Abstract
In the recent years, various food additives, medicinal plants, and their bioactive components have been utilized in anti-inflammatory and immunomodulatory therapy. Nigella sativa is a key dietary supplement and food additive which has a strong traditional background. It is also one of the most broadly studied seeds in the global pharmaceutical and nutraceutical sector. N. sativa seeds are potential sources of natural metabolite such as phenolic compounds and alkaloids. The anti-inflammatory and immunomodulatory abilities of these seeds, most peculiarly with reference to some inflammatory and immune mediators, are reviewed. N. sativa and its bioactive compounds modulate inflammatory and immunomodulatory mediators including tumor necrosis factor-alpha (TNF-α), interferon gamma (IFN-γ), nuclear factor kappa B (NF-kB) cyclooxygenase (COX), lipoxygenase (LOX), transforming growth factor beta (TGF-β), interleukins, and immunoglobulin levels. This paper comprehensively describes the biomarkers and signaling pathways underlying the anti-inflammatory and immunomodulatory potential of N. sativa. This review also explains the scientific basis and the pharmacological properties of core bioactive ingredients of N. sativa responsible for these biological activities which indicates that their bioactive components could be possibly regarded as favorable therapy for disorders linked to inflammation and immune-dysregulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pragmatically, all through the continents, several plants and their derived substances are used in the treatment and prevention of varieties of pathologies. The proof of the favorable curative and medicinal effect is observed in their incessant utility. The advancement of recent chemistry authenticated the isolation of chemicals from plant and plant seeds that have inevitably been an alternative and indispensable ample source for new drugs. Approximately ten percent (10%) of drugs are derivatives of natural plant materials endorsed by U.S. Food and Drug Administration (FDA) (Patrigde et al., 2016). Nigella sativa (N. sativa) is a chief therapeutic herb widely cultivated in Mediterranean, Middle East, South Asia, Europe, and North Africa (Khare et al., 2004; Hussain and Hussain, 2016; Hossain et al., 2021). N. sativa is a flowering plant classified scientifically as the Ranunculaceae-butter cup family whose common names are black seed or black cumin (Fig. 1). Others names include devil in a bush, love in a mist, fennel flower, etc. It is about twenty-to-fifty centimeters (20–50 cm) tall. It is identified as white petals and pale blue with its fruits consisting of several stark black seeds and it grows annually (Eid et al., 2017). N. sativa has been widely utilized as essential pharmaceutical and nutraceuticals agents as well as traditional remedy for many ailments (Periasamy et al., 2016). N. sativa also functions as food additives in diets, such as pickles, salads, bread, pastries, sauces, smoothies, oatmeal, and youghurt. It is also utilized as spices, food preservatives, and flavoring agents (Bashir et al., 2021) and abundant in bioactive phytochemicals. N. sativa utility can be traced to 1300 BC, and was unveiled in Egyptian pharaoh’s tomb known as Tutankhamun (Ali et al., 2003). An authentic saying from Prophet Mohammed revealed that black seed, indisputably, can be used to cure all diseases except “As Sam” (death) (Al-Bukhari et al., 1976; Hadith Al-Bukhari 7:591). The treasured “Canon of Medicine” encyclopedia by Avicenna, designed N. sativa study as a core curriculum for medical scholars. In Northern Africa, it is used as a natural remedy for treating several inflammatory disorders, allergies, and infections (Hussain and Hussain, 2016). The diverse utility of N. sativa in folklore motivated various researchers to distinguish its bioactive constituents. Acute and chronic toxicity studies on laboratory animals have reported that N. sativa, its oil and thymoquinone, the most widely studied active principle, are quite safe, particularly when given orally (Badary et al., 1998; Mansour et al., 2001; Al-Ali et al., 2008). Dollar et al. reported that the N. sativa (0.1and 1 g/kg/day) supplementation administered to male Sprague Dawley rats for a period of 28 days revealed no changes in hepatic enzyme levels (serum alanine aminotransferase (ALT) and aspartate-aminotransferase (AST)) and did not exert any toxic effect on the liver function (Dollah et al., 2013). The low acute toxicity of Nigella sativa fixed oil in mice, indicated by high values of oral and intraperitoneal lethal dose (LD50 = 28.8 ml/kg body wt., p.o.; LD50 value = 2.06 ml/kg body weight., i.p) values, principal hepatic enzyme stability and organ integrity, demonstrates a broad margin of safety for therapeutic doses of N. sativa oil. Additionally, chronic toxicity test experimented on rats treated daily with an oral dose of 2 ml/kg body weight for a period of 12 weeks showed no alteration in the hepatic enzyme levels [gamma-glutamyltransferase, alanine-aminotranferase (ALT), and aspartate-aminotransferase (AST)] as well as histopathological modifications in rats treated with N. sativa oil (Zaoui et al., 2002). A particular study also revealed that TQ at a dose level (30, 60, or 90 mg/kg/day.) administered for 90 days in drinking water demonstrated absence of mortality and showed no signs of toxicity in mice. Intriguingly, histological analysis also revealed no tissue damage. The plasma levels of total protein, urea, creatinine and triglycerides, and enzyme activities of ALT, lactate dehydrogenase (LDH), and creatine phosphokinase (CPK) were not altered (Badary et al., 1998) (Fig. 2).
Inflammation is a physiological response of the biological system to injury and can evolve from a physiological state to a pathological state. It is indicated by immune and non-immune cell activation which preserves the host from parasites, bacteria, and viruses (Kumari and Kumar, 2022). It can be grouped into two; acute and chronic inflammation. The normal process of inflammation occurs in three phases which includes: (1) vascular inflammatory response, which is concerned with healing of acute injury enhancing tissue recovery, elevated blood flow, as well as the transportation and mobilization of cells to the affected local area; (2) new collagen formation with the collagen fibers arranged in disorganized state in the form of scar with frail fiber linkage; (3) tissue remodeling and maturation, a healing process characterized by reduced collagen production but increased arrangement of collagen fibers coupled with strong fiber bond. This enhances cellular organization with regulated inflammatory response (Hench, 2005; Kotas and Medzhitov, 2015). In acute inflammation, it involves early response and the healing process commences when the body sends inflammatory cells to the site of the injury, but in chronic inflammation, the body continues to send inflammatory cells in an unregulated manner. Nevertheless, unregulated acute inflammation can lead to chronic, resulting in diverse inflammatory disorders. Immune disorder is a dysregulation of the immune system and it occurs from an abnormally low or over activity of immune response in the biological tissue or system. Chronic inflammatory and immune diseases, including diabetes mellitus, cancer, cardiac disorders, chronic respiratory diseases, arthritis, stroke, chronic kidney diseases, liver diseases, etc., are the most notable cause of mortality in the world and more than 50% of deaths have been attributed to it (Feigin and GBD., 2017; Furman et al., 2019; Pahwa et al., 2021). Oxidative stress occurs when reactive oxygen species overwhelms or overshadows the antioxidant defense system in the biological system. Oxidative stress elevates the various inflammatory genes expression and upregulates the inflammatory mediators’ cascade, such as the prooxidant molecules, neutrophils, lytic enzymes, cytokines, chemokines, and nitric oxide (Gholamnezhad et al., 2016). The excessive production of these mediators as well as both reactive nitrogen and oxygen species contributes to the pathogenesis of inflammatory and immune disorder (Abel-Salam, 2012; Shuid et al., 2012). Special interest has grown toward the immune modulatory abilities of N. sativa. Some detailed studies have explored the antioxidant, anti-inflammatory, and immunomodulatory abilities of this dynamic seed (Salem, 2005; Majdalawieh and Fayyad, 2015; Islam et al., 2021). However, some scientific findings have unraveled the signaling system inherent in the immunomodulatory and anti-inflammatory abilities of N. sativa (Kulyar, et al., 2021; Mahmoud et al., 2021; Montazeri et al., 2021). This review explicitly describes the pharmacological, anti-inflammatory, and immune- modulating potential of N. sativa and its constituents in folklore (Fig. 1).
Traditional uses of Nigella sativa
Nigella sativa (N. sativa) has been cultivated for thousands of years for the therapy of various infectious and non-infectious diseases. N. sativa is a principal traditional medicinal herb extensively cultivated in Mediterranean, Middle East, South Asia, Europe, and North Africa (Khare et al., 2004; Hussain et al., 2021). N. sativa is used as a flavoring agent in several pastries and beverages, and has been placed among the highly ranked and effective seeds. In the traditional system of medicine, it represents a promising natural remedy extensively recommended for ailments, including nasal congestion, headache, migraine, dizziness, dysentery, fever, cough, bronchitis, asthma, dysmenorrheal, obesity, rheumatoid arthritis, hypertension, and gastrointestinal problems. N. sativa has been applied topically on abscesses, nasal and diabetic ulcers, eczema, and swollen joints. The mixture of N. sativa oil with beeswax can serve as a therapy for skin infection, burns and wrinkles (Gilani et al, 2004; Hossain et al., 2021). N. sativa has also been utilized as a liver tonic, emmenagogue, diuretic, and diaphoretic. It can be prepared synergistically with other ingredients, including honey, milk, and syrup to treat nausea, prolonged hiccups, diarrhea, edema, high blood cholesterol, dyspepsia, dyspnea puerperal disease, and bad breath. The powdered pods are inhaled to reinstate sense of smell (El-Dakhakhny et al., 2002; Gilani et al, 2004; Botnick et al., 2012). Intriguingly, modern medicine has progressively shown interest in folk medicine, utilizing medicinal plants in the prevention and treatment of various diseases. To date, numerous bioactive compounds present in N. sativa have been isolated, identified, and described (Toma et al., 2015; Topcagic et al., 2017). The health-promoting properties of N. sativa could be attributed to their various bioactive constituents present in them including thymoquinone, phenolics, and alkaloids (El Mezayen et al., 2006; Kalantar et al., 2018; Dabeek et al., 2019; Anand et al., 2016).
Some active ingredients of Nigella sativa seeds and their biological activities
Nigella sativa (N. sativa) has been recognized as a medicinal remedy for over four millennia in many parts of the continents. The therapeutic and the antioxidant abilities of N. sativa have been ascribed to the bioactive phytochemicals present in them. In modern era, the seeds of N. sativa have been exposed to a broad variety of phytochemical investigation. Several studies have revealed the presence of diverse active phytochemical constituents present in the seeds of N. sativa. Thymoquinone (TQ) represents a major and abundant bioactive phytochemical in the essential oil of N. sativa (Kannan, 2022). TQ effectively modulates anti-inflammatory and immunomodulatory signaling pathways. The treatment with TQ via oral administration brought about a significant suppression in various pro-inflammatory mediators including tumor necrosis factor (TNF-α), Interleukin (6 and 1β), interferon gamma as well as prostaglandin E2 in rat model (Umar et al., 2012; Chen et al., 2018). More so, a study carried out by Ammar et al. demonstrated that TQ downregulated inducible nitric oxide synthase (NOS) messenger RNA’s expression inhibiting inflammatory pathways linked with asthma (Ammar et al., 2011). Another study revealed that TQ suppressed type 2 T helper cytokines. Nemmar and colleagues observed reduction in exaggerated white blood cell count and interleukin 6 levels in the plasma of diesel exhaust particle induced lung inflammation in mice treated with TQ via intraperitoneal injection (Nemmar et al., 2011). The anti-inflammatory ability of TQ in adjuvant induced- arthritis has been revealed to be caused by the suppression of TNF-α and IL-1β (Tekeoglu et al., 2007; Vaillancourt et al., 2011). In addition, TQ expends its effectiveness through COX modulation. Cyclooxygenase (COX) is excessively expressed during inflammation and regulated by cytokines. Chen and his team members revealed that the treatment with thymoquinone blocked the expression of PGE2, lowered COX-2 protein expression levels, and enhanced activated peroxisome proliferator-activated receptor γ expression in rats induced with spinal cord injury (Chen et al 2018). Also, this is consistent with a previous study which revealed that TQ remarkably decreased the expression of COX-2 and the recurring synthesis of prostaglandins in mice model of allergic airway inflammation (El Mezayen et al., 2006).
