Abstract
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that belongs to Phosphatidylinositol-3-kinase related kinase superfamily. The signaling pathways of mTOR are integrated through the protein complexes of mTORC1 and mTORC2. mTORC1 controls protein synthesis, cell growth, proliferation, autophagy, cell metabolism, and stress responses, whereas mTORC2 seems to regulate cell survival and polarity. Dysregulation of the mTOR pathway has been implicated in the pathophysiology of a number of disease conditions, including cancer, cardiovascular, neurodegenerative, and various renal diseases. The hyperactivation of the mTOR pathway leads to increase in cell growth and proliferation and also has been documented to stimulate tumor growth. Therefore, investigation of the involvement of mTOR and its downstream pathways in various diseases intensively preoccupied scientific community. The present review is focussed on recent advances in the understanding of the mTOR signaling pathway and its role in health and various diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian target of rapamycin (mTOR) is a signaling molecule that controls growth and metabolism by regulating catabolic and anabolic functions. Protein kinase mTOR was originally identified and purified after the discovery of Target of Rapamycin (TOR) from Saccharomyces cervisiae (Brown et al. 1994; Sabatini et al. 1994). The mTOR is a serine threonine protein kinase having molecular weight 289-kDa that belongs to the PI3K-related kinase (PIKK) superfamily. The mTOR is an intracellular kinase molecule which regulates protein synthesis via mRNA translation process. The proteins synthesized are necessary for many cellular functions such as cell metabolism, cell growth, cell division, and responses to cellular stresses such as hypoxia or DNA damage.
mTOR plays a major role in many signaling pathways activated by amino acids, insulin, growth factors, and stress mediators like starvation and hypoxia. The PI3K/Akt pathway is one of the major pathways that are involved in mTOR activation and functioning. mTOR activation depends upon both the availability of the materials required for cell growth, i.e. glucose, amino acids, energy, and the growth controlling signals from other cells and tissues, i.e. hormones, growth factors. Further it also depend on cellular stress conditions, i.e. hypoxia, DNA damage, heat shock, external pH, osmotic stress, and oxidative stress. In this way, the cell is fenced from signals outside the cell to augment and proliferate originating when the supply of nutrients and energy inside the cell are not adequate to support the effort.
The knowledge of the science about mTOR’s role as a controller of many cell processes and its emergence as a therapeutic target has opened the different treatment possibilities in several types of diseases. Thus the present article highlights the information of mTOR biology and provides new abilities into the role of mTOR in various diseases.
mTOR structure
mTOR consists of 2549 amino acids and constitutes several structural domains (Fig. 1). The N terminal contains 20 tandem HEAT repeats (for Huntington, EF3, A subunit of PP2A, TOR1), each of them consisting of two helices of ∼40 amino acids with a specific pattern of hydrophilic and hydrophobic residues. The tandem HEAT repeats are entwined in the protein–protein interactions. The C-terminal half contains the kinase domain, which is having the sequential equality with the catalytic domain of phosphatidylinositol 3-kinase (PI3K). On the upstream of the catalytic domain, there is FRB domain. On the other hand, mTOR contains a FAT (for FRAP, ATM, TRAP) domain, which is also present in other PIKK proteins. The C-terminal end also contains another FAT domain, named FATC. The FATC domain is essential for mTOR activity, and the deletion of even a single amino acid from this domain abrogates the activity (Peterson et al. 2000). It is reported that the FATC and FAT domains interact to provide a configuration that exposes the catalytic domain. mTOR also consists of a negative regulatory domain (NRD) between that of the catalytic domain and the FATC domain (Sekulic et al. 2000).
Organization of mTOR into various protein complexes
mTOR nucleates into two different protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2).
mTORC1
mTORC1 consists of five protein components: mTOR (catalytic subunit of the complex); Raptor (regulatory-associated protein of mTOR); mLST8 (mammalian lethal with Sec13 protein 8, also known as GbL); PRAS40 (prolinerich AKT substrate 40 kDa); and Deptor (DEP-domain-containing mTOR-interacting protein) (Peterson et al. 2009). Raptor is the defining component of mTORC1, acts as an essential scaffold for mTORC1-mediated phosphorylation of downstream target molecules such as 4E-BP1 and p70S6K. PRAS40 and Deptor have been characterized as negative regulators of mTORC1. mTORC1 controls cell growth and cell proliferation by encouraging many anabolic processes which include biosynthesis of lipids, proteins, and organelles, and by restricting the catabolic processes such as autophagy.
Upstream regulation of mTORC1 signaling pathway
The mTORC1 pathway receives inputs from various intracellular and extracellular cues such as-growth factors, amino acids, stress, energy status, and oxygen to regulate many processes, including protein synthesis, lipid synthesis, and autophagy. Tuberous sclerosis complex (TSC) is one of the most important sensors involved in the regulation of mTORC1 activity, which is a heterodimer that constitutes TSC1 and TSC2 (also known as hamartin and tuberin, respectively). The TSC1/2 functions as a Ras-related GTPase Rheb. The active, i.e. GTP-bound form of Rheb interacts directly with mTORC1 to promote its activity (Long et al. 2005). On being a Rheb-specific GAP (GTPase-activating protein), TSC1/2 negatively regulates mTORC1 signaling and converts Rheb into its inactive GDP-bound state (Tee et al. 2003).
Regulation of mTORC1 by growth factors
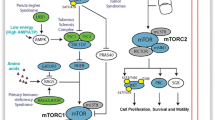
Growth factors such as IGF and insulin bind to their receptors and lead to activation of PI3K/Akt signaling (Fig. 2). The mTORC1 pathway is one of the most accepted downstream targets of Akt. Akt phosphorylates and inhibits TSC 1/2 (tuberous sclerosis complex), a GTPase-activating protein (GAP) for the small G-protein Rheb (Ras homolog enriched in brain) (Inoki et al. 2002; Tee et al. 2003). Inhibition of TSC1/2 leads to an increase in GTP-bound active Rheb which is a direct activator of mTORC1. Akt also phosphorylates PRAS40 and distinguishes PRAS40 from Raptor and further leads to the activation of mTORC1 (Sancak et al. 2007; Wang et al. 2007). Growth factors also regulate mTORC2 via PI3K and this mTORC2 further leads to the activation of Akt. mTORC2 phosphorylates Akt at a serine residue S473. Phosphorylation of the serine residue stimulates the phosphorylation of Akt at a threonine T308 residue by PDK1 and leads to full activation of Akt.
Regulation of mTORC1 by amino acids
Amino acids constitute the signals that positively regulate mTORC1. Leucine is an essential amino acid required for mTORC1 activation that is transported into cells in a glutamine-dependent manner (Fig. 3) (Nicklin et al. 2009). Glutamine is imported into cells through SLC1A5 [solute carrier family 1 (neutral amino acid transporter) member 5] and exchanged to import leucine through a heterodimeric system that consists of SLC7A5 [antiport solute carrier family 7 (cationic amino acid transporter, y+ system, member 5] and SLC3A2 [solute carrier family 3 (activators of dibasic and neutral amino acid transport) member 2]. Human vacuolar protein-sorting associated protein 34 (VPS34) is incriminated in some studies in nutrient sensing (Nobukuni et al. 2005). It has also been shown in recent studies that the Rag proteins which are a family of four related small GTPases interact with mTORC1 and are crucial for the activation of the mTORC1 pathway by amino acids (Kim et al. 2008; Sancak et al. 2008). In the presence of amino acids, Rag proteins bind to Raptor and promote the relocalization of mTORC1 from distinct locations throughout the cytoplasm to a perinuclear region that contains its activator Rheb (Sancak et al. 2008).
