Abstract
People of Pakistan have undisturbed customs for the employment of medicinal plants for healthcare requisites. Chloroform extract of F. hygrometrica (CE FH) was examined for its ability to reduce inflammation and to produce analgesia. Carrageenan and formalin-induced paw edema model for inflammatory activity, hot-plate and tail-flick methods to assess analgesic activity were executed. Phytochemical analysis was done by UHPLC–MS and GC–mass spectrometer. The results demonstrated that in carrageenan-induced paw edema, maximum reduction in inflammation was observed at 5th hour at the dose 100 mg/kg; while at doses 250 and 500 mg/kg, maximum response was observed at 5th and 6th hours. Analgesic activity results indicated that maximum analgesia was observed up to 120 min at 100 mg/kg, while up to 90 min in case of 250 and 500 mg/kg doses. The formalin-induced rat paw edema showed significant (p < 0.05) anti-inflammatory activity after 5 days treatment. After, testing period of 10 days, the biochemical parameters such as CBC, CRP, serum enzymes like CAT, SOD, GSH and inflammatory mediators like TNF-α, IL-6, IL-4 and IL-10 were estimated. The administration of formalin resulted in an increase in the level of leucocytes, total WBC, CRP, serum enzymes and in the diameters of paw thickness, while pre-treatment with CE FH at dose levels of 100, 250 and 500 mg/kg exhibited a diminution in the levels of SOD, GSH, CAT, total RBC and HB. Acute inflammatory mediators such as TNFα, IL -6 and IL-4 were reduced, and IL-10 was upregulated in the treated group as compared to the control. Many phytoconstituents, i.e., chitobiose, chlorovulone III, γ-tocotrienol, emmotin, cassine, hexacosanedioic acid, neophytadiene, fumaric acid, neophytadiene, hexadecanoic acid, phytol and stigmasterol were detected during UHPLC–MS and GC–MS analysis seems to be responsible for the said activity in correlation with the already reported data about these compounds. The results concluded that CE FH possess noteworthy anti-inflammatory and central analgesic action at different doses (100, 250 and 500 mg/kg).
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a physiological defensive response of the body that plays a key role in protection against microbial infections and physical injury. In case of acute inflammation, onset is rapid and short, represented by liquid exudate, plasma proteins and leucocytes at site of injury. While the persistent inflammation for prolonged duration results in the chronic inflammation. It plays an important role in the pathogenesis of certain diseases like cancer, neuro degenerative disease, rheumatoid arthritis, obesity, cardiovascular diseases etc. (Bouyahya et al. 2022). Pain, edema redness and, sometimes, loss of the tissue functions due to change in blood flow, increased vascular tissue permeability and tissue destruction are the common characteristics of inflammation. All these changes occur due to activation and migration of leucocytes along with the production of reactive oxygen species (ROS), local inflammatory mediators, i.e., prostaglandins leukotrienes platelet activating factor induced by COX, LOX, and phospholipase A2. (Anyasor et al. 2019). ROS and other free radicals are involved in the pathogenesis of different inflammatory diseases. During inflammation, WBCs engaged at the damage site results in the production of cytokines (IL-1beta, IL-8, IL-6) and TNF alpha promoting cell activation and cell infiltration causing inflammation progression. Enzymes responsible for body defense mainly SOD, CAT and GSH fight against the oxidative stress, thus providing shield to the body against this oxidative imbalance (Kim et al. 2021). Research has indicated that inflammation can be effectively control by regulating the production of mediators and cytokines. In the present era, inflammation is being treated by various chemical-based drugs which possess many side effects including hepatotoxicity, cardiotoxicity, different immunological dysfunctions (Jan et al. 2020). Therefore, to avoid these side effects, researchers are being attracted by anti-inflammatory compounds from natural sources. A larger number of natural products particularly plants have been exhibiting excellent anti-inflammatory potentials both in vivo and in vitro (Miranda et al. 2013).