Carvacrol, a phenolic monoterpene found in N. sativa possesses inhibitory potential on neutrophil elastase which could be a therapy for anti- inflammatory disease such as emphysema, arthritis, and cardiac disease (Kacem and Meraihi 2006). Quercetin has been recorded over time as an effective bioactive ingredient of N. sativa that have a potent anti-inflammatory ability. Weng et al. reported that the oral administration of quercetin capsule ameliorated nickel patch induced dermatitis as well as ultraviolet B-induced skin erythema in patients (Weng et al., 2012). A study by Pfeuffer et al. demonstrated that quercetin suppressed markers of inflammation including TNF-α and IL-8 in patients diagnosed with sarcoidosis (Pfeuffer et al., 2013). Quercetin possesses cytoprotective, mast cell stabilizing, as well as immunosuppressive ability on the accessory (dendritic) cells (Huang et al., 2010). It also prevents disorders such as cardiovascular disease, osteoporosis, and cancer of the lungs (Mirsafaei et al., 2020; Derakhshanian et al., 2013; Zhao and Zhang, 2015). A particular finding demonstrated that quercetin prevents lipopolysaccharide-induced TNF-α generation in macrophages and lipopolysaccharide-induced interleukin 8 generation in A549 lung epithelial cells of tumor model (Gerates et al., 2007). However, in neuroglia, quercetin was reported to suppress lipopolysaccharide-induced mRNA TNF-α levels and IL-1α. This activity of quercetin brought about a notable apoptotic cell death in the neurons triggered by microglial (the innate immune cells of the central nervous system) activation (Bureau et al., 2008). Quercetin prevents COX and LOX through the blockage of phosphatidylinositol-3-kinase tyrosine phosphorylation and Toll-like receptor production that suppresses downstream signaling in RAW 264.7 cells (Endale et al., 2013; Lhadiee et al. 2010). Interestingly, quercetin revealed protective ability against hydrogen peroxide-induced inflammation in the cells of human umbilical vein endothelium. This protective ability was mediated through the suppression of adhesion molecule 1 of vascular cells and the expression of cluster of differentiation 80 (Dabeek et al., 2019; Anand et al., 2016). Kaempferol, a type of antioxidant polyphenol, was reported to prevent the oxidative damage of cells and the pathogenesis of atherosclerosis by suppressing platelet formation in the blood and low-density lipoprotein oxidation. The phenolic compound, kaempferol, also prevented atherosclerosis by blocking the oxidation of low-density lipoprotein (LDL) and development of platelet in the blood (Dabeek et al., 2019; Anand et al., 2016). The mice administered with α-terpineol was reported to inhibit the expressions of some pro-inflammatory cytokines such as COX-2, iNOS, IL1β, and TNF-α in peritoneal macrophages and NF-κB transcription factor activation (Kalantar et al., 2018). The α-terpineol was also observed to suppress myeloperoxidase activities in vaginal tissues of mice (Kacem, 2011). Histopathological findings revealed that p-cymene significantly suppressed lipopolysaccharide (LPS)-induced neutrophils in the tissue of the lung when compared to the control group. The study also demonstrated that p-cymene had a preventive effect against LPS-induced acute lung injury-induced mice (Jayakumar et al., 2020). Jayakumar et al. revealed that oral administration with the p-cymene notably ameliorated inflammatory cells in the bronchoalveolar lavage fluid (BALF), enhanced superoxide dismutase (SOD) activity, and lowered the lungs’ NF-κB protein and myeloperoxidase levels (Jayakumar et al., 2020).
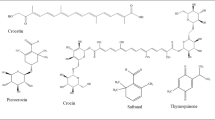
Nigellone, also known as dithymoquinone, is a dimerized structure of TQ. Nigellone, a crystalline bioactive compound, is the only constituent of the carbonyl fraction of the N. sativa oil. Nigellone is involved in histamine secretion causing relief in inflammatory conditions such as asthma (Wienkotteret al., 2008; Islam et al., 2017). The relaxation effect of black seed during inhalation in asthmatic patient could also be attributed to its bioactive component “nigellone” that prevents histamine release from mast cells (Chakravarty, 1993). Interestingly, phytonutrients studies have revealed that N. sativa are categorized according to their dual groups of alkaloid skeletons which include; isoquinoline alkaloids (nigellicimine-N-oxide, nigellicimine) and pyrazole or indazole alkaloids (nigellicine, nigellidine) (Fig. 3) (Akram Khan and Afzal, 2016; Ahmadi et al., 2016) as well as the Dolabellane-type diterpene alkaloids: nigellamines A1 (1), nigellamines A2 (2), nigellamines B1 (3), nigellamines B2(4) (Ambati et al., 2021; Hossain et al., 2021) promotes lipid metabolizing ability, thus modulating inflammatory pathways. N. sativa functions as a panacea showing a broadly diversified therapeutic properties which also have been ascribed to the chemistry of their alkaloid composition, although several studies have focused on the pharmacologic benefits of the phenolic constituents of N. sativa seed (Fig. 3).
Alkaloids present in Black Seed (N. sativa) (Akram Khan & Afzal, 2016)
Therapeutic and pharmacological values of black seed (N. sativa)
Several researchers have been investigated on the ability of N. sativa seed and its active constituents in the biological system (both in vitro and in vivo). The therapeutic and medicinal abilities of N. sativa seeds have been experimentally validated in pre-clinical and clinical trials (Table 1). Numerous findings have established the therapeutic and pharmacological abilities of these seeds as analgesic, immunomodulatory, anti-inflammatory, antioxidant, antipyretic, anticancer, antimicrobial, antiparasitic, antiviral, cardioprotective, and immune modulatory (Aikemu et al., 2013; Ahmad et al.2013; Feng et al., 2020; Koç et al., 2019; Randhawa et al., 2011; Ugur et al., 2016; Yimer et al., 2019; Umar et al., 2016) in folk medicine.
Antioxidant activity of black seed (N. sativa)
The anti-inflammatory and immunomodulatory properties of N. sativa have been well documented and have been reported to be hinged on the wheels of its antioxidant potentials. Some research studies have suggested the implication of reactive free radicals in immunomodulatory and anti-inflammatory functions (Islam et al., 2021). Oxidation reaction in foods is brought about by reactive oxygen species; reactive oxygen species (ROS) alter the functionalities of macromolecules like DNA, carbohydrates, proteins, and lipids (Yousefi et al., 2021). Oxidative stress, one of the key factors linked to inflammation and immune system dysfunction, results in the excessive production of reactive oxygen species when compared to the antioxidant defense system such as superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-s-transferase (GST), and catalase (CAT). Antioxidants prevent and protect cellular systems from the destructive activity of reactive oxygen and nitrogen species. Antioxidants plays the role of scavenging ROS. It can be produced by the cells either endogenously or through diet. N. sativa extract comprises various phenolics compounds responsible for its antioxidant property including carvacrol, gallic acid, kaempferol, etc. (Feng et al., 2020). Additionally, TQ and black seed-supplemented diets enhance enzymatic (SOD, CAT, GPX, and GST) and non-enzymatic activities (GSH and ascorbic acid) of antioxidants. N. sativa treatment caused a notable elevation in glutathione levels in the red blood cells (RBCs) and possesses a potent defensive ability against reactive oxygen and nitrogen species (ROS and RNS), along with inhibiting the mononuclear cells infiltration in the central nervous system (CNS) (Majdalawieh and Fayyad, 2015). N. sativa suppresses the reactive nitrogen species, hydrogen peroxide (H2O2), and hydroxyl (OH), superoxide (O2) radical’s generation (Gius and Spitz., 2006). The ROS synthesized in the joints has been implicated in arthritis as a result of the degradation of matrix constituents by biological oxidants via the latent collagenases activation (Luchian et al., 2022). Malondialdehyde (MDA) and thiobarbituric acid reactive substance (TBARS) are biological markers of lipid peroxidation. The oxidation of polyunsaturated fatty acids occurs via an array of chain reaction causing membrane destruction. N. sativa with potent antioxidant activities decreased the generation of ROS, thus, inhibiting lipid peroxidation (Burits et al., 2000; Al-Majed et al., 2006). N. sativa also lowered malondialdehyde (MDA) level in mice brain, liver, spleen, and blood plasma (Ardiana et al., 2020; Perera et al. 2021; Majdalawieh and Fayyad, 2015) (Table, 2, 3).
Effect of black seed (N. sativa) on anti-inflammation
The recent proof of anti-inflammatory ability of N. sativa is favorable. Diverse research studies have established the anti-inflammatory abilities of black seed in the biological system. The predominant inflammatory mediators (cytokines, prooxidants, lytic enzymes, nitric oxide, and eicosanoids including prostaglandins and leukotrienes) are often produced by neutrophils, lymphocytes, macrophages, as well as impaired tissue itself (Majdalawieh and Fayyad, 2015; Mahboubi et al., 2018). Excessive generation of pro-inflammatory cytokines, ROS, eosinophils, and neutrophils have been implicated in the pathophysiology of inflammation (Byun et al., 2021). N. sativa and its active phytochemical, TQ have been reported to significantly decrease the synthesis of inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), nuclear factor kappa B (NF-kB), lipoxygenase, cyclooxygenase-2, monocyte chemoattractant protein (MCP-1), C-reactive protein (CRP), and interleukin (IL-1β) in pancreatic ductal adenocarcinoma (PDA) cell in animal model (Sallehuddin et al., 2020; Mahmoud and Abdelrazek, 2019; Chehl et al., 2009; Al-Ghamdi 2001). The abilities of N. sativa oil and some of its active components have been recorded in various models of inflammation (including peritonitis, encephalomyelitis colitis, edema, and arthritis) to cause suppression of inflammatory mediators (Sallehuddin et al., 2020). Some studies revealed the inhibitory activity of N. sativa on protein synthesis and mRNA expression on IL-4, IL-5, and TNF-α, and also stimulated IFN-γ production (El Gazzar et al., 2006; Aslam et al., 2018). The Plasmodium parasite and ruptured erythrocytes activates inflammatory response in the host. Ojueromi et al reported that the dietary supplementation of N. sativa seed at percentage inclusion of 5% and 10% significantly suppressed TNF-α, IL-6 levels and increased IL-10 levels in Plasmodium berghei -infected mice (Ojueromi et al., 2022)
Intriguingly, the treatment of Algas-induced paw edema, with N. sativa oil (400 mg/kg), brought about a notable reduction in the pro-inflammatory cytokines including interleukins, and TNF-α in paw serum and pus of rats (Attia et al., 2016). However, topical medication with balm stick containing N. sativa oil (10%) in wistar rats with paw edema decreased inflammatory response with a significant edema inhibition (between 60 and 64%), decreased white blood cell count (approximately 44% lower than control), and TNF-α (55% lower than control) at the inflammatory site (Dwita et al., 2019). An in vivo finding done by Houghton et al. revealed that the administration of N. sativa fixed oil to rats brought about a significant inhibition in 5-LOX and COX of arachidonate metabolic pathway in the peritoneal leukocytes due to the suppression of leukotriene B4 (LTB4) and thromboxane B2 (TXB2) metabolites (Houghton et al. 1995; Salem, 2005). Most studies revealed that N. sativa seed and its biological constituents inhibit various inflammatory mediators and hence may be advantageous in mitigating autoimmune and inflammatory conditions. The seeds of N. sativa are efficient for treating patients with chronic anti-inflammatory disease (Eid et al., 2017; Koshak et al., 2018). Thymoquinone derived from N. sativa seeds can decrease oxidative stress and regulate the pro- and anti-inflammatory cytokines balance (Elsherbiny et al., 2017). The seeds were also observed to cause an elevation in the ratio of helper to suppressor T cells and to augment natural killer cell activity in human (Majdalawieh and Fayyad, 2015). A recent research study revealed the suppression of tumor necrosis factor-alpha (TNF-α) levels in septic peritonitis of murine model by thymoquinone (Theofanidou et al., 2021) and the distinctive immune modulatory ability of N. sativa. The capacity of TQ to regulate chemokines synthesis and boost immunity could be the major factor for its protective property in the liver against schistosomiasis (Mahmoud et al., 2021; Mushtaq, et al., 2021). Thriving evidence also showed that elevated NO levels have been implicated in the pathogenesis of inflammation and arthritis. Nitric oxide (NO) is a signaling molecule that performs a crucial function in the pathogenesis of inflammation. It plays a regulatory role in several inflammatory conditions. Conversely, NO is regarded as a pro-inflammatory mediator that incites inflammation and tissue damage as a result of excessive production in aberrant condition. Elevated serum NO levels and synovial fluid have been recorded in rheumatoid arthritis and osteoarthritis patients (Fonseca et al., 2019). However, reducing NO through inducible nitric oxide synthase (iNOS) inhibition decreased aggravated inflammatory response (Mahboubi et al., 2018). Bahrami and colleagues showed that N. sativa remarkably inhibited NO level in peritoneal macrophages and RAW264.7 cells (Bahrami et al., 2021). More so, treatment with N. sativa caused a significant reduction in NO, IL-6, and TNFα levels of lipopolysaccharide (LPS)- and IFNγ-induced peritoneal macrophages in dose-dependent manner in vitro (Hadi et al., 2015). These studies are in concordance with a finding which showed that N. sativa demonstrated inhibitory ability on NO level in murine macrophages Majdalawieh and Fayyad, 2015). Also, the findings done by El-Mahmoudy et al. revealed that TQ decrease the NO level of lipopolysaccharide-activated macrophages as well as preserving the cell’s viability (El-Mahmoudy et al., 2002). The levels of protein and mRNA of inducible nitric oxide synthase (iNOS) in peritoneal macrophages of the rats were also lowered by TQ.