Glucose-dependent regulation of mTORC1
Glucose is a crucial source of energy and glucose deprivation instigates autophagy in different cell types, which is accompanied by decreased activity of mTORC1 (Fig. 4). Whenever there is the reduction of cellular ATP levels, AMPK is activated, inhibits mTORC1, and induces autophagy in the absence of glucose. The cells directly sense intracellular glucose levels to regulate the mTORC1 pathway. Lysosomes are identified as activation site for mTORC1, and it has also been shown that deficiency of glucose causes mTORC1 to be diffusely distributed in the cytosol in HEK-293T cells (Efeyan et al. 2013).
In the heart, insulin has induced the mTOR activation which has been shown to require glucose, which suggests that glucose metabolism has an indispensible role in mTORC1 activation (Sen et al. 2013; Sharma et al. 2007). Glucose-dependent mTORC1 activation is independent of the hexosamine biosynthetic pathway. An ameliorating work load is associated with glucose-6-phosphate (G-6P) aggregating and mTORC1 activation in the heart (Sen et al. 2013; Sharma et al. 2007).
The first and foremost step of glycolysis is mediated by hexokinases (HKs), which produce G-6P by phosphorylating glucose (Pastorino and Hoek 2003; Wilson 2003). HK2 also plays a vital role in mTORC1 inhibition and protective autophagy in the absence of glucose. HK2 binds to mTORC1 through Raptor and this binding is largely increased by glucose withdrawal in the heart. GAPDH act as a Rheb binding protein and the binding of GAPDH to Rheb is enhanced by decreasing glucose concentration, which leads to sepration of mTORC1 from Rheb and ultimately the inhibition of mTORC1. The inhibitory binding of GAPDH to Rheb also contributes to GLUT1 upregulation-induced activation of mTORC1.
Downstream signaling of mTORC1 pathway
mTORC1 directly phosphorylates the translational regulators eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1), which, in revert, augments protein synthesis (Ma and Blenis 2009). The phosphorylation of 4E-BP1 demolishes its binding to eIF4E (cap-binding protein) and contributes in the formation of the eIF4F complex which is needed for the commencement of cap-dependent translation. The activation of S6K1 leads, with different effectors, to an increase in mRNA biogenesis, also translational initiation and elongation (Fig. 5a). mTORC1 also upregulates the protein synthesis machinery such as (1) It activates the regulatory element tripartite motif which holds protein-24 (TIF-1A), and helps to promote its interaction with RNA Polymerase I and the expression of ribosomal RNA (rRNA) (Mayer et al. 2004); and (2) mTORC1 phosphorylates and inhibits Maf1 which is a Pol III repressor; this promotes the transcription of 5S rRNA and transfer RNA (tRNA) (Kantidakis et al. 2010; Shor et al. 2010). The basic role of mTORC1 in the regulation of mRNA translation is highly important because specific, active-site inhibitors of mTOR that fully retard mTORC1 role, significantly deduce overall rates of protein synthesis in augmenting cells in culture (Thoreen et al. 2009; Yu et al. 2009).
Cellular processes downstream of mTORC1. a Phosphorylation of S6K1 and 4E-BP bY mTORC1 and regulation of protein synthesis. b mTORC1 acts via sterol regulatory element binding protein 1/2 (SREBP1/2) transcription factors which regulates the expression of various genes included in cholesterol and fatty acid synthesis
In addition to controlling the protein production, mTORC1 also regulates the lipid synthesis needed for proliferating cells for the generation of membranes (Laplante and Sabatini 2009). mTORC1 acts via sterol regulatory element binding protein 1/2 (SREBP1/2) transcription factors, which regulates the expression of various genes included in cholesterol and fatty acid synthesis (Fig. 5b). The inactive SREBPs stays on the endoplasmic reticulum and their proteolytic processing in revert to insulin or sterol abolishment releases an active form that goes to the nucleus to activate transcription. mTORC1 inhibition deduces SREBP1/2 levels also processing and markedly reduces the expression of lipogenic genes (Düvel et al. 2010; Wang et al. 2011). mTORC1 regulates SREBP function by various mechanisms, including, through S6K1 (Düvel et al. 2010; Wang et al. 2011). Moreover, mTORC1 causes Lipin-1 phosphorylation and prevents this from entering the nucleus and prohibiting SREBP1/2 role and levels (Peterson et al. 2011). mTORC1 also flourishes the expression and activity of PPAR-γ, the master regulator of adipogenesis (Kim and Chen 2004).
mTORC2
mTORC2 contains six protein components named as mTOR; rapamycin-insensitive companion of mTOR (Rictor); mammalian stress-activated protein kinase interacting protein (mSIN1); protein observed with Rictor-1 (Protor-1); mLST8; and Deptor. Evidence shows that Rictor and mSIN1 balance each other and establish the structural foundation of mTORC2 (Frias et al. 2006; Jacinto et al. 2006). Like its role in mTORC1, Deptor negatively regulates the activity of mTORC2 (Peterson et al. 2009); Deptor is also characterized as the single endogenous inhibitor of mTORC2.
Overview of mTORC2 signaling
mTORC2 signaling is insensible to nutrients, but does respond to growth factors like insulin via a mechanism that needs PI3K. Ribosomes have also been documented to play a crucial role in mTORC2 activation in a PI3K-dependent manner (Zinzalla et al. 2011).
mTORC2 controls various members of the AGC subfamily of kinases which includes Akt, serum and glucocorticoid-induced protein kinase 1 (SGK1), and protein kinase C-α (PKC-α) (Fig. 6). Akt regulates cellular processes such as metabolism, survival, apoptosis, growth, and proliferation by phosphorylating various effectors. mTORC2 activates Akt directly by phosphorylation at Ser473, which is a site needed for its maximal activation (Sarbassov et al. 2005). Phosphorylation of the serine leads to the stimulation of Akt phosphorylation at a threonine T308 residue via PDK1 and finally causes the full activation of Akt. Defect in phosphorylation of Akt-Ser473 linked with mTORC2 depletion disbalances the phosphorylation of some Akt targets, like forkhead box O1/3a (FoxO1/3a); on the other hand, Akt targets such as TSC2 and GSK3-β stay unaffected (Jacinto et al. 2006; Guertin et al. 2006). mTORC2 also directly activates SGK1, a kinase that controls growth and ion transport (García-Martínez and Alessi 2008), and also several members of PKC family, including PKCα (Sarbassov et al. 2004), PKCε (Ikenoue et al. 2008), PKCδ (Gan et al. 2012), and PKCζ (Li and Gao 2014). Recently, mTORC2 was found to phosphorylate MST1 which is a kinase in the Hippo signaling pathway (Sciarretta et al. 2015).
mTORC2 is reported to be involved in the regulation of cytoskeletal organization (Fig. 6). Studies have shown that knockdown of mTORC2-specific components in cultured cells results in the modification of the actin cytoskeleton. In addition, studies done in yeast suggested that mTORC2 regulate signaling to the actin cytoskeleton through Rho GTPases and PKCα (Jacinto et al. 2004; Sarbassov et al. 2004). These results reveal that mTORC2 controls the actin cytoskeleton by promoting phosphorylation of protein kinase Cα (PKCα), paxillin phosphorylation and its relocalization to focal adhesions, and the GTP loading of RhoA and Rac1.
Physiological roles of mTOR
mTOR, as a master regulator of metabolism
The unique capacity of mTOR to sense and integrate various environmental and hormonal signals makes it relevant to play a pivotal role in the regulation of metabolism. In this section, we will discuss the recent findings about the role of mTOR signaling in physiology and metabolism in some of the major metabolic tissues such as adipose, liver, pancreas, and muscle.
mTOR in adipose tissue
mTOR signaling plays an essential role in adipogenesis (Laplante and Sabatini 2009), the process which leads to the development of adipose tissue. In vitro inhibition of mTORC1 inhibits adipogenesis and disturbs the maintenance of fat cells (Kim and Chen 2004; Polak et al. 2008), although mTORC1 overactivation facilitates adipogenesis (Zhang et al. 2009). Many downstream effectors of mTORC1 are also involved in the regulation of adipogenesis. For example; S6K1 controls the commitment of embryonic stem cell to adipogenic progenitors by controlling the expression of early adipogenic transcription factors (Carnevalli et al. 2010) and the 4E-BPs regulate the terminal differentiation of adipocytes via translational control of PPAR-γ, the master regulator of adipogenesis (Carnevalli et al. 2010; Le Bacquer et al. 2007).