Bryophytes are small group of plants that lack true vascular system. They are rarely subjected to medicinal and chemical investigation. Several therapeutic compounds have been isolated from bryophytes like polysaccharides, terpenoids, amino acids, lipids, quinones, phenylpropanoids and other specialized metabolites. These compounds have been reported to possessed antimicrobial, antiviral, cytotoxic, nematocidal, insecticidal, effects on smooth and non-striated muscles, weight loss, plant growth regulators and allelopathic activities (Sabovljevic et al. 2017). Mosses are the major group of bryophytes that contribute significantly to the ecosystem supporting terrestrial biodiversity. In the past, mosses were used to cure wounds, burns, and many illnesses. Funaria is the largest genus in the moss family Funariaceae (Minozzomi et al. 2020). In ethnomedicine, it is frequently used for hemostasis, pulmonary tuberculosis, bruises and in skin infections (Yayintas and Irkin 2018). Many new biologically active compounds with potential for medicinal applications are derived from bryophytes, particularly moss and liverworts (Singh et al. 2007). Around 3.2% of mosses and 8.8% of liverworts have undergone chemical analysis. Funaria hygrometrica (FH) is found in the Punjab planes of Pakistan as a wild plant. The reports about the anti-inflammatory and analgesic potentials of this species and its mechanisms of action are lacking in the literature. The present study is innovated to vindicate the folk employment of FH by executing its anti-inflammatory and analgesic activeness scientifically.
Materials and methods
Plant materials
Whole Funaria hygrometrica (FH) plants were congregated from Khanspur and Nathigalli areas (in the northern region of Pakistan). The specimen of the plant species was identified and authenticated by Dr. Zaheer-ur-Khan, a plant taxonomist from Botany Department, Government College University Lahore. The plant specimen was deposited in the herbarium of Department of Botany, Government College University Lahore, Pakistan with voucher No. Cog-011 for further reference. The plant material was carefully dried, garbled, pulverized, and placed in a glass container.
Crude plant extract preparation
The grounded plant material (4 kg) was progressively macerated with n-hexane, chloroform, and methanol. The plant material was retained in each solvent for 7 days and 8 L of each solvent was utilized sequentially. Each solvent extract was inchoately percolated completely through muslin cloth, followed by filtering using Whatman-1 filter paper, and the filtration operation was repeated twice or three times to obtain the highest yield of each. Solvents from each extract was evaporated at low pressure (Abubakar et al. 2020). Resulting extracts, i.e., n-hexane, chloroform and methanol were light orange color, dark brown and greenish black in color, respectively. To obtain the semisolid extract, each extracted material underwent further drying in an oven set at 37 °C. Each extract was packaged with a label and stored between 25 and 30ºC. Chloroform extract (CE) was utilized for further investigation.
Chemicals and drugs
All chemicals were of analytical grade. These were formalin (Riedel–deHaen, Germany), carrageenan and acetic acid acetonitrile, formic acid (Sigma–Aldrich, USA). Diclofenac sodium was gifted from CCL Pharmaceuticals. Different doses of CE FH (100, 250 and 500 mg/kg) were prepared in 10% Tween 20 solution while diclofenac sodium 15 mg/kg were prepared in distilled water for administration.
UHPLC–MS analysis
Phytochemical profiling of CE FH was carried out using reverse-phase ultra-high-performance liquid chromatography mass spectrometry utilizing Agilent 1290 infinity UHPLC system coupled with Agilent 6520 accurate-mass Q-TOF mass spectrum equipped with dual ESI source. Agilent zorbax eclipse XDB-C18 (narrow19 bore 2.1 × 150 mm, 3.5 micron) was used for the analysis. Temperatures of the column and auto-sampler were maintained at 20 at 25 °C and 4 °C, respectively. Mobile phases X-formic acid (0.1% in water) and Y-acetonitrile (0.1%) were used at the rate of 0.5 mL/min during analysis. Each run was carried out for 25 min with injection volume of1.0 µL. Full-scan MS analysis was performed over the m/z 100–1000 range using an electrospray ion source in both negative mode and positive mode. Nitrogen was provided at flow rates of 25 and 600 L/hour, respectively, as nebulizing and drying gas (350 °C was the temperature of the drying gas). The voltage for fragmentation was calibrated to 125 V. An analysis was conducted using a 3500 V capillary voltage. Agilent Mass Hunter Qualitative Analysis B.05.00 was used to analyze the data (Method: Metabolomics3 2017-00.004.m). Compounds were identified by database search 4 METLIN AM PCDL-N- 170502.cdb: with parameters as: match tolerance 5 ppm, positive ions: + H, + Na, + NH4, negative-H (Saleem et al. 2020).