Lipoxygenase (LOXismailsogut) and cyclooxygenase (COX) are core lipid mediators of inflammatory pathways. They are essential enzymes that catalyzes the conversion of arachidonic acid to eicosanoids. N. sativa also lowered the lipoxygenase and cyclooxygenase activity of the arachidonate metabolic pathway, which have been involved in the generation of various inflammatory biomarkers (Montazeri et al., 2021). Furthermore, Al Wafai et al. assessed the synergistic ability of N. sativa and TQ supplementation in streptozotocin (STZ)-induced diabetic rats and it was observed to prevent COX-2 expression in pancreatic tissue (Al Wafai et al., 2013). Another study also reported the anti-oxidative and anti-inflammatory efficacy of N. sativa via the suppression of NF-kB and COX-2 expression as well as the preservation and up-regulation of protein expression of the cells of the bare mouse skin (Kundu et al., 2013). Some plausible anti-inflammatory mechanisms of N. sativa could be linked to the suppression of TNF-α-linked stimulation of NF-κB via lowering the ability of the NF-κB kinase (IKK) inhibitor, and also decreasing the transfer of NF-κB (transcription factor provoking inflammation) from the cytosol to the nucleus (Woo et al., 2012). This is in agreement with a study which revealed that TQ blocked TNF-α-induced nuclear factor kappa B initiation via the inhibition of I Kappa B Kinase (IKK) activity causing reduced phosphorylation and the breakdown IKB alpha inhibitor (Sethi et al., 2008). However, these molecular occurrences could significantly reduce the pro-inflammatory mediators like TNF-α, matrix metalloproteinases (MMPs), IL-1β, COX-2, and PGE2 expression (Majdalawieh & Fayyad, 2015). Interleukins (IL-6 and IL-18) have been reported to trigger some inflammatory disorder such as atherosclerosis (Coveney et al., 2021). One of the major anti-inflammatory cytokines observed in atherosclerosis and rheumatoid arthritis is Interleukin-10 (IL-10) synthesis. The IL-10 was shown to prevent age-connected elevation in oxidative stress and IL-6 levels (Girard et al., 2016). The oral administration of encapsulated N. sativa oil (1 g/patient/day) for 2 months caused a notable elevation in IL-10 level but significantly reduced TNFα levels in the serum, showing that N. sativa could attenuate the inflammatory responses in rheumatoid arthritis patients (Majdalawieh et al., 2010). Much emphasis has been laid on the inflammatory mechanism of N. sativa compared to its anti-inflammatory mechanism. Both inflammatory and anti-inflammatory role possess similar mechanism of action. Persistent inflammatory response can lead to chronic inflammation which can damage the biological tissue and cause the failure of the target organ.
Effect of black seed (N. sativa) on immunomodulation
Nigella sativa performs a pertinent role in the immune-boosting ability and treatment of disorders such as rheumatoid arthritis, Hashimoto’s thyroiditis, eczema, allergic asthma, etc. Drug resistance and residual of immunomodulatory drugs like thalidomide, lenalidomide, pomalidomide, and antibiotics are common upon administration against pathogenic organisms. However, natural therapy might be advantageous compared to synthetic drugs. N. sativa seeds and their purified bioactive ingredients have been extensively utilized in the treatment and prevention of several immune system disorders. N. sativa has a potentiating ability on the cellular immunity mediated by T cells which are connected to immune response (Fig. 5) (Salem, 2005). A particular study revealed that N. sativa oil decreases the anti-islet cell antibodies level, and the principal antibodies generated in autoimmune process and brought about significant reduction of immunological markers {Pan B-lymphocytes (CD19), Pan innate cells (CD 1 lb), and Pan T- lymphocytes (CD90)}in Streptozotocin-Induced type l diabetes rat model (Hmza et al., 2013). Paarakh reported that N. sativa oil enhances immune resistance in the geriatrics and T lymphocytes’ cell production (Paarakh, 2010). N. sativa significantly elevated total white blood cells (leukocytes) count and hemoglobin levels induced by cisplatin in mice model as well as stimulating the production of T-lymphocytes cells. Approximately 3% increase was observed in the total leukocyte count of mice treated with N. sativa (Tutuncu, 2020). Studies have shown that N. sativa stimulates T cells synthesis, thus, aiding the secretion of IL-3 but no stimulatory ability on IL-2 production. N. sativa protein elevated absolute lymphocytes count and, in this regard, possesses immunomodulatory ability in the biological system (Randhawa and Alghamdi, 2011; Salama, 2010). Some studies revealed that N. sativa elevated T-lymphocyte cell production, clusters of differentiation (CD3, CD4, CD8) surface antigens, and some immune cells. Due to this elevation, N. sativa and thymoquinone were observed to activate blood cellular component (hematopoiesis) and modulate immune-related cells formation (Rahmani and Aly, 2015; Tutuncu, 2020). This is consistent with a particular study which revealed that N. sativa enhances the total leukocytes and hemoglobin count induced by cisplatin in mice model. Also, this seed increased (cluster of differentiation 3) plus T-lymphocyte cells; (cluster of differentiation 4) plus helper T-lymphocyte cells; (cluster of differentiation 8) plus suppressor to cytotoxic T lymhocyte cells (Mills and Bone, 2010). Alsamarai et al. reported that the allergic rhinitis patients going through allergen-specific immunotherapy for a month combined with the consumption of N. sativa (2 g/day) along with immunotherapy experienced better progress (polymorphonuclear leukocytes functions (PMNs) and cluster of differentiation 8 (CD8 counts) than patients who received only immunotherapy for 2 months (Alsamarai et al., 2009). He concluded that administration of N. sativa contributed toward more effective immunotherapy.
The initial step of the inflammatory cascade entails the identification of tissue or endothelial injury. Macrophages become activated as a result of tissue damage triggering a complex series of cellular and inflammatory responses and greatly affected as a result of the magnitude, nature, and duration of the injury. The activated neutrophils, lymphocytes, eosinophils, inflammatory mediators [including cytokines, C-reactive protein (CRP), ROS, Nuclear factor kappa B (NF-kB), growth factors, prostaglandins, leukotrienes, and nitric oxide (NO)] are involved in inflammatory and immune response. Neutrophils induce proteolytic enzymes. These specific set of enzymes are implicated in extracellular matrix damage (ECM). Neutrophils mediate ECM remodeling by producing specific remodeling enzyme including matrix metalloproteinases (MMPs) and neutrophil elastase, leading to myriad of inflammatory and immune system dysfunction such as rheumatoid arthritis and asthma
The level of immunoglobulins (IgM and IgA) and complements (C3 and C4) revealed no notable differences in the control and N. sativa-treated groups of immunosuppressed rabbit induced with dexamethasone. However, the IgG level was significantly elevated in the immune-suppressed mice treated with N. sativa and remarkably suppressed in the untreated rabbits. N. sativa demonstrated a potent immune modulatory effect, thus enhancing cellular immunity in normal and immune-suppressed rabbits (Al-Saaidi et al., 2012). Furthermore, a notable increase in the weight of the kidney, lymph node, and liver were observed in the immunosuppressed rabbits. Also, there was reduction in the total white blood cell count, monocytes eosinophils, and lymphocytes but elevation in their neutrophil levels. Conversely, N. sativa seed reversed this effect by elevating lymphocyte and suppressing neutrophil levels. This finding is consistent with some studies which reported the effect of dexamethasone to stimulate high degranulation and aggregation of the inflammatory focus of the white blood cells (Salem and Hussain, 2000; Jeklova et al., 2008). This could be as a result of the secretion and translocation of white blood cells differentials (with the exception of neutrophils) from circulation to lymphatic tissue or hypergenesis. A particular finding by Al-Saaidi et al. revealed elevation in the phagocytic ability of male rabbits treated with N. sativa, which could be due to its immune’s regulatory potential (Al-Saaidi et al., 2012). This result also proved the elevated leukocytes inflammatory core degranulation by dexamethasone. The administration of lipopolysaccharide (LPS) elevated the total leukocyte, neutrophils, monocytes’ eosinophils, and basophils’ count and reduced lymphocytes count. However, treatment with the extract of N. sativa (200, 400 mg/kg) on LPS-induced lung injury in rats significantly reversed the effect in dose-dependent manner (Mokhtari-Zaer et al., 2020). Immunoglobulins (IgG and IgM) play a pivotal part in reducing parasitemia levels and ameliorating the enervating effect of Plasmodium parasites. Interestingly, our findings revealed that N. sativa supplemented diet enhanced IgG and IgM antibody levels in the serum of Plasmodium berghei-infected mice (Ojueromi et al., 2022).