The extension of adipose tissue that characterizes the obese state denotes the major risk factor for the occurrence of insulin resistance and type 2 diabetes and mTORC1 is exceedingly active in the tissues of obese and high-fat diet fed rodents (Khamzina et al. 2005; Um et al. 2004). Highly circulating levels of insulin, pro-inflammatory cytokines, and nutrients characterize driving forces, which boosts mTORC1 activity in obese animals. Adding-up to directly contributing to adipose tissue extension via activation of adipogenic or lipogenic factors, mTORC1 develops insulin resistance in adipose tissue via S6K1-mediated inhibition of insulin signaling (Um et al. 2004). The decrease in insulin action in adipose tissue possibly aggravates systemic insulin resistance by promoting FFA release by adipocytes, lipotoxicity, and ectopic fat deposition. The increased rate of protein synthesis linked with mTORC1 activation can also instigate insulin resistance by promoting ER stress and the unfolded protein response (UPR) (Ozcan et al. 2008).
mTOR in the liver
Liver is the main organ which plays a key role in regulating glucose and lipid homeostasis in response to fasting and feeding. mTORC1 regulates the hepatic production of the ketone bodies, used by peripheral tissues as energy sources during fasting (Sengupta et al. 2010). Activity of mTORC1 is less during fasting, and mice with constitutive mTORC1 activation in liver are not able to induce ketogenesis when fasted. mTORC1 deregulates the activity of PPAR-α, the major transcriptional controller of ketogenic genes, by stimulating the nuclear accretion of nuclear receptor co-repressor 1 (NcoR1). mTORC1 also promotes anabolism in the fed state by regulating hepatic lipogenesis via regulation of SREP1c expression (Li et al. 2010a; Yecies et al. 2011).
Like adipose tissue, mTORC1 and S6K1 activity is elevated in the livers of obese rodents that leads to the deterioration of IRS1 and hepatic insulin resistance (Khamzina et al. 2005; Tremblay et al. 2007). The deregulation of PI3K-Akt signaling in the liver facilitates gluconeogenesis and contributes to hyperglycemia and hyperinsulinemia noticed in insulin resistance or type 2 diabetes. Obesity is the main risk factor in the occurrence of non-alcoholic fatty liver disease, caused by fat deposition in the liver that can lead to serious complications such as cirrhosis and hepatocellular carcinoma. The deposition of triglycerides in the liver of obese humans is related to the stimulation of lipogenesis in hepatocytes (Donnelly et al. 2005). Controlled activation of mTORC1 in response to elevated circulating levels of nutrients and pro-inflammatory cytokines is likely to aggravate lipogenesis via SREBP1 activation. Continuing with this, liver-specific removal of mTORC1 considerably deregulates SREBP1 function and makes mice resistant to the hepatic steatosis and hypercholesterolemia generated by a western diet (Peterson et al. 2011). Thus, increased hepatic mTORC1 might elucidate why lipogenesis remains active while the inhibition of glucose production becomes insulin resistant in the liver of obese or insulin-resistant mice and humans (Brown and Goldstein 2008).
mTOR in the pancreas
The β-cells of the pancreas secrete insulin in revert to nutrients and are significant in controlling glucose homeostasis. mTORC1 signaling regulates growth in response to nutrients and has a potential role in the regulation of β-cell mass and function. The constitutive activation of mTORC1 in β-cells of mice causes a reduction in blood glucose, hyperinsulinemia, and enhances glucose tolerance (Rachdi et al. 2008; Shigeyama et al. 2008). This phenotype is linked with a rise in number and β-cell size and can be given back by rapamycin, representing that mTORC1 is a positive regulator of β-cell function and mass. S6K1 seems to arbitrate some effects of mTORC1, as mice with deletion of S6K1 have small β-cells and are intolerant to glucose, hypoinsulinemic, and have impaired insulin secretion (Pende et al. 2000).
mTORC2 loss in β-cells is associated with the decrease in Akt activity and to the activation of FoxO1 and leads to mild hyperglycemia and glucose intolerance due to decrease in β-cell mass, proliferation, and insulin production and secretion (Gu et al. 2011). Nutrient excess and peripheral insulin resistance raise the pressure on pancreatic β-cells for more insulin production. The increased demand for insulin causes β-cell hypertrophy and proliferation and enhances the formation of new β-cells from progenitors, which culminates in the increase of production and secretion of insulin. The process is thus termed as β-cell compensation. The constant (chronic) pressure on β-cells may cause their exhaustion and the occurrence of type 2 diabetes.
mTORC1 activity enhances in the β-cells of genetically obese or high fat given mice (Shigeyama et al. 2008). mTORC1 acts as a double-edged sword in β-cell mass regulation and function in reaction to nutrient overload and/or insulin resistance. Since mTORC1 positively regulates β-cell mass and insulin secretion, continued activation of mTORC1/S6K1 signaling aggravates insulin resistance in islets via feedback inhibition of IRS1 and IRS2, which decreases cell survival and elevates apoptosis (Elghazi et al. 2010; Shigeyama et al. 2008). Supporting this model, mice with constitutive activation of mTORC1 in β-cells have enlarged β-cells mass in the first phase of life but on aging become hyperglycemic and hypoinsulinemic because of the loss of β-cells (Shigeyama et al. 2008).
mTOR in muscles
In muscle, mTORC1 senses mechanical contraction, through an unknown mechanism which encourages synthesis of proteins to drive muscle hypertrophy (Philp et al. 2011). Muscle-specific loss of mTORC1 in mice decreases muscle mass and oxidative function and causes early death (Bentzinger et al. 2008). The expression of the mitochondrial transcriptional regulator PGC1-α reduces in such mice, which associates with a decrease in oxidative metabolism. Earlier study indicates a link between mTORC1 and PGC1-α as rapamycin inhibits the complex of PGC1-α with YY1 (Cunningham et al. 2007).
mTORC1 loss in muscle also decreases the strength of the negative feedback loop to IRS1, which causes increased Akt activation and stimulates glycogen storage in muscles. On the flip side, in vivo inhibition of mTORC2 in muscle has no structural impact (Bentzinger et al. 2008; Kumar et al. 2008), but it causes a decrease in glucose uptake and thus mild systemic glucose intolerance.
Skeletal muscle is the main site of glucose disposal in revert to food intake/insulin and an impairment of glucose uptake in this tissue leads to type 2 diabetes. The increased activation of mTORC1 in muscles of obese and high-fat given rodents operates S6K1- mediated feedback inhibition of insulin signaling, which decreases glucose uptake by the muscle and gives rise to systemic insulin resistance (Khamzina et al. 2005; Um et al. 2004). Ahead of its impact on glucose homeostasis, impairment of insulin signaling in muscle may also lead to the muscle loss seen in obesity/insulin resistance by enhancing protein catabolism via expression of ubiquitin ligases by FoxO1 (Wang et al. 2006). This facilitation of protein catabolism might explain why increased mTORC1 activity in the muscles of obese mice and humans does not convert into increased muscle mass. Weirdly, in spite of high mTORC1 activity, high-fat feeding, obesity, and type2 diabetes impair mitochondrial biogenesis and function in muscles (Patti et al. 2003; Sparks et al. 2005).