GC–MS analysis
GC–MS analysis was performed by Agilent-B-7890 equipped with mass spectrometer detector (5977-B using DB-5MS column having 100% dimethyl polysiloxane as stationary phase, having dimensions (L—30 m and D—0.25 mm) with pore size of 0.25 μm. The temperature of the injector was set at 250 °C, while an inter-phase temperature was 280° C. Helium was used as carrier gas and electron-impact ionization was used at −70 eV in full scan mood. Analysis time was 30 min. 2 μL of CE FH was performed in the split-less mode (Fan et al. 2021).
Experimental animals
Studies were conducted on albino rats weighing (150–200 g) and mice weighing 25–30 g of both the sexes. The animals were laid in Plexiglas cages (47 × 34 × 18 cm3) in the Research Laboratory of Pharmacology and Physiology, Faculty of Pharmacy, University of the Punjab Lahore, Pakistan. The laboratory temperature was sustained at 26 ± 2 °C and humidity was maintained at 50–55% along with 12-h light–dark cycle. All the animals were acclimatized for 7 days prior to study. They fed on standard animal feed and water ad libitum. All the experimental protocols were recapitulated by the Pharmacy Animal Ethics Committee with approval No. 2101 in the Faculty of Pharmacy, University of the Punjab Lahore, Pakistan.
Acute oral toxicity study
Using OECD criteria, the CE FH was assessed for acute oral toxicity prior to conducting the in vivo biological experiments (421 and 422). Mice were administered the extract at doses of 100, 250, 500, 750, 1000 and 2000 mg/kg for 14 days and after that, toxicity signs were noted. The mice were observed at 1-h interval for the first 12 h and daily, thereafter, for 14 days. The mortality was recorded after 48 h. The animals were observed for behavioral changes like tremors, convulsions, salivation, sweating, lacrimation, writhing reflex, convulsions, activity, and behavior pattern (Javed et al. 2020).
In vitro anti-inflammatory assay by inhibition of albumin denaturation
Anti-inflammatory activity was assessed using the inhibition of albumin denaturation technique (Leelaprakash et al. 2011). 5 mL of the reaction mixture consisted of 0.2 mL of eggs albumin (from hens’ egg), 2.8 mL of phosphate buffer saline (PBS, pH 6.4), and 2 mL of varying concentrations of the extract. A similar volume of double distilled water served as a control. Then, the mixture was incubated at 37 °C in a BOD incubator for about 15 min, then heated at 70 °C for 5 min. After cooling, the mixture absorbance was measured at 660 nm using against blank. Diclofenac sodium (standard drug) was used as a reference. The percentage inhibition of protein denaturation was calculated.
In vivo anti-inflammatory assay
For anti-inflammatory activity, animals were divided into six groups and each group containing five animals. Group I (control) received vehicle, Group II (disease control) did not receive any oral treatment, Group III (standard) animals were administered diclofenac sodium 15 mg/kg orally, Group IV, Group V and Group VI received CE FH at doses of 100 mg/kg, 250 mg/kg and 500 mg/kg, respectively.
Carrageenan-induced hind paw edema
0.1 mL of freshly prepared 1% carrageenan was injected into the right hind paw of mice except the control group. The normal paw size of mice was measured with a digital vernier calliper (Mitutoyo, Japan). Half an hour earlier, the mice were given vehicle (orally), diclofenac sodium and various doses of a CE FH (100, 250 and 500 mg/kg, orally). Paw thickness (in mm) was again measured after carrageenan injection at 0 h up to 6 h. The percentage inhibition of the paw was calculated according to the previously reported formula (Sudjarwo et al. 2005).
Formalin-induced edema in rat paw
Chronic anti-inflammatory consequences of different dosses of CE FH were evaluated by formaldehyde induced rat paw edema by following formerly delineate method.
Edema was induced by injecting 0.1 mL of 2% formaldehyde solution in distilled water into sub-planer region of hind paw of each mouse except for the control group (group-I) and repeated induction on day 3. Vehicle (group-I), disease control (group-II), diclofenac sodium 15 mg/kg as a standard drug (group-III) and the CE FH at the dosses of 100, 250 and 500 mg/kg/mL (group IV, group V and group VI) were administrated orally one hour prior to injecting the formaldehyde solution. Drug treatment was prolonged for 10 days. Paw thickness was measured from 0 to 10 days with a digital vernier caliper and edema was calculated (Chang et al. 2012). Percentage inhibition of paw edema was calculated using the above-mentioned equation. At the end of the 10-day study, the rats were killed, and blood was collected in serum tubes and centrifuged at 3000 rpm for 10 min at room temperature to obtain the serum. The serum was then tested for the presence of serum enzymes like catalase (CAT), superoxide dismutase (SOD), and glutathione (GSH) (John et al. 2012). In addition, TNF-α, IL-6, IL-4, IL-10, and hematological assays were also performed as described below.