The immune stimulating property of N. sativa on cell line of murine macrophage (J774A.1) was evaluated by Sheik et al. The result showed that N. sativa extract modulated immune function by elevating the rate of proliferation of macrophage population and stimulating phagocytic activity (Sheik et al., 2017; Dalli et al., 2022). The anti-oxidative and anti-inflammatory ability of the N. sativa may enhance its function in stimulating the immune and phagocytic cells (Salem, 2005; Abdulelah, 2007), its activity on the synthesis of DNA during cell proliferation, and scavenging ability of superoxide radicals (Musa, 2004). A particular study carried out by Salem et al. recorded the ability of N. sativa to decrease the deleterious side effects of dexamethasone which may be associated with the ability of the N. sativa proteins to regulate the production of IL-1 and IL-3 by lymphocytes, as it has been demonstrated when cultured with or without allogenic cell (Salem et al., 2005). The oral administration of encapsulated N. sativa oil (dosage of 40–80 mg/kg per day) in individuals diagnosed with asthma, eczema, and allergic rhinitis significantly suppressed the immunoglobulin levels (IgE), eosinophil count, and endogenous cortisol in the urine and blood plasma when compared with the control (Kalus et al. 2003). In addition, ovalbumin-induced asthmatic mice revealed significant decrease in T helper 2 cytokines, leukotrienes-B4, C4, and eosinophils in BALF. Interestingly, another study reveals that TQ mitigated ovalbumin-induced airway inflammation by preventing the expression of COX-2 and prostaglandin D2 production. This preventive effect on prostaglandins production is enhanced by a notable reduction in T helper type 2 cytokines, goblet cell hypergenesis, immunoglobulins, and activated eosinophils in OVA-induced mice (El Mezayen et al., 2006). Intriguingly, TQ was reported to inhibit IL-4, and suppress activated eosinophils in the airway and IgE level in the serum of murine allergen-induced mice (Renz et al., 1995).
Immunopathogenesis of asthma and the dietary intervention of black seed (Nigella sativa)
Asthma is characterized by prolonged inflammatory disorder of the respiratory system connected to a specific pattern of network of mutually interacting cytokines, inflammatory cells (mast cells, plasma cells, lymphocytes, eosinophils, neutrophils, etc.), and excessive synthesis of mucus. Asthma statistics worldwide revealed that approximately 300 million (300,000,000) individuals are affected around the globe and it is probable that additional 100 million (100,000,000) may be vulnerable by the year 2025 (Afzal et al., 2022). Asthma is prevalent in both children and adult. The use of glucocorticosteroids, a core anti-inflammatory therapy for asthma, gives rise to adverse reaction (reduced bone metabolism, stunted growth, and adrenal suppression) with non-specificity in their mechanism of action (Ora et al., 2020). More so, patients diagnosed of asthma react unsatisfactorily or appallingly to steroid therapy (Henderson et al., 2020). Therefore, efforts are channeled towards the need to invent and advance specific and safe therapeutic anti-asthmatic drugs made from natural products. Research findings suggest that some natural plant seeds which include N. sativa in folklore possess remarkable effect in decreasing the intensity of respiratory disorder Koshak et al., 2018; Ikhsan et al., 2018). The pathophysiology of asthma originates from diverse interconnection between cells and mediators of inflammatory and immune system disorder. The principal immune cells implicated in asthma-related inflammation were mast cells, dendritic cells, T helper cells (namely Th1, Th2, and Th9 cells, etc.), B cells, innate lymphoid cells, neutrophils, eosinophils, basophils, macrophages, and epithelial cells (Koshak et al., 2018; Ikhsan et al., 2018), and they function in the pathophysiology of airway and lung inflammation in asthma progression. Actually, Th2 cells also performs a pertinent function in the pathophysiology of allergic airway inflammation via the proliferation of cytokines (Chang et al., 2022; Chen et al., 2022). The result obtained from the study buttressed the point that Th2 inflammatory activity are majorly caused by the cytokines proliferation. In asthmatic condition, the pro-inflammatory mediators released by Th2 cells which include, IL-4, IL-5 and IL-13 as well as IFN-γ and IL-6 (Th1 cells-secreted cytokines) were observed to be the key cytokines stimulated in asthma (Beasley et al., 2019) (Fig. 5 & 6). Interleukin-6 (IL-6), a core pro-inflammatory mediator was significantly elevated in the bronchial epithelial cells, BALF, stimulated sputum in the serum of patients diagnosed of asthma (Ilmarinen et al., 2016; Neveu et al., 2010). Interleukin 6 also performs a central role in the adaptive immune response via the induction of Th2 cells development (Matucci et al., 2021). Pre-clinical and clinical studies demonstrated that N. sativa exhibits bronchodilatory and anti-allergic properties. Diverse research findings have assessed the effect of N. sativa and its bioactive constituents on the anti-inflammatory and immunomodulatory markers associated with asthma (Balaha et al., 2012; Saadat et al., 2015; Shahzad et al., 2009; El Gazzar et al., 2006). The mice treated with N. sativa was reported to potentially suppress IL-4, IL-5, IL-13, and MUC5, a gene expression in OVA-induced asthmatic mice (Azimi et al., 2016). Azimi et al. also revealed that N. sativa possessed anti-inflammatory, immunomodulatory and mucus hypersecretion potential as a result of eosinophil infiltration, inhibition of Th2 cytokine and also suppressed the overproduction in the airways of asthmatic mice (Azimi et al., 2016). Koshak et al. recorded that N. sativa and TQ enhanced immune function and decreased asthma-associated inflammatory mediators such as IL-2, IL-6, and PGE2 in primary human T-lymphocytes, monocytes, and A549 human lung epithelial cells (Koshak et al., 2018) This is in concordance with the study conducted by Shahzad et al. who investigated the immunomodulatory ability of N. sativa oil in allergic airway inflammation using rat model (Shahzad et al., 2009). It was observed that N. sativa mitigated OVA-induced airway inflammation via the suppression of T cells causing a remarkable reduction in Th2 cells, goblet cell hyperplasia, NO generation, cytokines (IL-4, IL-5, IL-6 and TGF-β1), immunoglobulin, and lung eosinophil level in OVA-induced rats. The TGF-β has been reported to stimulate the proliferation of goblet cells and excessive secretion of the mucus (Young et al., 2021). The elevation in eosinophil production as well as Immunoglobulins (IgG and IgE) levels can be traced to increased synthesis of interleukins (IL 4 & 5) levels in the rats (Shahzad et al., 2009). Dajani et al. revealed that the asthmatic symptoms (night wheezing, night coughing, and chest wheezing) decreased significantly after 45 days of treatment with the N. sativa extract (15 ml/kg/day) compared to the control (placebo) group (Dajani et al., 2018). Koshak, et al. carried out a randomized, double-blind, placebo-controlled trial in which N. sativa oil (500 mg twice daily) was utilized as a supplementary treatment in eighty (80) asthmatic patients. The N. sativa-treated group revealed remarkable improvement in asthma test scores and pulmonary function tests (PFTs) when compared with the placebo group after four (4) weeks of treatment. (Koshak et al., 2017) Remarkably, observed improvement was connected to a significant normalization of blood eosinophilia in the patients. Intriguingly, Boskabady et al. also reported a randomized double-blinded clinical trial that revealed a short bronchodilator activity in asthmatic patients after administration of a single dose of N. sativa (50 mg/kg) (Boskabady et al., 2010).
N. sativa downregulates inflammatory and immune signaling pathways [IRF 3 (Interferon regulatory factor 3); TBK-1 (TANK-binding kinase-1); ERK1/2 (Extracellular signal-regulated kinase 1/2); IRAK-linked AP1 (Interleukin-1 Receptor Associated Kinase Activator Protein-1); JAK/STAT (Janus kinase-signal transducer and activator of transcription) and JNK (c-Jun N-terminal kinase) phosphorylation} and transcription factor {NF-kB (Nuclear factor kappa B); HIF-1α (hypoxia-inducible factor 1); STAT1, STAT3, STAT4, STAT 6 (Signal transducer and activator of transcription 1, 3, 4, and 6), respectively]. During inflammatory and immune response, the excessive ROS generated inhibit antioxidant enzymes, modulate immune markers, and activate inflammatory mediators [interleukins (IL-2, 4, 5, 6, 8, 12, 13), TNF-α (tumor necrosis factor-alpha), CRP (C-reactive protein), MCP-1 (monocyte chemoattractant protein-1), LOX (lipooxygeenase), PGE2 (prostaglandin E2) NF-kB} and oxidative stress markers {Malondialdehyde (MDA) Myeloperoxidase (MPO), Peroxynitrite (ONOO)−]
Nitric oxide, a biomarker of lung inflammation, and elevated exhaled NO levels have been observed in asthmatic patients (Heaney et al., 2019; Duong-Quy et al., 2019). It was observed that the treatment with N. sativa oil remarkably suppressed NO generation in OVA-induced rats. Previous studies have shown the important alkaloid components (nigellidine, nigellimine, and nigellicine) present in N. sativa could alter NO production (Khaldi et al., 2017; Nayeem et al., 2022). The inhibitory ability of N. sativa oil on the inflammation of the airway could be as a result of the reduction in Th2 cytokine level and consequent mitigation of inflammatory mediators. Hence, N. sativa can be endorsed as a pharmacological substance for the treatment of allergic asthma.
Immunopathogenesis of rheumatoid arthritis and the dietary intervention of black seed (Nigella sativa)
Rheumatoid arthritis is a prolonged inflammatory disorder that affects the joint of synovial-lined diarthrodial, as well as joints of the hand and the wrist, and it lacks effective cure. Rheumatoid arthritis was first initiated in the year, 1859, by Garrod and stated as a systemic inflammatory disorder that is predominant in the female gender associated with rheumatoid factor and dayspring inflexibility which could lead to moderate or critical disability (Christian and Paget, 1978). Although there is dearth of information as regards the pathogenesis of rheumatoid arthritis, but the activation of broad range of the humoral and cellular components of the immune system contributes to its pathology. It is believed that immense activation of the B- and T-lymphocyte antigens as well as macrophage interactivity in the inflamed synovial membrane results in lymphocytes’ release from T cells and antibodies (immunoglobulins) from plasma cells. Immune dysfunction is one of the major factors that contributes to the progression of rheumatoid arthritis via the discovery of rheumatoid factors including anti-immunoglobulin antibodies (IgM, IgG, IgA, and IgE) formerly by Erik Waaler in the 1940s and later explicitly described by Devi and his colleagues (Devi et al., 2021; Sieghart Waaler, 2018). Bandilla et al. investigated the core antibody in rheumatoid arthritis patients in response to three antigens (sheep cell stroma and Brucellin) (Bandilla et al., 1970). A probable description for deficiency in IgM level by rheumatoid arthritis patients is the aspect of antigenic competition. This term has been used by Adler to describe the inhibition of production of antibody to a second antigen by a host intensively immunized with a prior antigen (Adler, 1964). Increased levels of immunoglobulins, high titers of rheumatoid factors, and low levels of complement in synovial fluid in patients with rheumatoid arthritis suggest that the patients are making an intense immune response to an unidentified antigenic stimulus. A significant suppression in the IgM response to Brucellin than sheep cell stroma was observed in rheumatoid arthritis patients (Bandilla et al., 1970). Oxidative stress and pro-inflammatory cytokines, such as IL-1, IL-6, IFN-γ, CRP, TNF-α, and GM-CSF, has been linked to the pathogenesis of rheumatoid arthritis (Lucas et al., 2020; Kim et al. 2019). However, the excessive production of TNF-α is proposed to be the core contributor to elevated release of ROS in patients diagnosed with rheumatoid arthritis. Conversely, Regulatory T cells (Tregs) suppressed immune response overactivity via the generation of anti-inflammatory cytokines of TGF-β and IL-10 and prevented exaggerated inflammatory states (Lucas et al., 2020). The findings by Kril et al. revealed that neutrophils and monocytes as the key target cells of the innate immune system that regulate the development and progression of inflammatory rheumatic diseases (Kril et al., 2020). Hadi et al. reported the supplementation N. sativa seed for eight weeks notably suppressed the pro-inflammatory cytokine (TNFα) and increased anti-inflammatory cytokine (IL-10) of patients diagnosed with rheumatoid arthritis (Hadi et al., 2016). A particular study on rheumatoid arthritis showed that administration of N. sativa orally significantly reduced TNF-α, NF-kβ, IL-6, MDA, NO, and increased IL-10 levels in animal model (Zielińska et al., 2021; Mahboubi et al., 2018; Khabbazi et al., 2020).