Role of mTOR in various disease conditions
mTOR signaling in cancer
mTOR signaling is proved to be activated in some cancer hallmarks (Hanahan and Weinberg 2011). The anomalous mTOR pathway activation via oncogene stimulation or failure of tumor suppressors leads to tumor growth, metastasis, and angiogenesis in many in vitro cell-lines and in vivo murine xenograft models (Faivre et al. 2006). Mutations in mTOR gene that presents constitutive activation of mTOR signaling, even under nutrient deficient conditions, were found to be present in some human cancers (Sato et al. 2010). Other than this, the upstream and downstream signaling components of mTORC1 are frequently changed in human tumors.
The PI3K/AKT signaling, upstream of mTOR is deregulated via a number of mechanisms, including overexpression or activation of GFRs (growth factor receptors), for example, HER-2 (human epidermal growth factor receptor 2) and IGFR (insulin-like growth factor receptor), mutations in PI3K and mutations/amplifications of AKT (Sato et al. 2010; Stemke-Hale et al. 2008). PTEN, which is the negative regulator of PI3K signaling, is downregulated via various mechanisms, including mutation, loss of heterozygosity, abnormal expression of regulatory micro RNA, and protein instability (Tamguney and Stokoe 2007).
The downstream effectors of mTOR such as, S6K1, 4EBP1, and eIF4E are insinuated in cellular transformation, and their overexpression is associated with poor cancer diagnosis (Armengol et al. 2007; Bärlund et al. 2000; De Benedetti and Graff 2004). The activation of mTOR signaling is also related to the development of various syndromes, including Cowden’s syndrome (PTEN mutations), Peutz-Jeghers syndrome (LKB1 mutations), and tuberous sclerosis (TSC1/2 mutations) (Inoki et al. 2005; Liaw et al. 1997; Shaw et al. 2004).
mTOR signaling is activated in conditions of proliferation deregulation and in various different cancer forms. Deregulation of multiple elements of the mTOR pathway (PI3K amplification/mutation, PTEN failure of function overexpression of AKT, eIF4E, S6K1, and 4EBP1) has been seen in cancer, like breast, ovarian, renal, colon, head and neck cancers.
Rapamycin, an inhibitor of mTOR acts as a cytostatic agent, slowing or arresting growth of cell lines derived from various tumor types like glioblastoma, osteosarcoma, prostate cancer, pancreatic cancer, small cell lung cancer, breast cancer, and B cell lymphoma. Adding up to direct anti-tumor effects, rapamycin also inhibits cell proliferation, survival, and angiogenesis.
mTOR signaling in cardiovascular system
The mTOR signaling pathway has both physiological and pathophysiological roles in the cardiovascular system. Preclinical studies depicting the loss of function of the mTOR complexes have revealed the involvement of mTOR in the embryonic cardiovascular development and in the regulation of fundamental cellular processes essential for normal postnatal growth and maintenance of cardiac function (Sciarretta et al. 2014). In addition, mTORC1 is necessary for cardiac adaptation to pressure overload and development of compensatory hypertrophy.
Role of mTOR signaling in the regulation of cardiac hypertrophy
The mTOR pathway plays a vital role in the progression of cardiac hypertrophy (Fig. 7). In cardiac hypertrophy there is a marked elevation in the synthesis of sarcomeric proteins and the mTOR pathway is the master promoter of protein synthesis. mTORC1 activity is documented to be increased in the cardiomyocyte hypertrophic response to β-adrenergic stimulation (Simm et al. 1998), angiotensin-II (Sadoshima and Izumo 1995), and insulin growth factor-1 (Lavandero et al. 1998).
Role of mTOR in cardiac hypertrophy. mTOR activation promotes pathological hypertrophy during pressure overload. However, mTOR kinase is also required for physiological mechanisms that are necessary for cardiac adaptation to cardiac overload. ERK indicates extracellular signal regulated kinase, NO nitric oxide, PRAS40 proline-rich Akt substrate 40, TAC transverse aortic constriction, and TRPC transient receptor potential channel
The PI3K/Akt pathway contributes to the activation of mTORC1 during the development of cardiac hypertrophy, specifically in revert to physical exercise (Völkers et al. 2013a; McMullen et al. 2003; Proud 2004). Along with β-adrenergic signaling, the ERK pathway and nitric oxide signaling are also included in the activation of mTORC1 during cardiac hypertrophy (Simm et al. 1998; Wang et al. 2001; Zhang et al. 2007). Moreover, biomechanical activation of transient receptor potential channels and focal adhesion kinase also promote mTORC1 activation during pressure overload (Seth et al. 2009; Clemente et al. 2007), and glucose-6-phosphate accumulation is another metabolic stimulus that contributes to mTORC1 activation in the overloaded heart (Sen et al. 2013). This entangled network of mechanical, biochemical, and metabolic signals is sensed by mTORC1 signaling during cardiac pressure overload and consequently leads to the development of hypertrophy.
mTOR inhibition significantly deduces the cardiac hypertrophy.
mTOR signaling and ischemia–reperfusion (I/R) injury
During ischemia
mTOR regulates the response of cardiomyocytes to deprivation of energy and ischemia. In lower organisms and mammalian cell lines, mTORC1 is inhibited during deprivation of energy (Laplante and Sabatini 2012, 2013; Johnson et al. 2013). mTORC1 inhibition stores the energy status with the reduction of cellular energy expenditure and activation of autophagy and, in this way, it promotes survival (Fig. 8). It is recently witnessed that mTORC1 is inhibited during cardiomyocyte energy deprivation and ischemia through the inhibition of Rheb (Sciarretta et al. 2012). Rheb is the important regulator of mTORC1 during energy stress condition in the cardiomyocyte and the inhibition of Rheb/mTORC1 is an adaptive response which enhances survival through the activation of autophagy. AMPK inhibits the activity of mTOR to promote autophagy in ischemia. AMPK is activated in revert to elevations of the AMP/ATP ratio and autophagy has been witnessed to be up-regulated as a result of reduced cellular content of ATP.
During reperfusion
mTORC1 is activated in the heart during the time of reperfusion. Rapamycin has been reported to decrease the infarct size in both ex vivo and in vivo models of ischemia/reperfusion via activation of the Janus kinase 2/signal (JNK) transducer and activator of transcription 3 signaling pathway (Das et al. 2012). It is also documented that the inhibition of GSK-3β in transgenic mice with cardiac-specific overexpression of dominant negative GSK-3β decreases reperfusion injury through mTORC1 activation (Fig. 9) (Zhai et al. 2011). These results and discussion suggests that mTORC1 may also exert some protective effects during the reperfusion phase.
mTORC1 inhibition is protective during ischemia through the upregulation of adaptive mechanisms, whereas mTOR is reactivated during reperfusion and takes part in the regulation of physiological processes. Tg-DN-GSK-3β, transgenic dominant negative glycogen synthase kinase-3β, mPTP mitochondrial permeability transition pore, ROS reactive oxygen species
Furthermore, mTORC1 is also reported to regulate mitochondrial permeability transition pore opening and enhance mitochondrial biogenesis that may also favor cardiac recovery after ischemia and promote the up-regulation of antioxidant genes through the activation of Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (Zhai et al. 2011; Cunningham et al. 2007; Lu et al. 2010).
The protective effects mediated by mTOR overexpression are dependent on mTORC2 activation, which is necessary for cardiomyocyte survival during ischemia and limitation of chronic ischemic remodeling (Völkers et al. 2013b). mTORC1 inhibition is protective during ischemia through the activation of autophagy, decrease of protein synthesis, and subsequent activation of mTORC2. Moreover, mTORC1 seems to potentiate physiological mechanisms during reperfusion.
mTOR and neurodegenerative diseases
mTOR controls survival, differentiation, and development of neurons. Axon growth and navigation, dendritic arborization, as well as synaptogenesis, depend on mTOR activity. In adult brain mTOR is essential for synaptic plasticity, learning and memory formation, and brain control of food uptake (Swiech et al. 2008). Recent studies show that mTOR activity is altered in many conditions, including brain tumors and various neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s diseases.