Estimation of TNF-α, IL-6, IL-4 and IL-10
The quantitative analysis of inflammatory mediator gene expression was performed with optimized qRT-PCR. The extraction of total RNA from blood samples treated was performed with a multi-type sample DNA/RNA extraction purification kit (Sansure Biotech Inc, China). The reverse transcription of RNA samples to cDNA was performed with Thermo-First cDNA kit (HRP013 100 T, ZOKEYO, China). A 2X SYBR qPCR Mixture (Zokeyo, China) containing a total reaction volume of 15 μL (10 μL of SYBR Green mix, primers at 0.5 μM each and 1 μL of cDNA as template with relevant primers in duplicates) was run in SLAN-96P Real-Time PCR System (Sansure Biotech Inc, China). The microplate was kept at 95 °C for 30 s for initial denaturation and annealing process occurred at 95 °C for 5 s and extension phase was carried out at 60 °C for 20 s. The relevant Ct’s of samples were compared against controls and control samples containing housekeeping genes (GAPDH) (Table 1).
Hematological studies
Rats were anesthetized with ketamine + xylazine and blood was drawn for biochemical evaluation by heart puncture following ten days of formalin-induced rat paw edema study. Some of the collected blood was used to perform hematological studies like complete blood count and CRP mg/L (Peerzada et al. 2020).
Analgesic activity
Mice (14–28 g) were used to determine the analgesic effect of CE FH by hot-plate and tail immersing test. Mice were divided into five groups containing five animals each. Vehicle was given to the control group, diclofenac sodium in 15 mg/kg was given to the standard group, while treated groups were administered 100, 250 and 500 mg/kg of the extract orally.
Hot-plate method
Hot-plate assay was performed by treating mice with different treatments and after 1 h of treatment/doses, the individual mouse from each group was put on the hot plate (VELP®, Italy) at fixed temperature of 50–55 °C. The pain latency time for the mice to show response by paw licking, lifting, or jumping was noted. The reaction time before the treatment and after 30, 60, 90 and 120 min of drug administration was recorded. 25 s was taken as cut-off time to prevent the injury to the paw (Javed et al. 2020).
Tail flicking method
A thermostat was adjusted to maintain the temperature of the water heater at 50–55 °C. The tail of the mice (3 cm) was dipped in hot water and the reaction time of tail withdrawing from hot water was noted. After that, testing drugs and different doses of the extract (100, 250 and 500 mg/kg) were given to the experimental animals to find out the reaction time again at 30, 60, 90 and 120 min. 25 s was taken as cut-off time to avoid the damage the tissues (Bhukya et al. 2009).
Statistical analysis
Result values were represented in mean ± SEM (n = 5). Two-way ANOVA followed by post hoc Dunnett’s test was performed using Graph Pad Prism 9 (San Diego, CA, USA) software. p-value of less than 0.05 was considered statistically significant.
Results
UHPLC–MS profiling
It is not feasible to evaluate the pharmacological effects of plant extracts without analyzing their chemistry. The bioactive chemicals that naturally occur are typically what determines the biological potential of therapeutic plants. So, both positive and negative ionization modalities of UHPLC–MS analysis of CE FH were performed to acquire an in-depth identification. The tentative presence of 30 distinct chemicals was detected in the CE FH, as stated in Table 2 and the total ion chromatograms (TICs) are displayed in Fig. 1.
GC–MS analysis
26 compounds were detected in GC–MS analysis. Many of these compounds have reported anti-inflammatory and analgesic properties Table 3 (Fig. 2).
Acute toxicity
Acute toxicity observations in mice with increasing doses of crude plant extract up to 2000 mg/kg were noted and found to be safe. There was no sign of toxicity in terms of altered behavior or mortality after 48 h.
Anti-inflammatory assay
In vitro anti-inflammatory assay
In the current study's in vitro anti-inflammatory test, the CE FH demonstrated a mean inhibition of protein denaturation of 15, 20, 27.50, 32.50, 40.00, 47.25 and 52.75% for doses of 50, 100, 150, 200, 250, 300 and 350 µg/mL, respectively; while, the standard diclofenac sodium showed a mean inhibition of protein denaturation of 25.26, 29.75, 36.25, 41.74, 47.00, 55.10 and 63.00% (Table 4). With a linear response, the CE FH demonstrated good anti-inflammatory action. Diclofenac sodium a common anti-inflammatory medicine, displayed the highest level of inhibition at 350 µg/ml.