Signaling pathway associated with inflammation and immune system disorders
Various findings have been carried out to unravel the signal transduction mechanism utilized by N. sativa and its bioactive constituents to demonstrate their anti-inflammatory and immunomodulatory abilities. Inflammatory disorders develop as a result of the elevation of pro-inflammatory cytokines (IL-1, TNF, IFN, NF-kB), granulocyte–macrophage-colony-stimulating factor and the suppression of anti-inflammatory cytokines including IL-10 and TGF-β. N. sativa was shown to activate the synthesis of T-lymphocytes and macrophages, thus, aiding the secretion of interleukin (Haq et al., 1995). The stimulatory and suppressive abilities of N. sativa on cultured lymphocytes to modulate the inflammatory cytokines levels including interleukins were also observed (Haq et al., 1999). In the biological system, the signal transduction pathways must have sufficient regulatory steps in a physiological state. The anti-inflammatory ability of N. sativa and their active constituents can be linked to various signaling pathways. N. sativa and bioactive phytochemicals, known for their immune modulatory ability, transduce their effect by targeting some specific signaling pathways. Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-ΚB) is a principal dimeric eukaryotic transcription factor that regulates genes crucially associated with several biological processes including immune and inflammatory response. The NF-kB proteins activation performs a main role in inflammation via the modulation of genes encoding pro-inflammatory mediators, and inducible enzymes including NO synthase and COX-2 (Ingawale and Mandlik, 2020). Hossen et al. revealed that N. sativa and TQ suppressed pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, NO, iNOS, and COX-2 in lipopolysaccharide (LPS)-induced murine macrophage-like RAW264.7 cells, via an inhibition mediated by interleukin-1 receptor-associated kinase 1 (IRAK)-linked AP-1/NF-κB signaling pathways (Hossen et al., 2017). The study also utilized a kinase activity experiment to investigate the effect of TQ on the enzymatic ability of IRAKs. TQ lowered the inflammatory responses by targeting the activity of the enzyme and break down of IRAK 1, thus suppressing the downstream ability of NF-κB and Activator protein 1 (AP-1) transcription factor (in vitro and in vivo) (Fig. 6). TQ blocked the activation of NF-kB and 5-LOX by attenuating the effect of these inflammatory agents (El Mezayen et al., 2006) The TNF-α-activated Ik-β phosphorylation and degradation brought about the suppression of NF-kβ (Sethi et al., 2008). The stimulation of inflammatory signaling cascades in the cell caused the activation of some specific immune cells including, mast cell, macrophage and NK-cell and also affected inflammatory response (Kotowski et al., 2017; Ismail et al., 2016). NF-κBP65 is a core biochemical pathway responsible for the pro-inflammatory biomarkers’ expression induced by LPS. In a recent finding, there was a notable elevation in expression of NF-κBP65 in the LPS- induced group when compared with the normal control group; p-cymene (bioactive compound of N. sativa) was found to downregulate NF-κBP65 (Hossen et al., 2017; Chen et al., 2014). Chen et al. assessed the effect of p-cymene on the inhibition of TNF-α and IL-6 by interfering with the activation of NF-κB. The NF-κB signaling has been observed to function in the pathogenesis of acute lung injury (ALI) (Chen et al., 2014). The extracellular signal-regulated kinase (ERK1/2) signaling performs a crucial function in inflammation and is not only limited to NF-kB up-regulation but also involved in inflammatory burst, leading to the activation of phagocytosis (Monick et al., 2008). Carvacrol, a phenolic monoterpene compound, identified in N. sativa demonstrated potent anti-inflammatory ability by suppressing ERK1/2 and NF-kB signaling pathway as well as decreasing NO generation in LPS- activated macrophages. However, carvacrol inhibited the activity of LPS on the translocation of NF-kβ (p65) from the cytoplasm to nucleus. The attenuation of the transactivation by carvacrol with or without LPS was able to suppress the basal activation of NF-k B in in RAW 264.7 macrophages.
Also, in the cells of LPS-induced murine macrophage-like RAW264.7, N. sativa and TQ suppressed IRF-3 signaling, enhanced TANK-binding kinase autophosphorylation and decreased mRNA expression of interferons (IFN-α, β) (Aziz et al., 2018). The suppression of IRF-3 activation plays a central role in innate immune responses via the modulation of type one interferon synthesis (Aziz and Cho 2018). Interferon (IFN) utilizes the Janus kinase (JAK, a non-receptor tyrosine kinases family that transmits signals effected by cytokines through the JAK–STAT pathway) and majorly activates signal transducer and activator of transcription 1 (STAT 1). Intriguingly, IL-10 in turn activates STAT 3, STAT 4, and STAT 6 which are important for Th1 and Th2 progression. The STATs proteins are downstream signaling target of the Janus Kinase 2 required for the modulation of broad spectrum of cytokines, growth factors, as well as various cellular activity including immune response, cell proliferation, and death (Xin et al., 2020). It performs a central function in inflammation and are critical mediators of immunity against pathogens, and progression of inflammatory disease (Habbel et al., 2020). It functions through the JAK/STAT signaling pathway modulating genes expression associated with inflammatory and immune response. STAT 1, 3, and 4 was observed in inflammatory bowel disease, colitis, Crohn’s disease, and rheumatoid arthritis elevating IL-6 level in mice model (Parrello et al., 2000; Hanada and Yoshimura, 2002). STAT-4-driven Th1 responses have been revealed to be paramount in human Crohn’s disease. Activation of STAT 1 and 5 were also noticed in epithelial cells of asthmatic patients (Zhu et al., 2021). STAT 3 is involved in inflammation via the activation of T helper cells, inflamed epithelial cells, and fibroblast stimulating the production of inflammatory cytokines. Some studies showed that conditional knockout of STAT 3 in macrophages and neutrophils caused prolonged enterocolitis (Gonzalez et al., 2022; Takeda et al., 1999) This knockout might be due to Th1 stimulation via the inhibition of Interleukin-10 (a potent anti-inflammatory cytokine) signaling, which uses STAT3. Some findings have revealed the preventive effect of TQ in the pathogenesis of cancer (Imran et al., 2015; Shanmugam, et al., 2018). TQ prevented the constitutive and IL-6 inducible STAT3 phosphorylation in multiple myeloma cells (Li et al., 2010). More so, TQ significantly reduced JAK 2 effect in the gastric cells of patients diagnosed with cancer and also suppressed the expression STAT3-regulated gene (Zhu, et al., 2016). The Janus Kinase 2 and STAT 3 functions as important signaling pathway in inflammation (Zhu et al., 2016). STAT 5 also functions in the pathophysiology of leukemia and cancer (Dalgıç et al., 2015). A study conducted by Al-Rawashde et al. revealed that TQ notably suppressed the proliferation of chronic myeloid leukemia cells (K562) through JAK2/STAT3 and STAT5 gene downregulation and decreasing the level of STAT 5 and JAK 2 proteins (Al-Rawashde et al., 2021).
Conclusion
The therapeutic potential of N. sativa has been reported by several researchers promoting its utility in folklore to suppress the biological markers that have been implicated in the pathogenesis of inflammatory and immune system disorder. Intriguingly, the most remarkable signaling pathways targeted at N. sativa and its compounds were those connected to anti-inflammatory, immunomodulatory, and the antioxidant defense system. However, the bioactive components present in N. sativa have often been attributed to its pharmacological properties, but there is dearth of information on the molecular and cellular mechanism underlying the immunomodulatory and anti-inflammatory effect of some other active component of N. sativa. Despite the fact that the fundamental mechanism of action of these bioactive components of these seeds was carefully demonstrated, some are still incipient, hence, needs additional clarification. Noteworthy, outstanding development as regards the antioxidant, anti-inflammatory, and immunomodulatory abilities of N. sativa have been reported, yet, there is still limited clinical implementation and application. Majority of the findings in this review were carried out at the pre-clinical stage; therefore, it is necessary to perform more clinical trial of these studies for translation into clinical usage, most especially to abate chronic inflammatory and immune system disorders.
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- N. sativa :
-
Nigella sativa
- TNF-α:
-
Tumor necrosis factor
- IL-6:
-
Interleukin 6
- LOX:
-
Lipooxygenase
- LDL:
-
Low-density lipoprotein
- BALF:
-
Bronchoalveolar lavage fluid
- SOD:
-
Superoxide dismutase
- GPx:
-
Glutathione peroxidase
- OH:
-
Hydroxyl radical
- TBARS:
-
Thiobarbituric acid reactive substance
- ROS:
-
Reactive oxygen species
- MCP:
-
Monocyte chemoattractant protein
- DNA:
-
Deoxyribonucleic Acid
- Ig:
-
Immunoglobulin
- IRAK:
-
Interleukin-1 receptor-associated kinase 1
- MUC5:
-
Mucin 5
- TGF-β:
-
Transforming growth factor β (TGF-β)
- ALI:
-
Acute lung injury
- TBK:
-
TANK-binding kinase
- AP 1:
-
Activator protein 1
- STAT:
-
Signal transducer and activator of transcription
- TQ:
-
Thymoquinine
- COX:
-
Cyclooxygenase
- PGE2:
-
Prostaglandin
- INOs:
-
Inducible nitic oxide synthase
- NF-ΚB:
-
Nuclear factor kappa B
- CAT:
-
Catalase
- NO:
-
Nitric oxide
- MDA:
-
Malondialdehyde
- RBCs:
-
Red blood cells
- PDA:
-
Pancreatic ductal adenocarcinoma
- CD:
-
Clusters of differentiation
- LPS:
-
Lipopolysaccharide
- Th cells:
-
T helper cells
- OVA:
-
Ovalbumin
- CRP:
-
C-reactive protein
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- JAK:
-
Janus kinase
- IFN-γ:
-
Interferon gamma
- GM-CSF:
-
Granulocyte macrophage-colony-stimulating factor
References
Abdulelah H, Zainal- Abidin B (2007) In vivo anti-malarial tests of Nigella sativa (Black seed) different extracts. Am J Pharm Toxicol 2(2):46–50
Abel-Salam BK (2012) Immunomodulatory effects of black seeds and garlic on alloxan-induced diabetes in albino rat. Allergol Immunopathol (madr) 40:336–340
Adler FL (1964) Competition of antigens. Progr Allerg 8:41–57
Afzal S, Ramzan K, Ullah S, Jamal A, Basit S, AlKattan KM, Waqar AB (2022) Association between 17q21 variants and asthma predisposition in pashtun population from Pakistan. J. Asthma, (just-accepted), 1–20
Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, Anwar F (2013) A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed 3(5):337–352
Ahmadi M, Scurtu M, Tulcan C, Boldura OM, Milovanov C, Hutu I, Dronca D (2016) Nigella sativa—a plant with personality in biochemistry and experimental medicine researches. Bull Univ Agric Sci Vet Med Cluj-Napoca. Vet Med 73(2).
Aikemu A, Xiaerfuding X, Shiwenhui C, Abudureyimu M, Maimaitiyiming D (2013) Immunomodulatory and anti-tumor effects of Nigella glandulifera freyn and sint seeds on ehrlich ascites carcinoma in mouse model Pharmacogn. Mag 9:187–191. https://doi.org/10.4103/0973-1296.113258
Akram Khan M, Afzal M (2016) Chemical composition of Nigella sativa Linn: part 2 recent advances. Inflammopharmacol 24(2):67–79
Al Wafai RJ (2013) Nigella sativa and thymoquinone suppress cyclooxygenase-2 and oxidative stress in pancreatic tissue of streptozotocin-induced diabetic rats. Pancreas 42(5):841–849
Al-Ali A, Alkhawajah AA, Randhawa MA, Shaikh NA (2008) Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad 20(2):25–27
Al-Bukhari MI (1976) Division (71) on medicine. In: Sahi Al-Bukhari, the collection of authentic sayings of Prophet Mohammad (Peace be upon him). 2nd ed. Hilal Yayinlari, Ankara, Turkey, 1976.