Alzheimer’s disease
Aβ peptide, which is obtained from amyloid precursor protein, (APP) has been identified to be central to the pathophysiology of AD, and aggregation of Aβ is thought to be the upstream of all pathological events of AD (Cavallucci et al. 2012). The evidence suggests that dysfunction of autophagy plays an important role in Aβ accumulation (Li et al. 2010b; Cai et al. 2012; Zhu et al. 2013). However, it has been revealed through various studies that autophagy–lysosome system is responsible for the clearance of Aβ and APP-C-terminal fragments (APP-CTF). Autophagy-related protein Atg5, Beclin1, and Ulk1 are implicated in the degradation of Aβ and APP-CTF (Tian et al. 2011). mTOR signaling negatively regulates the instigation of autophagy; thus, mTOR is a crucial regulator of generation of Aβ through regulating autophagy (Fig. 10).
Various studies show that Aβ accumulation activates mTOR pathway (Caccamo et al. 2010, 2011; Oddo 2012), and this might be the most important reason for mTOR hyperactivation in AD patients. It is documented that Aβ can increase the PI3K-Akt pathway, which is a key activator to mTOR (Ito et al. 2007, 2006). The process of Aβ-induced mTOR hyperactivity is mediated by PRAS40. Aβ stimulates the phosphorylation of PRAS40 and releases its inhibitory effect on mTOR (Caccamo et al. 2011). Taking these together, it is suggested that Aβ increases mTOR activity through the PRAS40-mediated mechanism. Hyperactive mTOR inhibits autophagy and further aggravates the formation of amyloid plaques.
Like with Aβ plaques, mTOR signaling pathway plays a vital role in tau degradation, phosphorylation, and tau-induced neurofibrillary degeneration, and autophagy impairment is directly related to tau aggregation and NFTs formation (Zhu et al. 2013; Caccamo et al. 2013). Inhibition of mTOR signaling with rapamycin ameliorates tau pathology via mediating the increasing of autophagy, and autophagy induction is needed for the rapamycin-mediated effects (Caccamo et al. 2010; Berger et al. 2006).
Parkinson’s disease
Recently, it is evidenced that mTOR signaling is altered during the progression of Parkinson’s disease (Bockaert and Marin 2015). There are various genes associated with PD which includes, leucine-rich repeat kinase 2 (LRRK2), PTEN-induced putative kinase 1 (PINK1), RING domain-containing E3 ubiquitin ligase (Parkin), DJ-1, and ubiquitin carboxyl-terminal esterase L1 (UCHL1) (Fig. 11). It is documented that REDD1 is a substrate of Parkin and loss of Parkin causes neurodegeneration through up-regulation of REDD1 and following down-regulation of mTORC1 (Romani-Aumedes et al. 2013). Apart from Parkin, PINK1 causes phosphorylation of Rictor which is a unique element in mTORC2 and leads to the activation of mTORC2 (Murata et al. 2011). UCHL1 is another gene that regulates the activity of both the mTOR complexes. It is demonstrated that UCHL1 attenuates the kinase activity of mTORC1 for S6K1 and 4E-BP1 and increases mTORC2 activity for Akt (Hussain et al. 2013). LRRK2 can also phosphorylate 4E-BP1, a substrate of mTORC1 and results in dysregulated protein translation and loss of dopaminergic neurons in Drosophila (Imai et al. 2008).
mTOR signaling in PD. As a negative regulator of autophagy, active mTORC1 could be associated with α-synuclein accumulation. Parkin regulates the activity of mTORC1 via REDD1. PINK1 activates mTORC2 via phosphorylation of Rictor. UCHL1 attenuates the kinase activity of mTORC1 for S6K1/4E-BP1, whereas it enhances mTORC2 activity for Akt. Like mTORC1, LRRK2 can also phosphorylate 4E-BP1
The pathological hallmark of PD is the aggregation of α-synuclein (Lin et al. 2012). Activation of autophagy enhances the clearance of cytoplasmic protein aggregates like α-synuclein (Menzies et al. 2015). Thus, autophagy may serve as a survival strategy for neurodegenerative diseases like PD. Being a negative regulator of autophagy, mTOR seems to modulate autophagy via regulation of autophagy-related genes (Atg). Thus, activation of autophagy via inhibition of mTOR prevents the formation of cytoplasmic protein aggregates like α-synuclein (Fig. 11) (Jiang et al. 2013; Decressac et al. 2013; Pérez-Revuelta et al. 2014). However, autophagy can play a double role that can either promote cell survival or potentiate neuronal lesion in PD. Under the condition of oxidative stress, induction of autophagy with mTOR inhibition can also lead to neuronal cell death (Choi et al. 2010).
The mTOR pathway is also believed to control cell death mechanisms in PD via interaction with a stress response protein RTP801, activated by oxidative stress, and lead to apoptotic cell death. Rapamycin has been witnessed to inhibit expression of RTP801 and simultaneously protect against neuronal death in in vitro and in vivo models of PD (Malagelada et al. 2010).
In contrast, the anti-parkinsonian drug, levodopa, shows motor side effects (dyskinesia) through the activation of mTOR signaling in the animal model of PD (Santini et al. 2009; Subramaniam et al. 2012). mTOR inhibition with rapamycin has been reported to prevent the development of L-DOPA-induced dyskinesia in animal models of PD (Santini et al. 2009; Decressac and Björklund 2013).
Huntington’s disease
Huntington’s disease is an autosomal prevailing disorder which involves relatively selective neurodegeneration in the basal ganglia and cortex, correlated to trinucleotide repeat extension of polyglutamine on huntingtin protein. The mutant huntingtin protein is not efficiently cleaned from neurons, leading to buildup of toxic intracellular aggregates and linked neuronal death. In the human brains and mouse models of Huntington’s disease, mTOR is reported to be sequestered in polyglutamine aggregates. mTOR inhibitors are able to increase autophagy and as a result decrease huntingtin aggregation and related neuronal death in various cellular and animal models of Huntington’s disease (Ravikumar et al. 2004; Floto et al. 2007). Additionally, rapamycin has also been reported to improve motor deficits in mouse models of Huntington’s disease (Ravikumar et al. 2004).
mTOR and renal disorders
The mTOR signaling network is one of the major pathways that controls renal epithelial cells along the entire nephron (Grahammer et al. 2014). Both mTOR complexes, mTORC1 and mTORC2, are responsible for regulation of renal processes such as glomerular filtration and maintaining tubular electrolyte balance. The dysregulation of mTOR signaling contributes to prevalent kidney diseases like diabetic nephropathy and other progressive kidney diseases.
Acute kidney injury
After the acute kidney injury (AKI) induced by ischemic or toxic injury, the complete restoration of renal morphology and function can occur (Nigam and Lieberthal 2000; Megyesi et al. 2002). It is verified that mTOR plays a vital role in mediating the process of renewal and recovery after experimental AKI (Lieberthal et al. 2001, 2006). The activity of mTOR is low or absent in the normal kidney and increases after ischemia–reperfusion injury (Lieberthal et al. 2001). Additionally, the inhibition of mTOR by rapamycin delays renal recovery and repair (Lieberthal et al. 2001). This effect of rapamycin is because of the dual effects of inhibition of proliferation and initiation of apoptosis of tubular cells (Lieberthal et al. 2001, 2006).
mTOR is activated by growth factors, amino acids and inhibited by ATP reduction. mTOR activity may be highly repressed during the ischemic period of I/R injury, when the accessibility of growth factors, amino acids, and cell ATP all are expected to be reduced, whereas, the mechanistic aspects behind the activation of mTOR during reperfusion period after I/R injury remain to be explained (Lieberthal et al. 2001, 2006).
It is reported that rapamycin causes or exacerbates delayed graft function in human renal transplant recipients (McTaggart et al. 2003; Fuller et al. 2003). After the identification of adverse effects of rapamycin on revival from delayed graft function, its administration has been routinely delayed until the transplanted kidney is functional in patients having renal transplants and acute kidney injury (McTaggart et al. 2003; Fuller et al. 2003).