Carrageenan-induced hind paw edema
The results indicated that CE FH extract had an anti-inflammatory effect at different doses (100, 250, and 500 mg/kg). Maximum reduction in inflammation was observed at 5th hour at the dose 100 mg/kg while at doses 250 and 500 mg/kg maximum response was observed at 5th and 6th hours. The standard drug exhibits a significant anti-inflammatory response from 4 to 6 h (Fig. 3).
Paw diameter in carrageenan-induced edema was significantly reduced in the carrageenan-induced inflammation analysis at various plant dosages extract. Every result value is expressed in mean and SEM. n = 5. p < 0.05 (*) in comparison to the controlled group. Two-way ANOVA is applied using post hoc Dunnett’s test. All the groups are compared with disease control at one time interval
Formalin-induced rat paw edema
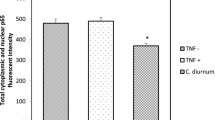
The formalin-induced mice paw edema showed a significant decrease (p < 0.05) in the paw size and diameter after 5 days treatment with CE FH (Fig. 4). The administration of formalin resulted in an increase in the level of leucocytes, total WBC, CRP in control group, while a decrease in the level of these parameters were observed with CE FH pre-treatment (Tables 5, 6). Formalin processed experimental animals exhibited a diminution in the levels of SOD, GSH, CAT, total RBC and Hb and these parameters in animals pre-treated with CE FH at dose levels of 100, 250 and 500 mg/kg were significantly normalized (Fig. 5). Acute inflammatory mediators such as TNF, IL-6, IL-were reduced and IL-10 up regulated in the treated group as compared to the control (Fig. 6).
Paw thickness in formalin-induced edema was significantly reduced by various plant extract dose treatments. Every result value is expressing in mean and SEM. n = 5. p < 0.05 (*) in comparison to the controlled group. Two-way ANOVA is applied using post hoc Dunnett’s test. All the groups are compared with disease control at one time interval
Analgesic activity
Hot-plate method
At various time intervals (30, 60, 90, and 120 min), the CE FH displayed considerable pain dormancy at doses of 100, 250, and 500 mg/kg. Results indicated that maximum analgesia was observed up to 120 min at 100 mg/kg while up to 90 min in case of 250 and 500 mg/kg doses. Diclofenac sodium, a standard medication, also greatly lessens the pain throughout all observational intervals (Fig. 7).
Tail flick method
Tail immersion method was used to assess the analgesic effect, and all dosages (100, 250 and 500 mg/kg) significantly reduced pain latency compared to the control (Fig. 7).
Discussion
Ethnobotanical investigations have shown that many plants are employed traditionally in various regions of the world as analgesics and anti-inflammatory agents (Yu et al.2019; Paramita et al 2017). The current study was designed to substantiate scientifically the traditional usage of Funaria hygrometrica. In vitro protein (albumin) denaturation assay is based upon the formation of antigens that triggers type III hypersensitivity reactions resulting in inflammation. Percent inhibition of albumin denaturation is directly linked with the anti-inflammatory potentials.
The chloroform extract of Funaria hygrometrica (CE FH) inhibited protein denaturation and the results were comparable with standard diclofenac sodium (Table 4). From the results of a linear response, the CE FH demonstrated significant in vitro anti-inflammatory action.
Hot-plate and tail-flick assays were employed to evaluate the central analgesic effectiveness of CE FH. These are acute models of pain and both types of stimuli induce pain by heat-mediated damage of tissues and are selective for chemicals like opioids (Andurkar and Gulati 2011). Results revealed that mice treated with doses of 100, 250, and 500 mg/kg of CE FH (Fig. 7) experienced a significant analgesic effect (p < 0.05) which may be due to the presence of components identified by UHPLC–MS and GC–MS analysis (Tables 2, 3). Fumaric acid (Shakya et al. 2014), steroids like stigmasterol and campesterol (Toma et al. 2003), Benzoic acid, 3,5-dicyclohexyl-4-hydroxy-, methyl ester (Subramanian et al. 2020) elucidated by UHPLC–MS may contribute to the analgesic activity. Fumaric acid or its esters have frequently been identified as the bioactive components of various medicinal plants, and it has long been recognized that fumaric acid has antioxidant, anti-inflammatory and analgesic potential (Shakya et al. 2014).