Al-Ghamdi MS (2001) The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol 76(1):45–48
Ali BH, Blunden G (2003) Pharmacological and toxicological properties of Nigella sativa. Phyther Res 17:299–305
Al-Majed AA, Al-Omar FA, Nagi MN (2006) Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus [J]. Eur J Pharmacol 543(1–3):40–47
Al-Rawashde FA, Wan Taib WR, Ismail I, Johan MF, Al-Wajeeh AS, Al-Jamal HAN (2021) Thymoquinone Induces downregulation of BCR-ABL/JAK/STAT pathway and apoptosis in K562 leukemia cells. Asian Pac J Cancer Prevent 22(12):3959–3965
Al-Saaidi JAA, Dawood KA, Latif AD (2012) Immunomodulatory effect of Nigella sativa seed extract in male rabbits treated with dexamethasone. Iraqi J Vet Sci 26:141–149
Alsamarai S, Adams JM, Murphy MK, Post MD, Hayden DL, Hall JE, Welt CK (2009) Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metabol 94(12):4961–4970
Ambati RR, Ramadan MF (2021) Nigella sativa Seed extracts in functional foods and nutraceutical applications. In: Black cumin (Nigella sativa) seeds: Springer, Cham. Chem. Technol. Functionality, pp 501–520.
Ammar ESM, Gameil NM, Shawky NM, Nader MA (2011) Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. Intl Immunopharmacol 11(12):2232–2236
Anand David AV, Arulmoli R, Parasuraman S (2016) Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 10(20):84–89
Ardiana M, Pikir B, Santoso A, Hermawan H, Al-Farabi MJ, (2020) Effect of Nigella sativa supplementation on oxidative stress and antioxidant parameters: a meta-analysis of randomized controlled trials. Sci World J: 239–706.
Asiaei F et al (2017) Neuroprotective effects of Nigella sativa extract upon the hippocampus in PTU-induced hypothyroidism juvenile rats: a stereological study. Metab Brain Dis 32(5):1755–1765
Aslam H, Shahzad M, Shabbir A, Irshad S (2018) Immunomodulatory effect of thymoquinone on atopic dermatitis. Mol Immunol 101:276–283
Ates MB, Ortatatli M (2021) The effects of Nigella sativa seeds and thymoquinone on aflatoxin phase-2 detoxification through glutathione and glutathione-S-transferase alpha-3, and the relationship between aflatoxin B1-DNA adducts in broilers. Toxicon 193:86–92
Attia HN, Ibrahim FM, Maklad YA, Ahmed KA, Ramadan MF (2016) Characterization of antiradical and anti-inflammatory activities of some cold pressed oils in carrageenan-induced rat model of acute inflammation. Der Pharma Chem 8:148–158
Azimi E, Athari SM, Afshari F, Eftekhari A, Athari SS (2016) Effects of black seed (Nigella sativa) on type 2 cytokines gene expression and mucus production in the airways of asthmatic mice. Arch Med Lab Sci 2(2).
Aziz N, Son YJ, Cho JY (2018) Thymoquinone suppresses IRF-3-mediated expression of type I interferons via suppression of TBK1. Intl J Mol Sci 19(5):1–13
Badary OA, Al-Shabanah OA, Nagi MN, Al-Bekairi AM, Elmazar M (1998) Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res 44(2–3):56–61
Bahrami M, Ghazavi A, Ganji A, Mosayebi G (2021) Anti-inflammatory activity of S. Marianum and N. Sativa extracts on macrophages. Rep Biochem Mol Biol 10(2):288
Balaha MF, Tanaka H, Yamashita H, Rahman MNA, Inagaki N (2012) Oral Nigella sativa oil ameliorates ovalbumin-induced bronchial asthma in mice. Intl Immunopharmacol 14(2):224–231
Bandilla KK, Pitts NC, Mcduffie FC (1970) Immunoglobulin M deficiency in the immune response of patients with rheumatoid arthritis. Arthrit Rheumat off J Am College Rheumatol 13(3):214–221
Beasley R, Harper J, Bird G, Maijers I, Weatherall M, Pavord ID (2019) Inhaled corticosteroid therapy in adult asthma. Time for a new therapeutic dose terminology. Am J Respir Crit Care Med 199(12):1471–1477
Bashir O, Jan N, Gani G, Naik HR, Hussain SZ, Reshi M, Amin T, (2021) Food Applications of Nigella sativa Seeds. In Black cumin (Nigella sativa) seeds: Chem. Technol. Functional. App. Springer, Cham. 191–207.
Boskabady MH, Mohsenpoor N, Takaloo L (2010) Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomed 17:707–713
Botnick I, Xue W, Bar E, Ibdah M, Schwartz A, Joel DM, Lev E, Fait A, Lewinsohn E (2012) Distribution of primary and specialized metabolites in Nigella sativa seeds, a spice with vast traditional and historical uses. Molecules 17(9):10159–10177
Bruce SO, Nwafor OI, Omoirri MA, Adione NM, Onyeka IP, Ezeoru VC (2021) GC-MS, FTIR and Antiulcer screening of aqueous seed extract and oil of Nigella sativa in Wistar rats. J Drug Deliv Therap 11(6):48–60
Bureau G, Longpre F, Martinoli MG (2008) Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res 86:403–410
Burits M, Bucar F (2000) Antioxidant activity of Nigella sativa essential oil [J]. Phytother Res 14(5):323–328
Byun J, Kim SK, Ban JY (2021) Anti-inflammatory and anti-oxidant effects of korean ginseng berry extract in LPS-activated RAW264. 7 macrophages. Am J Chin Med 49(03):719–735
Chakravarty N (1993) Inhibition of histamine release from mast cells by nigellone. Ann Allergy 70(3):237–242
Chang JH, Chuang HC, Hsiao G, Hou TY, Wang CC, Huang SC, Lee YL (2022) Acteoside exerts immunomodulatory effects on dendritic cells via aryl hydrocarbon receptor activation and ameliorates Th2-mediated allergic asthma by inducing Foxp3+ regulatory T cells. Intl Immunopharmacol 106:10860
Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA (2009) Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB 11(5):373–381
Chen L, Zhao L, Zhang C, Lan Z (2014) Protective effect of p-cymene on lipopolysaccharide-induced acute lung injury in mice. Inflammation 37(2):358–364
Chen Y, Wang B, Zhao H (2018) Thymoquinone reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways. Exp Therap Med 15(6):4987–4994
Chen X, Bi M, Yang J, Cai J, Zhang H, Zhu Y, Zhang Z (2022) Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J Haz Mater 421:126–704
Christian CL, Paget SA (1978) Rheumatoid arthritis. Immunological 6. Dumonde DC, Glynn LE: The production of arthritis in rabDiseases, 3rd ed (Samter M, ed), Boston, Little, Brown & bits by immunological reaction to fibrin. Br J Exp Pathol Co 1061.
Coveney S, McCabe JJ, Murphy S, Belton O, Gray C, Cassidy T, Kelly PJ (2021) Dose-dependent association of inflammatory cytokines with carotid atherosclerosis in transient ischaemic attack: implications for clinical trials. Cerebrovasc Dis: 1–10.
Dabeek WM, Marra MV (2019) Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutr 11(10):2288
Dajani EZ, Shahwan TG, Dajani NE (2018) Overview of the human investigations of Nigella sativa (Black Seeds): a complementary drug with historical and clinical significance. Gen Internal Med Clin Innov 4:1–16
Dalgıç CT, Kaymaz BT, Özkan MC et al (2015) Investigating the role of JAK/STAT pathway on dasatinib-induced apoptosis for CML cell model K562. Clin Lymphoma Myeloma Leuk 15:161–166
Dalli M, Bekkouch O, Azizi SE, Azghar A, Gseyra N, Kim B (2022) Nigella sativa L. phytochemistry and pharmacological activities: a review (2019–2021). Biomol 12(1), 20.
Derakhshanian H, Djalali M, Djazayery A, Nourijelyani K, Ghadbeigi S, Pishva H, Dehpour AR (2013) Quercetin prevents experimental glucocorticoid-induced osteoporosis: a comparative study with alendronate. Can J Physiol Pharmacol 91(5):380–385
Devi DC, Ravichandran DR, Selvaraj D, Ramesh DS, Aarthipriya DT (2021) Comparative study of rheumatoid factor-igm autoantibody testing by latex agglutination nephelometry and elisa in patients with rheumatoid arthritis. Eur J Mol Clin Med 7(8):4521–4527
Dollah MA, Parhizkar S, Latiff LA, Hassan MHB (2013) Toxicity effect of Nigella sativa on the liver function of rats. Advanc Pharm Bull 3(1):97
Duong-Quy S (2019) Clinical utility of the exhaled nitric oxide (NO) measurement with portable devices in the management of allergic airway inflammation and asthma. J Asthma Allergy 12:331
Dwita LP, Yati K, Gantini SN (2019) The anti-inflammatory activity of Nigella sativa balm sticks. Sci Pharm 87:3
Eid AM, Elmarzugi NA, Abu Ayyash LM et al (2017) A review on the cosmeceutical and external applications of Nigella sativa. J Trop Med. https://doi.org/10.1155/2017/7092514
El-Dakhakhny M, Madi NJ, Lembert N, Ammon HPT (2002) Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol 81(2):161–164
El Gazzar M, El Mezayen R, Nicolls MR, Marecki JC, Dreskin SC (2006) Downregulation of leukotriene biosynthesis by thymoquinone attenuates airway inflammation in a mouse model of allergic asthma. Biochem De Biophys Acta Gen Subj 1760:1088–1095
El-Mahmoudy A, Matsuyama H, Borgan MA, Shimizu Y, El-Sayed MG, Minamoto N, Takewaki T (2002) Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Intl J Immunopharmacol 2(11):1603–1611
El Mezayen R, El Gazzar M, Nicolls MR, Marecki JC, Dreskin SC, Nomiyama H (2006) Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol Letts 106(1):72–81
Elsherbiny NM, Maysarah NM, El-Sherbiny M, Al-Gayyar MM (2017) Renal protective effects of thymoquinone against sodium nitrite-induced chronic toxicity in rats: impact on inflammation and apoptosis. Life Sci 180:1–8. https://doi.org/10.1016/j.lfs.2017.05.005
Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, Rhee MH (2013) Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators’ production in RAW 264.7 cells. Immunobiol 218:1452–1467
Feigin V, GBD (2017) Causes of death collaborators: Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study. Lancet 392, 1736–1788
Feng Y, Dunshea FR, Suleria HA (2020) Lc-esi-qtof/ms characterization of bioactive compounds from black spices and their potential antioxidant activities. J Food Sci Technol 57(12):4671–4687
Fonseca LJSD, Nunes-Souza V, Goulart MOF, Rabelo LA (2019) Oxidative stress in rheumatoid arthritis: what the future might hold regarding novel biomarkers and add-on therapies. Ox Med Cell Longev
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Slavich GM (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 25(12):1822–1832
Gerates L, Moonen HJJ, Brauers K, Wouters EFM, Bast A, Hageman GJ (2007) Dietary flavones and flavonols are inhibitor of poly (ADP-ribose) polymerase-1 in pulmonary epithelial cells. J Nutr 137:2190–2195
Gholamnezhad Z, Havakhah S, Boskabady MH (2016) Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: a review. J Ethnopharmacol 190:372–386
Gilani AUH, Jabeen Q, Khan MAU (2004) A review of medicinal uses and pharmacological activities of Nigella sativa. Pak J Biol Sci 7(4):441–451
Girard D, Tardif JC, Boisclair Demarble J, D’Antono B (2016) Trait hostility and acute inflammatory responses to stress in the laboratory. PLoS ONE 11(6):e0156329
Gius D, Spitz DR (2006) Redox signaling in cancer biology. Antioxid Redox Signal 8(7–8):1249–1252
Gonzalez CG, Mills RH, Kordahi M, Carrillo-Terrazas M, Secaira-Morocho H, Widjaja CE, Dulai PS (2022) Ulcerative colitis host-microbiome response to hyperbaric oxygen Therapy. medRxiv.
