, Shidfar F, Safari M et al (2019) Efficacy of Melissa officinalis L. (lemon balm) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a randomized, double-blind, clinical trial. Phytother Res 33(3):651–659
Atkin M, Laight D, Cummings MH (2016) The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress, and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double-blind randomized placebo controlled trial. J Diabetes Complicat 30(4):723–727
Azimi P, Ghiasvand R, Feizi A et al (2014) Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud 11(3–4):258–266
Bailey CJ (2017) Metformin: historical overview. Diabetologia 60(9):1566–1576
Bańkowski S, Petr M, Rozpara M et al (2022) Effect of 6-week curcumin supplementation on aerobic capacity, antioxidant status and sirtuin 3 level in middle-aged amateur long-distance runners. Redox Rep 27(1):186–192
Basu A, Newman ED, Bryant AL et al (2013) Pomegranate polyphenols lower lipid peroxidation in adults with type 2 diabetes but have no effects in healthy volunteers: a pilot study. J Nutr Metab 2013:1
Bazyar H, Hosseini SA, Saradar S et al (2021) Effects of epigallocatechin-3-gallate of Camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: a clinical trial. J Complementary Integr Med 18(2):405–411
Bengmark S (2006) Curcumin, an atoxic antioxidant and natural NfκB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. J Parenter Enter Nutr 30(1):45–51
Chan JC, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P et al (2020) The lancet commission on diabetes: using data to transform diabetes care and patient lives. Lancet 396(10267):2019–2082
Chan SW, Chu TTW, Choi SW, Benzie IFF, Tomlinson B (2021) Impact of short-term bilberry supplementation on glycemic control, cardiovascular disease risk factors, and antioxidant status in Chinese patients with type 2 diabetes. Phytother Res 35(6):3236–3245
Chandra K, Jain V, Jabin A et al (2020) Effect of Cichorium intybus seeds supplementation on the markers of glycemic control, oxidative stress, inflammation, and lipid profile in type 2 diabetes mellitus: a randomized, double-blind placebo study. Phytother Res 34(7):1609–1618
Dalli E, Colomer E, Tormos MC et al (2011) Crataegus laevigata decreases neutrophil elastase and has hypolipidemic effect: a randomized, double-blind, placebo-controlled trial. Phytomedicine 18(8–9):769–775
Darmian MA, Hoseini R, Amiri E, Golshani S (2022) Downregulated hs-CRP and MAD, upregulated GSH and TAC, and improved metabolic status following combined exercise and turmeric supplementation: a clinical trial in middle-aged women with hyperlipidemic type 2 diabetes. J Diabetes Metab Disord 21(1):275–283
Derosa G, Maffioli P, Simental-Mendía LE, Bo S, Sahebkar A (2016) Effect of curcumin on circulating interleukin-6 concentrations: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 111:394–404
Ebrahimi F, Sahebkar A, Aryaeian N et al (2019) Effects of saffron supplementation on inflammation and metabolic responses in type 2 diabetic patients: a randomized, double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes 12:2107
Ebrahimpour Koujan S, Gargari BP, Mobasseri M, Valizadeh H, Asghari-Jafarabadi M (2015) Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: a randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine 22(2):290–296
Fallahzadeh MK, Dormanesh B, Sagheb MM et al (2012) Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis 60(6):896–903
Ghafouri A, Hajiluian G, Karegar SJ et al (2020) The effect of aqueous, ethanolic extracts of Rheum ribes on insulin sensitivity, inflammation, oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. J Herbal Med 24:100389
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070
Gorabi AM, Razi B, Aslani S et al (2021) Effect of curcumin on proinflammatory cytokines: a meta-analysis of randomized controlled trials. Cytokine 143:155541
Grabež M, Škrbić R, Stojiljković MP et al (2022) A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev Cardiovasc Med 23(2):57
Hadi S, Mirmiran P, Daryabeygi-Khotbesara R, Hadi V (2018) Effect of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress among people with type 2 diabetes mellitus: a randomized, double-blind, placebo controlled trial. Prog Nutr 20:127–133
Haidari F, Zakerkish M, Borazjani F, Ahmadi Angali K, Amoochi Foroushani G (2020) The effects of Anethum graveolens (dill) powder supplementation on clinical and metabolic status in patients with type 2 diabetes. Trials. https://doi.org/10.1186/s13063-020-04401-3
Hemmatabadi M, Abdollahi M, Bakhshayeshi S et al (2009) Benefits of Semelil (ANGIPARS™) on oxidant-antioxidant balance in diabetic patients; a randomized, double-blind placebo controlled clinical trial. Daru 17(SUPPL. 1):50–55
Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Higgins JP, Thomas J, Chandler J et al (2019) Cochrane handbook for systematic reviews of interventions, 2nd edn. John Wiley & Sons, Chichester (UK)
Howick J, Chalmers I, Glasziou P, et al (2022) OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Oxford Centre for Evidence-Based Medicine Available at: http://www.cebmnet/indexaspx?o=5653. accessed: 2022. 2011