Chronic kidney injury
The mTOR pathway plays an essential role in the mechanisms underlying the development of chronic kidney disease caused by diabetes and other causes. The inhibition of mTOR by rapamycin ameliorates the fibrosis, interstitial inflammation, and loss of renal function in Chronic Kidney Disease.
Diabetic nephropathy (DN)
The typical morphologic changes in diabetic nephropathy comprises glomerular hypertrophy, thickening of basement membrane, and the buildup of mesangial matrix (Estacio and Schrier 2000; Molitch et al. 2004). One of the first structural changes in DN is renal enlargement, because of the hypertrophy of existing glomerular and tubular cells rather than to cellular proliferation (Estacio and Schrier 2000; Molitch et al. 2004; Hostetter 1995, 2003). The activation of mTOR plays a crucial role in pathologic and physiologic forms of hypertrophy in kidney and other organs, also including the renal hypertrophy characteristic of DN (Fig. 12) (Lee et al. 2007a; Chen et al. 2005).
mTOR is activated within the kidney in DN. Hyperglycemia activates PI3K and Akt and inhibits AMPK. The activation of Akt and inhibition of AMPK lead to activation of mTORC1. Activation of mTORC1 contributes to the renal changes characteristic of DN, including glomerular hypertrophy, glomerular basement membrane (GBM) thickening, and the accumulation of mesangial matrix
mTOR-dependent changes increase the matrix protein synthesis that causes thickening of basement membrane and the buildup of mesangial matrix feature of DN (Sataranatarajan et al. 2007; Mariappan et al. 2007).
mTOR activity increases during the hyperglycemia by collective effects of Akt activation and AMPK inhibition (Sataranatarajan et al. 2007; Inoki 2008; Fraenkel et al. 2008; Lee et al. 2007b). The significance of mTOR in mediating the renal changes linked with DN is also established experimentally by assessing the effect of rapamycin on the DN in rats with streptozotocin-induced diabetes (Sakaguchi et al. 2006; Lloberas et al. 2006).
Rapamycin decreases the mTOR activity in this model and also reduces the glomerular changes characteristic of DN, including hypertrophy, basement membrane thickening, and mesangial matrix buildup (Sakaguchi et al. 2006; Lloberas et al. 2006). Rapamycin noticeably inhibited the influx of inflammatory cells, mainly lymphocytes and macrophages, linked with DN (Sakaguchi et al. 2006; Lloberas et al. 2006). This effect can be attributed to rapamycin-induced inhibition of the proliferation and expansion of B and T lymphocytes (Hay and Sonenberg 2004; Fingar and Blenis 2004). Rapamycin also reduces the release of proinflammatory cytokines and chemokines in the kidney, such as monocyte chemoattractant protein-1, RANTES, IL-8, and fractaline, which aggravate the inflammatory process in DN.
Perspectives
The mTOR pathway is figured out to be the critical signalling pathway in the etiology of cancer and various cardiac, neurological, and metabolic diseases. The knowledge of the upstream and downstream targets of mTOR explains the process of development and progression of these diseases. The link between mTOR pathway and cancer is clearly revealed, as many upstream and downstream components of mTOR are directly involved in initiation and progression of cancer. Rapamycin or its analogs act as a cytostatic agent, slows down or arrests the growth of various tumor cells, and also inhibits cell proliferation, survival, and angiogenesis. mTORC1 activation is maladaptive during development of cardiac hypertrophy, myocardial ischemia, and in obesity and metabolic syndrome. Rapamycin, the optimal mTORC1 inhibitor is proved to be beneficial under these conditions. In case of neurodegenerative diseases like AD and PD, autophagy plays an important role and mTOR is the negative regulator of autophagy. Hence, it is found out to be involved in accumulation of Aβ, aggregation of tau, and accumulation of α-synuclein, and its inhibition by rapamycin is proved to be beneficial in animal models of various neurodegenerative diseases. Interestingly, whereas the activation of mTOR plays an adaptive role in acute kidney injury, its activation in many other renal diseases has been shown to be deleterious. There are still various concepts related to the regulation of mTOR which have not been revealed yet. Particularly, the mechanism by which mTOR is activated by Rheb still remains to be unresolved, and this mechanism needs to be resolved in future. Another theory to be unmasked is the identification of the downstream targets of S6K, which stimulate cell growth by promoting translation of TOP mRNAs. Further studies on the integration between mTORC1 and mTORC2 signaling pathways are required. The presence of additional mTOR complexes that regulate any other biological processes still remains elusive. Thus a better understanding of the function of mTOR and its downstream interacting proteins may lead to more selective pharmacological intervention with respect to various diseases.
References
Armengol G, Rojo F, Castellví J, Iglesias C, Cuatrecasas M, Pons B, Baselga J, y Cajal SR (2007) 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res 67(16):7551–7555
Bärlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, Torhorst J, Haas P, Bucher C, Sauter G, Kallioniemi OP (2000) Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst 92(15):1252–1259
Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F (2008) Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8(5):411–424
Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O’Kane CJ, Rubinsztein DC (2006) Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet 15(3):433–442
Bockaert J, Marin P (2015) mTOR in brain physiology and pathologies. Physiol Rev 95(4):1157–1187
Brown MS, Goldstein JL (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7(2):95–96
Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL (1994) A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369(6483):756–758
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and tau effects on cognitive impairments. J Biol Chem 285(17):13107–13120
Caccamo A, Maldonado MA, Majumder S, Medina DX, Holbein W, Magrí A, Oddo S (2011) Naturally secreted amyloid-β increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism. J Biol Chem 286(11):8924–8932
Caccamo A, Magrì A, Medina DX, Wisely EV, López-Aranda MF, Silva AJ, Oddo S (2013) mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell 12(3):370–380
Cai Z, Zhao B, Li K, Zhang L, Li C, Quazi SH, Tan Y (2012) Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer’s disease? J Neurosci Res 90(6):1105–1118
Carnevalli LS, Masuda K, Frigerio F, Le Bacquer O, Um SH, Gandin V, Topisirovic I, Sonenberg N, Thomas G, Kozma SC (2010) S6K1 plays a critical role in early adipocyte differentiation. Dev Cell 18(5):763–774
Cavallucci V, D’Amelio M, Cecconi F (2012) Aβ toxicity in Alzheimer’s disease. Mol Neurobiol 45(2):366–378
Chen JK, Chen J, Neilson EG, Harris RC (2005) Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol 16(5):1384–1391
Choi KC, Kim SH, Ha JY, Kim ST, Son JH (2010) A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem 112(2):366–376
Clemente CF, Tornatore TF, Theizen TH, Deckmann AC, Pereira TC, Lopes-Cendes I, Souza JRM, Franchini KG (2007) Targeting focal adhesion kinase with small interfering RNA prevents and reverses load-induced cardiac hypertrophy in mice. Circ Res 101(12):1339–1348
Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P (2007) mTOR controls mitochondrial oxidative function through a YY1–PGC-1α transcriptional complex. Nature 450(7170):736–740
Das A, Salloum FN, Durrant D, Ockaili R, Kukreja RC (2012) Rapamycin protects against myocardial ischemia–reperfusion injury through JAK2–STAT3 signaling pathway. J Mol Cell Cardiol 53(6):858–869
De Benedetti A, Graff JR (2004) eIF-4E expression and its role in malignancies and metastases. Oncogene 23(18):3189–3199
Decressac M, Björklund A (2013) mTOR inhibition alleviates L-DOPA-induced dyskinesia in parkinsonian rats. J Parkinson’s Dis 3(1):13–17
Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A (2013) TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci 110(19):E1817–E1826
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Investig 115(5):1343–1351
Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39(2):171–183
Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM (2013) Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493(7434):679–683
Elghazi L, Balcazar N, Blandino-Rosano M, Cras-Méneur C, Fatrai S, Gould AP, Chi MM, Moley KH, Bernal-Mizrachi E (2010) Decreased IRS signaling impairs β-cell cycle progression and survival in transgenic mice overexpressing S6K in β-cells. Diabetes 59(10):2390–2399
Estacio RO, Schrier RW (2000) Diabetic nephropathy: pathogenesis, diagnosis, and prevention of progression. Adv Intern Med 46:359–408
Faivre S, Kroemer G, Raymond E (2006) Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 5(8):671–688