Habbel J, Arnold L, Chen Y, Möllmann M, Bruderek K, Brandau S, Hanoun M (2020) Inflammation-driven activation of JAK/STAT signaling reversibly accelerates acute myeloid leukemia in vitro. Blood Adv 4(13):3000–3010
Hadi V, Kheirouri S, Alizadeh M, Khabbazi A, Hosseini H (2015) Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed 6(1):34
Hadi V, Kheirouri S, Alizadeh M, Khabbazi A, Hosseini H (2016) Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed 6(1):34
Hadi V, Pahlavani N, Malekahmadi M, Nattagh-Eshtivani E, Navashenaq JG, Hadi S, Norouzy A (2021) Nigella sativa in controlling Type 2 diabetes, cardiovascular, and rheumatoid arthritis diseases: molecular aspects. J Res Med Sci Off J Isfahan Uni Med Sci. 26.
Hanada T, Yoshimura A (2002) Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev 13(4–5):413–421
Haq A, Abdullatif M, Lobo PI, Khabar KS, Sheth KV, Al-Sedairy ST (1995) Nigella sativa: effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity. Immunopharmacol 30(2):147–155
Haq A, Lobo PI, Al-Tufail M, Rama NR, Al-Sedairy ST (1999) Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Intl J Immunopharmacol 21(4):283–295
Heaney LG, Busby J, Bradding P, Chaudhuri R, Mansur AH, Niven R, Costello RW (2019) Remotely monitored therapy and nitric oxide suppression identifies nonadherence in severe asthma. Am J Resp Crit Care Med 199(4):454–464
Hench JW (2005) Inflammation and wound healing. In: Biomaterials, Artificial Organs and Tissue Engineering (pp. 71–76). Woodhead Publishing.
Henderson I, Caiazzo E, McSharry C, Guzik TJ, Maffia P (2020) Why do some asthma patients respond poorly to glucocorticoid therapy? Pharm Res 160:105–189
Hmza AJ, Osman MT, Adnan A, Omar E (2013) Immunomodulatory effect of Nigella sativa oil in the disease process of type 1 diabetic rats. Res J Pharm Biol Chem Sci 4(1):980–988
Hossain MS, Sharfaraz A, Dutta A, Ahsan A, Masud MA, Ahmed IA, Ming LC (2021) A review of ethnobotany, phytochemistry, antimicrobial pharmacology and toxicology of Nigella sativa L. Biomed Pharmacother 143:112–182
Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim JH, Cho JY (2017) Thymoquinone: an IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep 7(1):1–12
Houghton PJ, Zarka R, de las Heras B, Hoult JRS (1995) Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Medica 61(01), 33–36
Huang RY, Yu YL, Cheng WC, OuYang CN, Fu E, Chu CL (2010) Immunosuppressive effect of quercetin on dendritic cell activiation and function. J Immunol 184:6815–6821
Hussain DA, Hussain MM (2016) Nigella sativa (black seed) is an effective herbal remedy for every disease except death-a Prophetic statement which modern scientists confirm unanimously: a review. Adv Med Plant Res 4(2):27–57
Ikhsan M, Hiedayati N, Maeyama K, Nurwidya F (2018) Nigella sativa as an anti-inflammatory agent in asthma. BMC Res Notes 11(1):1–5
Ikram S, Suleman S, Ahmad SN, Nasir M, Kanwal MA, Raees K, Ahmad KR (2021) Nephroprotective role of Nigella sativa oil in bifenthrin-intoxicated mice. Fluoride 54(2).
Ilmarinen P, Tuomisto LE, Niemelä O, Danielsson J, Haanpää J, Kankaanranta T et al (2016) Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J 48:1052–1062. https://doi.org/10.1183/13993003.02198-2015
Imran M, Rauf A, Khan IA, Shahbaz M, Qaisrani TB, Fatmawati S, Gondal TA (2015) Thymoquinone: a novel strategy to combat cancer: a review. Biomed Pharmacother 106:390–402
Ingawale DK, Mandlik SK (2020) New insights into the novel anti-inflammatory mode of action of glucocorticoids. Immunopharmacol Immunotoxicol 42(2):59–73
Islam MT (2017) Nigella sativa, a promising source of respiratory medicines. Boletim Informativo Geum 8(2):16
Islam MN, Hossain KS, Sarker PP, Ferdous J, Hannan MA, Rahman MM, Uddin MJ (2021) Revisiting pharmacological potentials of Nigella sativa seed: a promising option for COVID-19 prevention and cure. Phytother Res 3:1329–1344
Ismail N, Ismail M, Azmi NH, Abu Bakar MF, Basri H, Abdullah MA, (2016) Modulation of hydrogen peroxide-induced oxidative stress in human neuronal cells by thymoquinone-rich fraction and thymoquinone via transcriptomic regulation of antioxidant and apoptotic signaling genes. Ox Med Cell Longev
Jayakumar T, Huang HC, Hsia CW, Fong TH, Khamrang T, Velusamy M, Hsia CH (2020) Ruthenium derivatives attenuate LPS-induced inflammatory responses and liver injury via suppressing NF-κB signaling and free radical production. Bioorganic Chem 96:103–639
Jaykare SC, Motghare VM, Padwal SL, Deshmukh VS, Patil JR, Pise HN, Pore RR (2013) Evaluation of anticonvulsant activity of the seed oil extract of Nigella sativa: an experimental study. Hygeia J Drugs Med 5:21–26
Jeklova E, Leva L, Jaglic Z, Faldyna M (2008) Dexamethasone-induced immunosuppression: a rabbit model. Vet Immunol Immunopathol 122:231–240
Kacem R (2011) Effects of Nigella (Nigella sativa L.) seed extract on human neutrophil elastase activity. In: Nuts and Seeds in Health and Disease Prevention (pp. 823–829). Academic Press.
Kacem R, Meraihi Z (2006) Effects of essential oil extracted from Nigella sativa (L) seeds and its main components on human neutrophil elastase activity Yakugaku Zasshi. J Pharm Soc Jpn 126(4):301–305
Kalantar K, Gholijani N, Mousaei N, Yazdani M, Amirghofran Z (2018) Investigation of Dracocephalum kotschyi plant extract on the effective inflammatory transcription factors and mediators in activated macrophages. Anti Inflamm Anti Allergy Agents Med. Chem. (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents) 17(1): 39–49
Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, Kiesewetter H (2003) Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res 17(10):1209–1214
Kannan R, George S, PS, B C, Maliakel B, Ittiyavirah S, Krishnakumar IM (2022) Thymoquinone-rich black cumin oil improves sleep quality, alleviates anxiety/stress on healthy subjects with sleep disturbances–a pilot polysomnography study. J Herb Med 32: 100507
Khabbazi A, Javadivala Z, Seyedsadjadi N, Mahdavi AM (2020) A systematic review of the potential effects of Nigella sativa on rheumatoid arthritis. Planta Med 86(07):457–469
Khaldi T, Rouibah Z, Rouag M, Messarah M, Boumendjel A (2017) Protective effects of Nigella sativa Oil on IL-4 and nitric oxide levels in a model of experimental asthma in wistar rat. In Euro-Mediterranean Conference for Environmental Integration (pp. 1249–1251). Springer, Cham.
Khan MA (1999) Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology 7(1):15–35
Khare CP (2004) Encyclopedia of Indian medicinal plants. Springer, Berlin Heidelberg New York
Koç A, Güven N, Öztürk SB, Dedeoğlu BD, Feyza H, Büyükhelvacigil RB, Koparal B (2019) Therapeutic trials of Nigella sativa. J Pharmacy Pharmacol 7(7):564–577
Koshak A, Wei L, Koshak E, Wali S, Alamoudi O et al (2017) Nigella sativa supplementation improves asthma control and biomarkers: a randomized, doubleblind, placebo-controlled trial. Phytotherap Res 31:403–409
Koshak AE, Yousif NM, Fiebich BL, Koshak EA, Heinrich M (2018) Comparative immunomodulatory activity of Nigella sativa L. preparations on proinflammatory mediators: a focus on asthma. Front Pharmacol 9:1075
Kotas ME, Medzhitov R (2015) Homeostasis, inflammation, and disease susceptibility. Cell 160(5):816–827
Kotowski U, Heiduschka G, Kadletz L et al (2017) Effect of thymoquinone on head and neck squamous cell carcinoma cells in vitro: synergism with radiation. Oncol Lett 14(1):1147–1151
Kril I, Havrylyuk A, Potomkina H, Chopyak V (2020) Apoptosis and secondary necrosis of neutrophils and monocytes in the immunopathogenesis of rheumatoid arthritis: a cohort study. Rheumatol Intl 40(9):1449–1454
Kulyar MFEA, Li R, Mehmood K, Waqas M, Li K, Li J (2021) Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: a hope to decelerate the COVID-19 pandemic. Phytomed 85:153–277
Kumari P, Kumar H (2022) Dimensions of inflammation in host defense and diseases. Intl Rev Immunol 41(1):1–3
Kundu JK, Liu L, Shin JW, Surh YJ (2013) Thymoquinone inhibits phorbol ester-induced activation of NF-κB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochem Biophys Res Commun 438:721–727
Li F, Rajendran P, Sethi G (2010) Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br J Pharmacol 2010(161):541–554
Lucas C, Perdriger A, Amé P (2020) Definition of B cell helper T cells in rheumatoid arthritis and their behavior during treatment. In: Seminars in Arthritis and Rheumatism. WB Saunders.
Luchian I, Goriuc A, Sandu D, Covasa M (2022) The role of matrix metalloproteinases (MMP-8, MMP-9, MMP-13) in periodontal and peri-implant pathological processes. Intl J Mol Sci 23(3):1806
Mahboubi M, Kashani LMT, Mahboubi M (2018) Nigella sativa fixed oil as alternative treatment in management of pain in arthritis rheumatoid. Phytomed 46:69–77
Mahmoud YK, Abdelrazek HM (2019) Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed Pharmacother 115:108783
Mahmoud HS, Almallah AA, El-Hak HNG, Aldayel TS, Abdelrazek HM, Khaled HE (2021) The effect of dietary supplementation with Nigella sativa (black seeds) mediates immunological function in male Wistar rats. Sci Rep 11(1):1–13
Majdalawieh AF, Fayyad MW (2015) Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Intl Immunopharmacol 28(1):295–304
Majdalawieh AF, Hmaidan R, Carr RI (2010) Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J Ethnopharmacol 131(2):268–275
Mansour MA, Ginawi OT, El-Hadiyah T, El-Khatib AS, Al-Shabanah OA, Al-Sawaf HA (2001) Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol 110(3–4):239–252
Matucci A, Bormioli S, Nencini F, Maggi E, Vultaggio A (2021) The emerging role of type 2 inflammation in asthma. Exp Rev Clin Immunol 17(1):63–71
Mills S, Bone K (2010) Principles and practice of phytotherapy. In: Modern herbal medicine: Churchill Livingstone
Mirsafaei L, Reiner Ž, Shafabakhsh R, Asemi Z (2020) Molecular and biological functions of quercetin as a natural solution for cardiovascular disease prevention and treatment. Plant Foods Hum Nutr 75(3):307–315
Mokhtari-Zaer A, Norouzi F, Askari VR, Khazdair MR, Roshan NM, Boskabady M, Boskabady MH (2020) The protective effect of Nigella sativa extract on lung inflammation and oxidative stress induced by lipopolysaccharide in rats. J Ethnopharmacol 253:112653
Monick MM et al (2008) Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity (in eng). J Immunol 180(11):7485–7496
Montazeri RS, Fatahi S, Sohouli MH, Abu-Zaid A, Santos HO, Găman MA, Shidfar F (2021) The effect of Nigella sativa on biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomized controlled trials. JFBC 45(4):e13625
Musa D, Duisiz N, Gumushan H, Ulakoglu G, Muharrem B (2004) Antitumor activity of an ethanol extract of Nigella sativa seeds. Biol Bratisl 59:635–670
Mushtaq A, Aslam B, Muhammad F, Khan JA (2021) Hepatoprotective activity of Nigella sativa and Piper nigrum against Concanavalin A-induced acute liver injury in mouse model. Pakistan Vet J 41(1).