Carrageenan-induced paw edema is a well-established model of acute inflammation which induces biphasic acute inflammatory response with the release of histamine, serotonin, kinins in the first phase and later prostaglandins (Yu et al. 2019). The clinically relevant steroidal and nonsteroidal anti-inflammatory drugs can have an impact on the second phase (Amdekar et al. 2011). Our results showed that CE FH significantly reduced the inflammation caused by carrageenan (Fig. 3) which may have blocked the production of prostaglandins in the second phase of acute inflammation. The phytochemical profiling by GC–MS and UHPLC–MS identified presence of established anti-inflammatory compounds like Hexadecanoic acid, Phytol, chlorovulone III (Ciufolini and Zhu 1998), γ-Tocotrienol (Yap et al. 2008), emmotin A (Da Silva et al 2012) and cassine (John and shobana 2012). Hexacosanedioic, a specific inhibitor of phospholipase A2, decreases inflammation through suppression of arachidonic acid pathway (Aparna, et al. 2012). Phytol also possesses prominent antioxidant and anti-inflammatory potential (Silva et al. 2014). Stigmasterol interacts with glucocorticoid receptors, thereby suggesting that the CE FH extract may interact with these receptors and reduces inflammatory burden (Maceyka and Speigel 2014).
In the current study, chronic inflammation was induced with formalin. In-inflammatory conditions, there is excessive superoxide radical production which can cause tissue damage through the production of hydrogen peroxide and hydroxy radicals responsible for membrane destruction (Jantaratnotai et al. 2022). Amongst the antioxidant enzymes, SOD is the most significant enzyme present in mitochondria and protects against superoxide anions. Glutathione is another essential endogenous antioxidant that functions as part of the glutathione redox system to protect cells from oxidative damage. Tissue glutathione depletion induces lipid peroxidation in the cells (Allagui et al. 2022). Our results showed that CE FH treatment significantly upregulated antioxidant enzymes (Fig. 5). Therefore, it could be suggested that CE FH protects against oxidative damage in chronic inflammation. Inflammatory mediators were also downregulated with prior CE FH treatment as compared to the diseased group (Fig. 6). The levels of inflammatory mediators including IL-6, IL-4 and TNF-α were declined and IL-10 was increased. IL-10 is a powerful macrophage deactivator that prevents human monocytes from producing TNF α, IL-1, IL-6, IL-4, or GM-CSF (Trushin et al. 2003).
Stigmasterol and campesterol detected in GC–MS analysis downregulated the mRNA expression of IL-6 and TNF-α reported in previous studies (Bakrim et al. 2022; Utami et al. 2020) and our results coincide with these findings (Fig. 6). Hematological findings supported the protective effect of CE FH on different parameters like RBC, hemoglobin, WBC and C-reactive protein. RBC and hemoglobin are important components of the oxygen transport system. Formalin treatment causes reduction in the RBC and Hb level which can lead to anemia (John and Shobana 2012). These levels were returned to normal after pre-treatment with the CE FH in various doses (100, 250 and 500 mg/kg, Table 5). WBCs are the key element of the body's defence system and increase in WBC count during inflammation occurs due to increased concentration of interleukins and macrophages. The current investigation showed that formalin administration elevates the count of neutrophils, eosinophils, and C-reactive protein. Different doses of CE FH (100, 250 and 500 mg/kg) lowered the neutrophil and eosinophil populations along with decrease in C-reactive protein (Table 6) indicating a considerable reduction in the inflammatory process (John and Shobana 2012). Terpenoids, hydrocarbons, esters, and fatty acids detected in phytochemical analysis are some of the phytoconstituents that have beneficial pharmacological effects in acute and chronic inflammation (Andrikopoulos et al. 2003).
Conclusion
The current phytochemical and biochemical investigations point to the potential analgesic and anti-inflammatory action of CE FH. Therefore, it can be concluded that this plant will make a viable choice for the treatment of illnesses linked to inflammation and pain. However, further investigation of molecular targets reducing inflammation may be elucidated in future.
Data availability
All data generated or investigated in the study are included in this article.