Fingar DC, Blenis J (2004) Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23(18):3151–3171
Floto RA, Sarkar S, Perlstein EO, Kampmann B, Schreiber SL, Rubinsztein DC (2007) Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington’s disease models and enhance killing of mycobacteria by macrophages. Autophagy 3(6):620–622
Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G (2008) mTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 57(4):945–957
Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM (2006) mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol 16(18):1865–1870
Fuller TF, Freise CE, Serkova N, Niemann CU, Olson JL, Feng S (2003) Sirolimus delays recovery of rat kidney transplants after ischemia-reperfusion injury. Transplantation 76(11):1594–1599
Gan X, Wang J, Wang C, Sommer E, Kozasa T, Srinivasula S, Alessi D, Offermanns S, Simon MI, Wu D (2012) PRR5L degradation promotes mTORC2-mediated PKC-[delta] phosphorylation and cell migration downstream of G [alpha] 12. Nat Cell Biol 14(7):686–696
García-Martínez JM, Alessi DR (2008) mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum-and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J 416(3):375–385
Grahammer F, Wanner N, Huber TB (2014) mTOR controls kidney epithelia in health and disease. Nephrol Dial Transplant 29(suppl 1):i9–i18
Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA (2011) Rictor/mTORC2 is essential for maintaining a balance between β-cell proliferation and cell size. Diabetes 60(3):827–837
Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell 11(6):859–871
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18(16):1926–1945
Hostetter TH (1995) Progression of renal disease and renal hypertrophy. Annu Rev Physiol 57(1):263–278
Hostetter TH (2003) Hyperfiltration and glomerulosclerosis. WB Saunders. Semin Nephrol 23:194–199
Hussain S, Feldman AL, Das C, Ziesmer SC, Ansell SM, Galardy PJ (2013) Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Mol Cell Biol 33(6):1188–1197
Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL (2008) Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J 27(14):1919–1931
Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, Lu B (2008) Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J 27(18):2432–2443
Inoki K (2008) Role of TSC–mTOR pathway in diabetic nephropathy. Diabetes Res Clin Pract 82:S59–S62
Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4(9):648–657
Inoki K, Corradetti MN, Guan KL (2005) Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 37(1):19–24
Ito S, Sawada M, Haneda M, Ishida Y, Isobe KI (2006) Amyloid-β peptides induce several chemokine mRNA expressions in the primary microglia and Ra2 cell line via the PI3K/Akt and/or ERK pathway. Neurosci Res 56(3):294–299
Ito S, Kimura K, Haneda M, Ishida Y, Sawada M, Isobe KI (2007) Induction of matrix metalloproteinases (MMP3, MMP12 and MMP13) expression in the microglia by amyloid-β stimulation via the PI3K/Akt pathway. Exp Gerontol 42(6):532–537
Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6:1122–1128
Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127(1):125–137
Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian LP, Liu J, Ding JQ, Chen SD (2013) Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J NeuroImmune Pharmacol 8(1):356–369
Johnson SC, Rabinovitch PS, Kaeberlein M (2013) mTOR is a key modulator of ageing and age-related disease. Nature 493(7432):338–345
Kantidakis T, Ramsbottom BA, Birch JL, Dowding SN, White RJ (2010) mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci 107(26):11823–11828
Khamzina L, Veilleux A, Bergeron S, Marette A (2005) Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146(3):1473–1481
Kim JE, Chen J (2004) Regulation of peroxisome proliferator–activated receptor-γ activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 53(11):2748–2756
Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL (2008) Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10(8):935–945
Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC (2008) Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol 28(1):61–70
Laplante M, Sabatini DM (2009) An emerging role of mTOR in lipid biosynthesis. Curr Biol 19(22):R1046–R1052
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293
Laplante M, Sabatini DM (2013) Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126(8):1713–1719
Lavandero S, Foncea R, Pérez V, Sapag-Hagar M (1998) Effect of inhibitors of signal transduction on IGF-1-induced protein synthesis associated with hypertrophy in cultured neonatal rat ventricular myocytes. FEBS Lett 422(2):193–196
Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N (2007) Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Investig 117(2):387–396
Lee CH, Inoki K, Guan KL (2007a) mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol 47:443–467
Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS (2007b) A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292(2):F617–F627
Li X, Gao T (2014) mTORC2 phosphorylates protein kinase Cζ to regulate its stability and activity. EMBO Rep 15(2):191–198
Li L, Zhang X, Le W (2010a) Autophagy dysfunction in Alzheimer’s disease. Neurodegener Dis 7(4):265–271
Li S, Brown MS, Goldstein JL (2010b) Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci 107(8):3441–3446
Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacoke M, Eng C (1997) Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16(1):64–67
Lieberthal W, Fuhro R, Andry CC, Rennke H, Abernathy VE, Koh JS, Valeri R, Levine JS (2001) Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Ren Physiol 281(4):F693–F706
Lieberthal W, Fuhro R, Andry C, Patel V, Levine JS (2006) Rapamycin delays but does not prevent recovery from acute renal failure: role of acquired tubular resistance. Transplantation 82(1):17–22
Lin X, Parisiadou L, Sgobio C, Liu G, Yu J, Sun L, Shim H, Gu XL, Luo J, Long CX, Ding J (2012) Conditional expression of Parkinson’s disease-related mutant α-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci 32(27):9248–9264
Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyó JM (2006) Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17(5):1395–1404
Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J (2005) Rheb binds and regulates the mTOR kinase. Curr Biol 15(8):702–713
Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y (2010) PGC-1α regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal 13(7):1011–1022
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10(5):307–318
Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA (2010) Rapamycin Protects against Neuron Death in In Vitro and In Vivo Models of Parkinson’s Disease. J Neurosci 30(3):1166–1175
Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS (2007) High glucose, high insulin, and their combination rapidly induce laminin-β1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes 56(2):476–485
Mayer C, Zhao J, Yuan X, Grummt I (2004) mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev 18(4):423–434
McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S (2003) Phosphoinositide 3-kinase (p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci 100(21):12355–12360
McTaggart RA, Gottlieb D, Brooks J, Bacchetti P, Roberts JP, Tomlanovich S, Feng S (2003) Sirolimus prolongs recovery from delayed graft function after cadaveric renal transplantation. Am J Transplant 3(4):416–423
Megyesi J, Andrade L, Vieira JM, Safirstein RL, Price PM (2002) Coordination of the cell cycle is an important determinant of the syndrome of acute renal failure. Am J Physiol Ren Physiol 283(4):F810–F816
Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16(6):345–357
Molitch ME, Defronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW (2004) Nephropathy in diabetes. Diabetes Care 27:S79–S83
Murata H, Sakaguchi M, Jin Y, Sakaguchi Y, Futami JI, Yamada H, Kataoka K, Huh NH (2011) A New Cytosolic Pathway from a Parkinson Disease-associated Kinase, BRPK/PINK1 ACTIVATION OF AKT VIA MTORC2. J Biol Chem 286(9):7182–7189
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136(3):521–534
Nigam SK, Lieberthal W (2000) Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Ren Physiol 279(1):F3–F11
Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ (2005) Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 102(40):14238–14243
Oddo S (2012) The role of mTOR signaling in Alzheimer disease. Front Biosci (Scholar edition) 4:941
Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, Manning BD, Hotamisligil GS (2008) Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29(5):541–551
Pastorino JG, Hoek JB (2003) Hexokinase II: the integration of energy metabolism and control of apoptosis. Curr Med Chem 10(16):1535–1551
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci 100(14):8466–8471
Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G (2000) Hypoinsulinaemia, glucose intolerance and diminished β-cell size in S6K1-deficient mice. Nature 408(6815):994–997