National Center for Biotechnology Information (2022) PubChem Compound Summary for CID 5280343, Phenolic Compounds. Retrieved July 5, 2022 from https://pubchem.ncbi.nlm.nih.gov/compound/5280343.
Nayeem M, Ahmed MK, Jawed A, Alshahrani S, Makeen HA, Taha MM, Khan A (2022) A meta-analysis of Nigella sativa in respiratory disorders. In: Black Seeds (Nigella sativa) (pp. 177–196). Elsevier.
Nemmar A, Al-Salam S, Zia S, Marzouqi F, Al-Dhaheri A, Subramaniyan D, Kazzam EE (2011) Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Brit J Pharmacol 164(7):1871–1882
Neveu WA, Allard JL, Raymond DM, Bourassa LM, Burns SM, Bunn JY et al (2010) Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res 11:28
Ojueromi OO, Oboh G, Ademosun AO (2022) Effect of black seeds (Nigella sativa) on inflammatory and immunomodulatory markers in Plasmodium berghei- infected mice. JFBC :e14300
Okeola VO, Adaramoye OA, Nneji CM, Falade CO, Farombi EO (2011) Antimalarial and antioxidant activities of methanolic extract of Nigella sativa seeds (black cumin) in mice infected with Plasmodium yoelli nigeriensis. Parasitol Res 108:1507–1512
Ora J, Calzetta L, Matera MG, Cazzola M, Rogliani P (2020) Advances with glucocorticoids in the treatment of asthma: state of the art. Expert Opin Pharmacother 21(18):2305–2316
Paarakh PM (2010) Nigella sativa Linn.—a comprehensive review. Indian J Natl Prod 1:409–429
Pahwa R, Goyal A, Bansal P, Jialal I (2021) Chronic inflammation. [Updated 2020 Nov 20]. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Parrello T, Monteleone G, Cucchiara S et al (2000) Up-regulation of the IL-12 receptor beta 2 chain in Crohn’s disease. J Immunol 165:7234–7239
Patridge E, Gareiss P, Kinch MS, Hoyer D (2016) An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today 21(2):204–207
Perera WPRT, Liyanage JA, Dissanayake KGC, Chandrasiri WAL, Gunathilaka H (2021) A review on pharmacological activities and anti-microbial properties of Nigella sativa and isolated Thymoquinone. J Med Plants 9(3):118–122
Periasamy VS, Athinarayanan J, Alshatwi AA (2016) Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrasonic Sonochem 31:449–455
Pfeuffer M et al (2013) Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr Metab Cardiovasc Dis 23:403–409
Rahmani AH, Aly SM (2015) Nigella sativa and its active constituents thymoquinone show pivotal role in the diseases prevention and treatment. Asian J Pharm Clin Res 8(1):48–53
Randhawa MA, Alghamdi MS (2011) Anticancer activity of Nigella sativa (black seed)—a review. Am J Chin Med 39(06):1075–1091
Renz H, Ensslek K, Lauffer L, Kurrle R, Gelfand EW (1995) Inhibition of allergen-induced IgE and IgG1 production by soluble IL-4 receptor. Int Arch Allergy Immunol 106:46–54
Saadat S, Mohammadi M, Fallahi M, Aslani MR (2015) The protective effect of α-hederin, the active constituent of Nigella sativa, on tracheal responsiveness and lung inflammation in ovalbumin-sensitized guinea pigs. J Physiol Sci 65(3):285–292
Salama RHM (2010) Clinical and therapeutic trials of Nigella sativa. AF Prevent Med Bull 9(5):513–522
Salem M (2005) Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. J Int Immunopharmacol 5:1749–1770
Salem M, Hussain M (2000) Protective effect of black seed oil from Nigella sativa against murine Cytomegalo virus infection. J Immunopharmacol 22(9):729–740
Sallehuddin N, Nordin A, Bt Hj Idrus R, Fauzi MB (2020) Nigella sativa and its active compound, thymoquinone, accelerate wound healing in an in vivo animal model: a comprehensive review. Int J Environ Res Pub Health 17(11): 41–60
Sethi G, Sung B, Aggarwal BB (2008) TNF: a master switch for inflammation to cancer. Front Biosci 13(2):5094–5107
Shahid F, Farooqui Z, Khan AA, Khan F (2018) Oral Nigella sativa oil and thymoquinone administration ameliorates the effect of long-term cisplatin treatment on the enzymes of carbohydrate metabolism, brush border membrane, and antioxidant defense in rat intestine. Naunyn-Schmiedeberg’s Arch Pharmacol 391(2):145–157
Shahzad M, Yang X, Asim MR, Sun Q, Han Y, Zhang F, Lu S (2009) Black seed oil ameliorates allergic airway inflammation by inhibiting T-cell proliferation in rats. Pulm Pharmacol Ther 22(1):37–43
Shanmugam MK, Arfuso F, Kumar AP, Wang L, Goh BC, Ahn KS, Sethi G (2018) Modulation of diverse oncogenic transcription factors by thymoquinone, an essential oil compound isolated from the seeds of Nigella sativa Linn. Pharmacol Res 129:357–364
Sheik NMM, Jaikumar K, Marimuthu S, John WW, Anand D, Saravanan P (2017) In vitro immunostimulation activity of Nigella sativa Linn. and psoralea Corylifolia Linn. seeds using a murine macrophage cell line. In Vitro 10(3):329
Shuid AN, Mohamed N, Mohamed IN, Othman F, Suhaimi F, Ramli ESM, Muhammad N, Soelaiman N (2012) Nigella sativa: a potential antiosteoporotic agent. Evid Based Compl Altern Med 2012:1–6
Sieghart D, Platzer A, Studenic P, Alasti F, Grundhuber M, Swiniarski S, Steiner G (2018) Determination of autoantibody isotypes increases the sensitivity of serodiagnostics in rheumatoid arthritis. Front Immunol 9:876
Takeda K, Clausen BE, Kaisho T et al (1999) Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of STAT3 in macrophages and neutrophils. Immunity 10:39–49
Tekeoglu I, Dogan A, Ediz L, Budancamanak M, Demirel A (2007) Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res Intl J Pharm Toxicol Nat Prod Deriv 21(9):895–897
Theofanidou T (2021) Anti-inflammatory properties of selected essential oils from aromatic plants
Toma CC, Olah NK, Vlase L, Mogoșan C, Mocan A (2015) Comparative studies on polyphenolic composition, antioxidant and diuretic effects of Nigella sativa L. (black cumin) and Nigella damascena L. (lady-in-a-mist) seeds. Mol 20(6):9560–9574
Topcagic A, Zeljkovic SC, Karalija E, Galijasevic S, Sofic E (2017) Evaluation of phenolic profile, enzyme inhibitory and antimicrobial activities of Nigella sativa L. seed extracts. Bosn J Basic Med Sci 17(4):286
Tutuncu S (2020) Black Seed (Nigella sativa) and immunomodulatory effect. IJVAR 3(1):6–9
Ugur AR, Dagi HT, Ozturk B, Tekin G, Findik D (2016) Assessment of in vitro antibacterial activity and cytotoxicity effect of Nigella sativa oil. Pharmacogn Mag 12(Suppl 4):S471
Umar S, Zargan J, Umar K, Ahmad S, Katiyar CK, Khan HA (2012) Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem Biol Interact 197(1):40–46
Umar S, Munir MT, Subhan S, Azam T, Nisa Q, Khan MI, Shah MA (2016) Protective and antiviral activities of Nigella sativa against avian influenza (H9N2) in turkeys. J Saudi Soc Agricl Sci. https://doi.org/10.1016/j.jssas.2016.09.004
Vaillancourt F, Silva P, Shi Q, Fahmi H, Fernandes JC, Benderdour M (2011) Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J Cell Biochem 112(1):107–117
Vaz NP, De Oliveira DR, Abouelella GA, Khater HF (2018) The black seed, Nigella sativa (Ranunculaceae), for prevention and treatment of hypertension. JN Govil Bhardwaj N 48:221–244
Weng Z et al (2012) Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS ONE 7:e33805
Wienkotter N, Hopner D, Schutte U, Bauer K, Begrow F, El-Dakhakhny M, Verspohl EJ (2008) The effect of nigellone and thymoquinone on inhibiting trachea contraction and mucociliary clearance. Planta Med 74:105–108
Woo YC, Tso AW, Xu A, Law LS, Fong CH, Lam TH, Lam KS (2012) Combined use of serum adiponectin and tumor necrosis factor-alpha receptor 2 levels was comparable to 2-hour post-load glucose in diabetes prediction. PLoS ONE 7(5):e36868
Xin P, Xu X, Deng C et al (2020) The role of JAK/STAT signaling pathway and its inhibitors in diseases. Intl Immunopharmacol 80:106210
Yimer EM, Tuem KB, Karim A, Ur-Rehman N, Anwar F (2019) Nigella sativa L. (black cumin): a promising natural remedy for wide range of illnesses. Evid Based Complem Alt Med. https://doi.org/10.1155/2019/1528635
Young S, McDonald K, Newberry R, Clarke L (2021) Evaluating the role of goblet cell associated antigen passages (gaps) in the development of mucosal immune tolerance in the cftr ko intestine. Inflammatory Bowel Dis 27(Supplement_1):S33–S33
Yousefi M, Adineh H, Reverter M, Hamidi MK, Vatnikov YA, Kulikov EV, Van Doan H (2021) Protective effects of black seed (Nigella sativa) diet supplementation in common carp (Cyprinus carpio) against immune depression, oxidative stress and metabolism dysfunction induced by glyphosate. Fish Shellf Immunol 109:12–19
Zaoui A, Cherrah Y, Mahassini N, Alaoui K, Amarouch H, Hassar M (2002) Acute and chronic toxicity of Nigella sativa fixed oil. Phytomed 9(1):69–74
Zhao X, Zhang J (2015) Mechanisms for quercetin in prevention of lung cancer cell growth and metastasis. Zhong nan da xue xue bao. Yi xue ban J Cent South Univ Med Sci 40(6): 592–597
Zhu W-Q, Wang J, Guo X-F et al (2016) Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J Gastroenterol 22:4149
Zhu MX, Huang LH, Zhu YK, Cai XJ (2021) LncRNA NEAT1 promotes airway smooth muscle cell inflammation by activating the JAK3/STAT5 pathway through targeting of miR-139. Exp Lung Res 47(4):161–172
Zielińska M, Dereń K, Polak-Szczybyło E, Stępień AE (2021) The role of bioactive compounds of Nigella sativa in rheumatoid arthritis therapy—current reports. Nutrients 13(10):3369
Funding
No funding was involved.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by OO and all authors commented on previous versions of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ojueromi, O.O., Oboh, G. & Ademosun, A.O. Black Seed (Nigella sativa): A Favourable Alternative Therapy for Inflammatory and Immune System Disorders. Inflammopharmacol 30, 1623–1643 (2022). https://doi.org/10.1007/s10787-022-01035-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01035-6