References
Abubakar AR, Haque M (2020) Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci 12(1):1
Allagui I, Horchani M, Zammel N, Jalouli M, Elfeki A, Kallel C, Hcini K (2022) Phytochemical characterization, antioxidant and anti-Inflammatory effects of Cleome arabica L. fruits extract against formalin induced chronic inflammation in female wistar rat: biochemical, histological, and in silico studies. Molecules 28(1):26
Amdekar S, Singh V, Singh R, Sharma P, Keshav P, Kumar A (2011) Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines. J Clin Immunol 31:147–154
Andrikopoulos NK, Kaliora AC, Assimopoulou AN, Papapeorgiou VP (2003) Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytother Res 17:501–507
Anyasor GN, Okanlawon AA, Ogunbiyi B (2019) Evaluation of anti-inflammatory activity of Justicia secunda Vahl leaf extract using in vitro and in vivo inflammation models. Clin Phytosci 5:1–13
Andurkar SV, Gulati A (2011) Assessment of the analgesic effect of centhaquin in mouse tail flick and hot-plate tests. Pharmacol 88:233–241
Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M (2012) Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Des 80:434–439
Bakrim S, Benkhaira N, Bourais I, Benali T, Lee LH, El Omari N, Bouyahya A (2022) Health benefits and pharmacological properties of stigmasterol. Antioxidants 11(10):1912
Bhukya B, Anreddy RNR, William CM, Gottumukkala KM (2009) Analgesic and anti-inflammatory activities of leaf extract of Kydia calycina Roxb. Bangladesh J 4:101–104
Bouyahya A, Guaouguaou FE, El Omari N, El Menyiy N, Balahbib A, El-Shazly M, Bakri Y (2022) Anti-inflammatory and analgesic properties of Moroccan medicinal plants: phytochemistry, in vitro and in vivo investigations, mechanism insights, clinical evidences and perspectives. J Pharm Anal 12(1):35–57
Chang CW, Chang WT, Liao JC, Chiu YJ et al (2012) Analgesic and anti-inflammatory activities of methanol extract of Cissus repens in mice. Evid Based Complement Altern Med 2012:1–10
Ciufolini MA, Zhu S (1998) Practical synthesis of (±)-chlorovulone II. J Org Chem Res 63:1668–1675
Da Silva KA, Manjavachi MN, Paszcuk AF, Pivatto M, Viegas C Jr, Bolzani VS, Calixto JB (2012) Plant derived alkaloid (−)-cassine induces anti-inflammatory and anti-hyperalgesics effects in both acute and chronic inflammatory and neuropathic pain models. Curr Neuropharmacol 62:967–977
Fan X, Jiao X, Liu J, Jia M, Blanchard C, Zhou Z (2021) Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Int Food Res J 140:109975
Jan HA, Jan S, Bussmann RW, Wali S, Sisto F, Ahmad L (2020) Complementary and alternative medicine research, prospects and limitations in Pakistan: a literature review. Acta Ecol Sin 40:451–463
Jantaratnotai N, Thampithak A, Utaisincharoen P, Pinthong D, Sanvarinda P (2022) Inhibition of LPS-induced microglial activation by the ethyl acetate extract of Pueraria mirifica. Int J Environ Res Public Health 19:12920
John NAA, Shobana G (2012) Antiinflammatory activity of of Talinum fruticosum l. On formalin induced paw edema in albino rats. J Appl Pharm Sci 02:123–127
Javed F, Jabeen Q, Aslam N, Awan AM (2020) Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of Indigofera argentea. Ethnopharmacol 256:112–966
Kim SY, Hong M, Kim TH, Lee KY, Park SJ, Hong SH, Sowndhararajan K, Kim S (2021) Anti-Inflammatory effect of liverwort Marchantia polymorpha L. and Racomitrium Moss Racomitrium canescens hedw brid growing in Korea. Plants 10(10):2075
Leelaprakash G, Dass SM (2011) Invitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int J Drug Dev Res 3:189–196
Maceyka M, Spiegel S (2014) Sphingolipid metabolites in inflammatory disease. Nature 510:58–67
Miranda AS, Brant F, Rocha NP, Cisalpino D, Rodrigues DH, Souza DG, Machado FS, Rachid MA, Teixeira AL Jr, Campos AC (2013) Further evidence for an anti-inflammatory role of artesunate in experimental cerebral malaria. Malaria J 12:388