Pérez-Revuelta BI, Hettich MM, Ciociaro A, Rotermund C, Kahle PJ, Krauss S, Di Monte DA (2014) Metformin lowers Ser-129 phosphorylated α-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis 5(5):e1209
Peterson RT, Beal PA, Comb MJ, Schreiber SL (2000) FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem 275(10):7416–7423
Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM (2009) DEPTOR is an mTOR inhibitor frequently overeexpressed in multiple myeloma cells and required for their survival. Cell 137(5):873–886
Peterson TR, Sengupta SS, Harris TE, Carmack A, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM (2011) mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146(3):408–420
Philp A, Hamilton DL, Baar K (2011) Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol 110(2):561–568
Polak P, Cybulski N, Feige JN, Auwerx J, Rüegg MA, Hall MN (2008) Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 8(5):399–410
Proud CG (2004) Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc Res 63(3):403–413
Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E (2008) Disruption of Tsc2 in pancreatic β cells induces β cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci 105(27):9250–9255
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36(6):585–595
Romani-Aumedes J, Canal M, Martin-Flores N, Sun X, Perez-Fernandez V, Wewering S, Fernandez-Santiago R, Ezquerra M, Pont-Sunyer C, Lafuente A, Alberch J (2013) Parkin loss of function contributes to RTP801 elevation and neurodegeneration in Parkinson’s disease. Cell Death Dis 5(8):e1364
Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH (1994) RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78(1):35–43
Sadoshima J, Izumo S (1995) Rapamycin selectively inhibits angiotensin II–induced increase in protein synthesis in cardiac myocytes in vitro potential role of 70-kD S6 kinase in angiotensin II–induced cardiac hypertrophy. Circ Res 77(6):1040–1052
Sakaguchi M, Isono M, Isshiki K, Sugimoto T, Koya D, Kashiwagi A (2006) Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice. Biochem Biophys Res Commun 340(1):296–301
Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM (2007) PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25(6):903–915
Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320(5882):1496–1501
Santini E, Heiman M, Greengard P, Valjent E, Fisone G (2009) Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA–induced dyskinesia. Sci Signal 2(80):ra36
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14(14):1296–1302
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307(5712):1098–1101
Sataranatarajan K, Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, Kasinath BS (2007) Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol 171(6):1733–1742
Sato T, Nakashima A, Guo L, Coffman K, Tamanoi F (2010) Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene 29(18):2746–2752
Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J (2012) Rheb is a critical regulator of autophagy during myocardial ischemia pathophysiological implications in obesity and metabolic syndrome. Circulation 125(9):1134–1146
Sciarretta S, Volpe M, Sadoshima J (2014) Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res 114(3):549–564
Sciarretta S, Zhai P, Maejima Y, Del Re DP, Nagarajan N, Yee D, Liu T, Magnuson MA, Volpe M, Frati G, Li H (2015) mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep 11(1):125–136
Sekulić A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT (2000) A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res 60(13):3504–3513
Sen S, Kundu BK, Wu HCJ, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, Scott B (2013) Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J Am Heart Assoc 2(3):e004796
Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM (2010) mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468(7327):1100–1104
Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L (2009) TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res 105(10):1023–1030
Sharma S, Guthrie PH, Chan SS, Haq S, Taegtmeyer H (2007) Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart. Cardiovasc Res 76(1):71–80
Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC (2004) The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6(1):91–99
Shigeyama Y, Kobayashi T, Kido Y, Hashimoto N, Asahara SI, Matsuda T, Takeda A, Inoue T, Shibutani Y, Koyanagi M, Uchida T (2008) Biphasic response of pancreatic β-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol Cell Biol 28(9):2971–2979
Shor B, Wu J, Shakey Q, Toral-Barza L, Shi C, Follettie M, Yu K (2010) Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III-dependent transcription in cancer cells. J Biol Chem 285(20):15380–15392
Simm A, Schlüter KD, Diez C, Piper HM, Hoppe J (1998) Activation of p70 S6 Kinase by β-adrenoceptor Agonists on Adult Cardiomyocytes. J Mol Cell Cardiol 30(10):2059–2067
Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR (2005) A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54(7):1926–1933
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68(15):6084–6091
Subramaniam S, Napolitano F, Mealer RG, Kim S, Errico F, Barrow R, Shahani N, Tyagi R, Snyder SH, Usiello A (2012) Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nat Neurosci 15(2):191–193
Swiech L, Perycz M, Malik A, Jaworski J (2008) Role of mTOR in physiology and pathology of the nervous system. Biochim Et Biophys Acta (BBA) Proteins Proteom 1784(1):116–132
Tamguney T, Stokoe D (2007) New insights into PTEN. J Cell Sci 120(23):4071–4079
Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J (2003) Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13(15):1259–1268
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284(12):8023–8032
Tian Y, Bustos V, Flajolet M, Greengard P (2011) A small-molecule enhancer of autophagy decreases levels of Aβ and APP-CTF via Atg5-dependent autophagy pathway. FASEB J 25(6):1934–1942
Tremblay F, Brûlé S, Um SH, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A (2007) Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient-and obesity-induced insulin resistance. Proc Natl Acad Sci 104(35):14056–14061
Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G (2004) Absence of S6K1 protects against age-and diet-induced obesity while enhancing insulin sensitivity. Nature 431(7005):200–205
Völkers M, Konstandin MH, Doroudgar S, Toko H, Quijada P, Din S, Joyo A, Ornelas L, Samse K, Thuerauf DJ, Gude N (2013a) Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation 128(19):2132–2144
Völkers M, Toko H, Doroudgar S, Din S, Quijada P, Joyo AY, Ornelas L, Joyo E, Thuerauf DJ, Konstandin MH, Gude N (2013b) Pathological hypertrophy amelioration by PRAS40-mediated inhibition of mTORC1. Proc Natl Acad Sci 110(31):12661–12666
Wang L, Gout I, Proud CG (2001) Cross-talk between the ERK and p70 S6 kinase (S6K) signaling pathways MEK-dependent activation of S6K2 in cardiomyocytes. J Biol Chem 276(35):32670–32677
Wang X, Hu Z, Hu J, Du J, Mitch WE (2006) Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147(9):4160–4168
Wang L, Harris TE, Roth RA, Lawrence JC (2007) PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem 282(27):20036–20044
Wang BT, Ducker GS, Barczak AJ, Barbeau R, Erle DJ, Shokat KM (2011) The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc Natl Acad Sci 108(37):15201–15206
Wilson JE (2003) Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206(12):2049–2057
Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD (2011) Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab 14(1):21–32
Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, Kim J, Verheijen J, Curran K, Malwitz DJ, Cole DC (2009) Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res 69(15):6232–6240
Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J (2011) Differential roles of GSK-3β during myocardial ischemia and ischemia/reperfusion. Circ Res 109(5):502–511
Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y (2007) Inducible Nitric Oxide Synthase Deficiency Protects the Heart From Systolic Overload–Induced Ventricular Hypertrophy and Congestive Heart Failure. Circ Res 100(7):1089–1098
Zhang HH, Huang J, Düvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD (2009) Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One 4(7):e6189
Zhu XC, Yu JT, Jiang T, Tan L (2013) Autophagy modulation for Alzheimer’s disease therapy. Mol Neurobiol 48(3):702–714
Zinzalla V, Stracka D, Oppliger W, Hall MN (2011) Activation of mTORC2 by association with the ribosome. Cell 144(5):757–768
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaur, A., Sharma, S. Mammalian target of rapamycin (mTOR) as a potential therapeutic target in various diseases. Inflammopharmacol 25, 293–312 (2017). https://doi.org/10.1007/s10787-017-0336-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0336-1