Ioannidis JP (2008) Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract 14(5):951–957
Javid AZ, Bazyar H, Gholinezhad H et al (2019) The effects of ginger supplementation on inflammatory, antioxidant, and periodontal parameters in type 2 diabetes mellitus patients with chronic periodontitis under non-surgical periodontal therapy. A double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes Targets Ther 12:1751–1761
Kanellos PT, Kaliora AC, Tentolouris NK et al (2014) A pilot, randomized controlled trial to examine the health outcomes of raisin consumption in patients with diabetes. Nutrition 30(3):358–364
Kar P, Laight D, Rooprai HK, Shaw KM, Cummings M (2009) Effects of grape seed extract in type 2 diabetic subjects at high cardiovascular risk: a double-blind randomized placebo-controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med 26(5):526–531
Kazemi S, Yaghooblou F, Siassi F et al (2017) Cardamom supplementation improves inflammatory and oxidative stress biomarkers in hyperlipidemic, overweight, and obese pre-diabetic women: a randomized double-blind clinical trial. J Sci Food Agric 97(15):5296–5301
Kempf K, Herder C, Erlund I et al (2010) Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr 91(4):950–957
Kim HJ, Yoon KH, Kang MJ et al (2012) A six-month supplementation of mulberry, Korean red ginseng, and Banaba decreases biomarkers of systemic low-grade inflammation in subjects with impaired glucose tolerance and type 2 diabetes. Evid Based Complement Alternat Med 2012:735191
Kim H, Simbo SY, Fang C et al (2018) Açaí (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct 9(6):3097–3103
Kooshki A, Tofighiyan T, Rastgoo N, Rakhshani MH, Miri M (2020) Effect of Nigella sativa oil supplement on risk factors for cardiovascular diseases in patients with type 2 diabetes mellitus. Phytother Res 34(10):2706–2711
Legiawati L, Bramono K, Indriatmi W et al (2020) Oral and topical Centella asiatica in type 2 diabetes mellitus patients with dry skin: a three-arm prospective randomized double-blind controlled trial. Evid Based Complement Alternat Med 2020:1
Maithili Karpaga Selvi N, Sridhar MG, Swaminathan RP, Sripradha R (2015) Efficacy of turmeric as adjuvant therapy in type 2 diabetic patients. Indian J Clin Biochem 30(2):180–186
Mao Q-Q, Xu X-Y, Cao S-Y et al (2019) Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 8(6):185
Mohammad A, Falahi E, Yusof B-NM et al (2021) The effects of the ginger supplements on inflammatory parameters in type 2 diabetes patients: a systematic review and meta-analysis of randomised controlled trials. Clin Nutr ESPEN 46:66–72
Musolino V, Gliozzi M, Bombardelli E et al (2020) The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J Tradit Complement Med 10(3):268–274
Nair AR, Mariappan N, Stull AJ, Francis J (2017) Blueberry supplementation attenuates oxidative stress within monocytes and modulates immune cell levels in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Food Funct 8(11):4118–4128
Neyestani TR, Shariatzade N, Kalayi A et al (2010) Regular daily intake of black tea improves oxidative stress biomarkers and decreases serum C-reactive protein levels in type 2 diabetic patients. Ann Nutr Metab 57(1):40–49
Nieto G (2020) How are medicinal plants useful when added to foods? Medicines (Basel) 7:58. https://doi.org/10.3390/medicines7090058
Nigam V, Nambiar VS (2019) Aegle marmelos leaf juice as a complementary therapy to control type 2 diabetes—randomised controlled trial in Gujarat. India Adv Integr Med 6(1):11–22
Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y (2001) Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord 12(3):232–236
Organization WH (2013) WHO traditional medicine strategy: 2014–2023: World Health Organization
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10(1):1–11
Park K, Kim Y, Kim J et al (2020) Supplementation with Korean red ginseng improves current perception threshold in Korean type 2 diabetes patients: a randomized, double-blind placebo-controlled trial. J Diabetes Res 2020:5295328
Pingali U, Ali MA, Gundagani S, Nutalapati C (2020a) Evaluation of the effect of an aqueous extract of Azadirachta indica (Neem) leaves and twigs on glycemic control, endothelial dysfunction and systemic inflammation in subjects with type 2 diabetes mellitus—a randomized, double-blind, placebo-controlled clinical study. Diabetes Metab Syndr Obes 13:4401–4412
Pingali U, Sukumaran D, Nutalapati C (2020b) Effect of an aqueous extract of Terminalia chebula on endothelial dysfunction, systemic inflammation, and lipid profile in type 2 diabetes mellitus: a randomized double-blind, placebo-controlled clinical study. Phytother Res 34(12):3226–3235
Ricklefs-Johnson K, Johnston CS, Sweazea KL (2017) Ground flaxseed increased nitric oxide levels in adults with type 2 diabetes: a randomized comparative effectiveness study of supplemental flaxseed and psyllium fiber. Obes Med 5:16–24