Minozzo MM, Metz GF, de Vargas MVM, Pereira AB, de Carvalho Victoria F (2020) Phylogenetic and selection pressure analyses of cold stress-associated PAL-Like and Lec-RLK genes in antarctic mosses. Curr Plant Biol 24:100178
Paramita S, Kosala K, Dzulkifli D, Saputri DI, Wijayanti E (2017) Anti-inflammatory activities of ethnomedicinal plants from Dayak Abai in North Kalimantan. Indonesia Biodivers J 18:1556–1561
Peerzada S, Khan MT, Akhtar MF, Saleem A, Akhtar HI, B, et al (2020) Phytochemical, anti-inflammatory, anti-nociceptive and cytotoxic basis for the use of Haloxylon stocksii. Pak J Pharm Sci 33:887–894
Sabovljevic MS, Vujičić M, Wang X, Garraffo HM, Bewley CA, Sabovljević A (2017) Production of the macrocyclic bis-bibenzyls in axenically farmed and wild liverwort Marchantia polymorpha L. subsp. ruderalis Bischl. et Boisselier. Plant Biosyst 151(3):414–418
Saleem H, Sarfraz M, Khan KM, Anwar MA, Zengin G, Ahmad I, Ahemad N (2020) UHPLC-MS phytochemical profiling, biological propensities and in-silico studies of Alhagi maurorum roots: a medicinal herb with multifunctional properties. Drug Dev Ind Pharm 46:861–868
Shakya A, Singh GK, Chatterjee SS, Kumar V (2014) Role of fumaric acid in anti-inflammatory and analgesic activities of a Fumaria indica extracts. J Intercult Ethnopharmacol 3:173
Silva RO, Sousa FBM, Damasceno SR, Carvalho NS, Silva VG, Oliveira FRM, Sousa DP, Aragão KS, Barbosa AL, Freitas RM (2014) Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam Clin Pharmacol 28:455–464
Subramanian S, Dowlath MJH, Karuppannan SK, Saravanan M, Arunachalam KD (2020) Effect of solvent on the phytochemical extraction and GC-MS analysis of Gymnema sylvestre. Pharmacogn J 12:749–761
Sudjarwo SA (2005) The potency of piperine as anti-inflammatory and analgesic in rats and mice. FMI 41:190–194
Toma W, Gracioso JDS, Hiruma-Lima CA, Andrade FD, Vilegas W, Brito AS (2003) Evaluation of the analgesic and antiedematogenic activities of Quassia amara bark extract. J Ethnopharmacol 85:19–23
Trushin SA, Pennington KN, Carmona EM, Asin S, Savoy DN, Billadeau DD, Paya CV (2003) Protein kinase Cα (PKCα) acts upstream of PKCθ to activate IκB kinase and NF-κB in T lymphocytes. Mol Cell Biol 23:7068–7081
Utami W, Aziz HA, Fitriani IN, Zikri AT, Mayasri A, Nasrudin D (2020) In silico anti-inflammatory activity evaluation of some bioactive compound from Ficus religiosa through molecular docking approach. J Physc Conf Ser 1563(1):2024
Yap WN, Chang PN, Han HY, Lee DTW, Ling MT, Wong YC, Yap YL (2008) γ-Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br J Cancer 99:1832–1841
Yayintas OT, Irkin LC (2018) Bryophytes as hidden treasure. J Sci Perspect 2:71–82
Yu HH, Lin Y, Zeng R, Li X, Zhang T, Tasneem S, Chen C, Qiu YX, Li B, Liao J, Wang YH (2019) Analgesic and anti-inflammatory effects and molecular mechanisms of Kadsura heteroclita stems, an anti-arthritic Chinese Tujia ethnomedicinal herb. J Ethnopharmacol 238:111902
Acknowledgements
Authors acknowledged Dr. Saiqa Ishtiaq, Head of department of Pharmacognosy, Punjab University College of Pharmacy, University of the Punjab, Pakistan for providing the lab facilities. We also acknowledged Dr. Sairah Hafeez Kamran for performing helping in compilation of data.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
AA, RW, SFF and SR performed the experimental work and wrote the manuscript. SI: planned the project. AA and SI: Elucidation of the GC–MS and UHPLC results. SHK: Proof reading. AA and SI: Manuscript formatting. SHK: Statistical analysis. All authors carefully read and approved the manuscript.
Corresponding author
Ethics declarations
Competing of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asif, A., Ishtiaq, S., Kamran, S.H. et al. UHPLC–MS and GC–MS phytochemical profiling, amelioration of pain and inflammation with chloroform extract of Funaria hygrometrica Hedw. via modulation of inflammatory biomarkers. Inflammopharmacol 31, 1879–1892 (2023). https://doi.org/10.1007/s10787-023-01207-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01207-y