Sakhaei R, Nadjarzadeh A, Esmaeili A et al (2021) Cardiovascular and renal effects of Hibiscus sabdariffa Linnaeus. In patients with diabetic nephropathy: a randomized, double-blind, controlled trial. J Nutr Food Secur 6(2):116–26
Sanaei M, Ebrahimi M, Banazadeh Z et al (2015) Consequences of Aphanizomenon flos-aquae (AFA) extract (Stemtech(TM)) on metabolic profile of patients with type 2 diabetes. J Diabetes Metab Disord. https://doi.org/10.1186/s40200-015-0177-7
Shahbazian H, Aleali AM, Amani R et al (2019) Effects of saffron on homocysteine, and antioxidant and inflammatory biomarkers levels in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Avic J Phytomed 9(5):436–445
Shidfar F, Rajab A, Rahideh T, Khandouzi N, Hosseini S, Shidfar S (2015) The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J Complementary Integr Med 12(2):165–170
Smitha Grace S, Chandran G, Chauhan JB (2019) Terpenoids: an activator of “fuel-sensing enzyme AMPK” with special emphasis on antidiabetic activity. Plant Hum Health 2:227–244
Soleimani Z, Hashemdokht F, Bahmani F, Taghizadeh M, Memarzadeh MR, Asemi Z (2017) Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complicat 31(9):1394–1400
Soltani D, Azizi B, Rahimi R, Talasaz A, Rezaeizadeh H, Vasheghani-Farahani A (2022) Mechanism-based targeting of cardiac arrhythmias by phytochemicals and medicinal herbs: a comprehensive review of preclinical and clinical evidence. Front Cardiovasc Med 9:990063. https://doi.org/10.3389/fcvm.2022.990063
Somanah J, Aruoma OI, Gunness TK et al (2012) Effects of a short-term supplementation of a fermented papaya preparation on biomarkers of diabetes mellitus in a randomized Mauritian population. Prev Med 54(Suppl):S90–S97
Sun C, Zhao C, Guven EC et al (2020) Dietary polyphenols as antidiabetic agents: advances and opportunities. Food Frontiers 1(1):18–44
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB et al (2022) IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119. https://doi.org/10.1016/j.diabres.2021.109119
Taghizadeh M, Soleimani A, Bahmani F et al (2017) Metabolic response to mulberry extract supplementation in patients with diabetic nephropathy: a randomized controlled trial. Iran J Kidney Dis 11(6):438–446
Tavakoly R, Maracy MR, Karimifar M et al (2018) Does fenugreek (Trigonella foenum-graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial. Eur J Integr Med 18:13–17
Thomford NE, Senthebane DA, Rowe A et al (2018) Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int J Mol Sci 19(6):1578
Usharani P, Fatima N, Muralidhar N (2013) Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, controlled study. Diabetes Metab Syndr Obes 6:275–284
Usharani P, Kishan PV, Fatima N, Uday KC (2014) A comparative study to evaluate the effect of highly standardised aqueous extracts of Phyllanthus emblica, Withania somnifera and their combination on endothelial dysfunction and biomarkers in patients with type II diabetes mellitus. Int J Pharm Sci Res 5(7):2687–2697
Vassalle C, Gaggini M (2022) Type 2 diabetes and oxidative stress and inflammation: pathophysiological mechanisms and possible therapeutic options. Antioxidants (Basel) 11(5):953. https://doi.org/10.3390/antiox11050953
Yuan H, Ma Q, Cui H et al (2017) How can synergism of traditional medicines benefit from network pharmacology? Molecules 22(7):1135
Zhang H, Tsao R (2016) Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci 8:33–42
Acknowledgements
All authors thank Endocrinology and Metabolism Clinical Sciences Institute for its financial support. It should be noted that the institute had no role in any part of the study, writing of the manuscript, or the decision to submit.
Funding
This work was supported by the Diabetes Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran [Grant IDs 1399-01-97-991].
Author information
Authors and Affiliations
Contributions
OT-M: supervision, data curation, methodology, validation, writing, reviewing, and editing; BA and SM: methodology, data curation, writing—original draft preparation; FE: methodology, data curation, reviewing and editing; MQ: methodology, validation, reviewing and editing; MK, EN, ZN: data curation, reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Neither ethics approval nor participant consent was required, as this study was based solely on the summary results of previously published articles. Individual patient data were not obtained or accessed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10787_2023_1315_MOESM2_ESM.docx
Supplementary file2 Table S2. Subgroup analyses for the effects of herbal medicines on the primary and secondary outcomes (DOCX 16 KB)
10787_2023_1315_MOESM4_ESM.docx
Supplementary file4 Fig. S2 Forest plot of antioxidative enzymes’ activity in the random effect meta-analysis (DOCX 97 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azizi, B., Mohseni, S., Tabatabaei-Malazy, O. et al. Meta-analysis of the anti-oxidative and anti-inflammatory effects of hypoglycaemic plant-derived medicines. Inflammopharmacol 31, 2521–2539 (2023). https://doi.org/10.1007/s10787-023-01315-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01315-9